ABSTRACT
Immune imprinting is a phenomenon that stems from the fundamentals of immunological memory. Upon recurrent exposures to an evolving pathogen, the immune system must weigh the benefits of rapidly recalling established antibody repertoires with greater affinity to the initial variant or invest additional time and energy in producing de novo responses specific to the emerging variant. In this review, we delve into the mechanistic complexities of immune imprinting and its role in shaping subsequent immune responses, both de novo and recall, against rapidly evolving respiratory viruses such as influenza and coronaviruses. By exploring the duality of immune imprinting, we examine its potential to both enhance or hinder immune protection against disease, while emphasizing the role of host and viral factors. Finally, we explore how different vaccine platforms may affect immune imprinting and comment on vaccine strategies that can favor de novo variant-specific antibody responses.
KEYWORDS: Original antigenic sin, immune imprinting, influenza, coronaviruses, SARS-CoV-2, antigenic seniority, antibody feedback
Immune imprinting
Constant exposure to evolving microbes represents a lifelong battle between the host and pathogen, beginning in childhood and continuing into old age. The cornerstone of adaptive humoral immunity relies on the induction of progressively compounding and efficient antibody responses following recurrent antigenic exposures, an effective mechanism exploited by seasonal or booster vaccinations.1 Yet, this prompts the question: To what degree does our first exposure to a virus, through either natural infection or vaccination, shape subsequent immune responses to later encounters with antigenically related variants? This query has perplexed immunologists since a series of pivotal studies in the 1950s examined humoral response dynamics across distinct age ranges before and after infection or vaccination with different influenza strains.2–4 Key observations were: 1) potent recall antibody responses induced against viral antigens not included in the vaccine formulation, and 2) the dominant antibody responses were against influenza strains encountered earliest in life. Consequently, Thomas Francis postulated the doctrine of original antigenic sin (OAS), describing an immune “imprint established by the original virus infection [that] governs the antibody response thereafter.”5
Although OAS or immune imprinting has primarily been observed with influenza due to its recurrent seasonality and high mutability, it has also been noted in other viral infections, including dengue virus, norovirus, hepatitis C (HCV), respiratory syncytial virus (RSV), and more recently, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).6–11 Evidence for imprinting in non-respiratory viruses includes the induction of antibody recall responses to the initially encountered antigen and the production of cross-reactive antibodies following reinfection with heterologous noroviruses genotypes or HCV subtypes. Differential infection risks and worsened clinical outcomes have been observed following sequential exposures to heterologous viral strains. For instance, increased hemorrhagic fever risk has been noted in individuals following secondary dengue infections with a heterologous serotype to the primary infecting serotype, suggesting immune imprinting effects.12 However, antibody-dependent enhancement (ADE) has also been observed where preexisting antibodies form complexes that facilitate viral entry into target immune cells via Fc receptor binding.6 Enhanced RSV disease severity was observed among children vaccinated against RSV that were subsequently infected.7 Thus, differential humoral responses following heterologous viral exposures along with specific evidence of immune imprinting in recurrent influenza infections and from recurrent SARS-CoV-2 infections or vaccinations, highlight the critical need for ongoing research on immune imprinting.
Mechanistic intricacies of immune imprinting
Circulating antibodies offer a unique snapshot of an individual’s distinctive history of infection. Following infection, the host’s B cells can directly recognize native (unprocessed) antigens in secondary lymphoid organs via their binding to the B cell receptor (BCR), and processed antigens that are displayed on MHC class II molecules on the surface of antigen-presenting cells (APC).13,14 Interactions with the antigen in both of these scenarios initiates B cell activation through receptor-induced signaling, which can be considerably enhanced by cross-linking multiple BCRs with multivalent antigens or repeating epitopes.13,14 Although most soluble protein antigens do not contain repetitive epitopes, particulate antigens like viruses or viral-like particles (VLP) often have repetitive arrays of antigen on their surface and can promote BCR cross-linking through higher affinity binding that enhances priming of B cells prior to receiving T cell help.14 Efficient interactions with the T cell receptor (TCR) and CD40 ligand (CD40L) expressed on antigen-specific helper T cells lead to further B cell activation, proliferation, and differentiation. In the early phase of the humoral response, following the first antigenic exposure, a portion of activated B cells differentiate into plasmablasts and provide an immediate but short-lived source of low-affinity antibodies to eliminate acute infections (Figure 1a). As the humoral response develops, more specialized responses are induced. Remaining activated B cells will differentiate into memory B cells (MBC) or migrate to secondary lymphoid tissues to form germinal centers (GC), where they will undergo isotype class switching, somatic hypermutation, and affinity maturation.15 Selected B cells with high affinity for the antigen can differentiate into plasma cells (LLPC), which are long-lived and continuous secretors of antibodies, or into MBCs, which will rapidly respond upon reencountering the viral antigen. These become the dominant source of antibodies in subsequent exposures to the antigen (Figure 1a). Thus, both LLPCs and MBCs are desirable cell types to elicit by vaccination, as they can rapidly respond following re-exposure. Therefore, with each new exposure to an antigenically distant viral variant, the induced humoral response may comprise both recall and de novo antibody responses, depending on the antigenic similarity between prior and subsequent antigens (Figure 1c).
Figure 1.
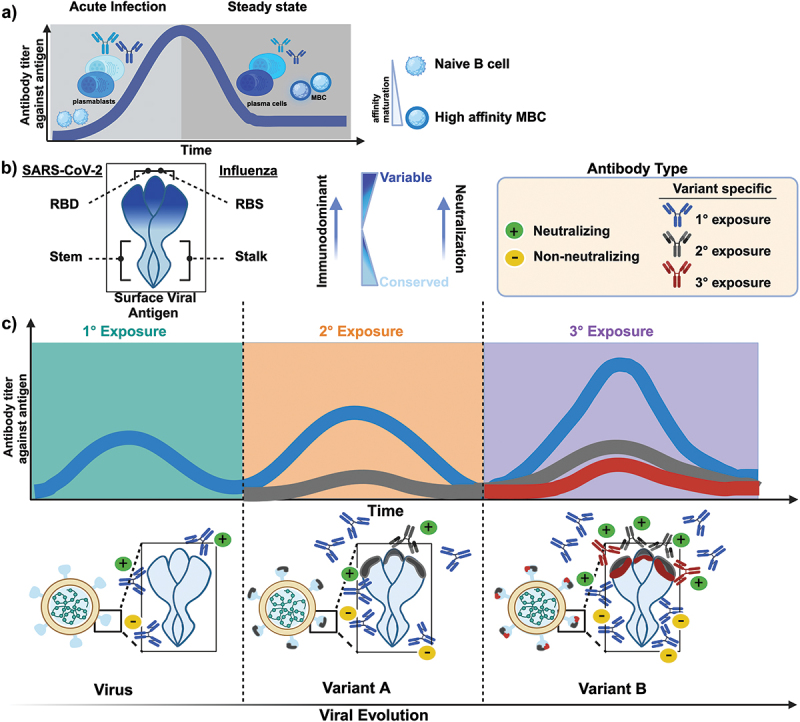
The dynamics of antibody responses following primary and subsequent infections with antigenically distant viruses. a) schematic illustration depicts the dynamics of the humoral response following infection or vaccination. Initially, after the first exposure, a portion of activated B cells differentiate into plasmablasts, which provide an immediate but short-lived source of low-affinity antibodies to limit acute infections. As the humoral response progresses, more specialized responses generate long-lived plasma cells and memory B cells (MBC) which persist at steady state. These cells will rapidly proliferate upon a reencounter with the antigen and become the dominant source of antibodies for subsequent exposures. b) schematic illustration of SARS-CoV-2 and influenza surface viral proteins highlighting the receptor-binding domain (RBD) and/or the receptor-binding site (RBS) as well as the stem and stalk domain, respectively. The gradient represents the more variable, immunodominant domain (dark blue) relative to the more conserved domain (light blue) of the surface viral protein. The RBD/RBS contains numerous immunodominant epitopes and is an important neutralization target given its role in cell-mediated entry. c) the kinetics of antibody responses following primary and subsequent infections with antigenically distant viruses are depicted. The induced humoral response may compromise both recall and de novo responses, depending on the antigenic similarity and cross-reactivity between the infecting variant and previously encountered variants. Primary exposure to an antigen elicits a polyclonal antibody response consisting of both neutralizing and non-neutralizing antibodies. Each subsequent exposure to an antigenically distant viral variant significantly boosts antibodies targeting conserved epitopes while also inducing de novo variant-specific neutralizing responses, albeit at much lower magnitudes. Exposure to an antigenically distinct antigen (variant B) alleviates immunological imprinting and promotes de novo responses targeted to variant-specific epitopes.
The emergence of SARS-CoV-2 and the characterization of population immunity following repeated infections and vaccine boosters offer a unique opportunity to disentangle immune imprinting. Most infections and vaccines induce antibodies targeting the immunodominant spike protein, an envelope glycoprotein involved in viral fusion and entry into permissive cells. Influenza’s hemagglutinin (HA) protein serves a similar role. Both are trimeric proteins composed of a variable receptor-binding domain (RBD) or receptor-binding site (RBS) and a more conserved stem or stalk, respectively (Figure 1b). Given the roles of the RBD or RBS in receptor binding and cell entry, these regions contain the majority of antigenic sites where antibody binding can directly inhibit infection, leading to virus neutralization. Neutralizing antibodies are considered a strong correlate of protection against SARS-CoV-2 and influenza infection.16,17 Epitopes within the RBD or RBS that are critical for virus binding are also, generally, highly immunogenic and attract the humoral response toward them. This targeted immunodominance creates a strong selection pressure that favors the emergence of mutations, also called antigenic drift. In new viral variants, immune escape mutations commonly arise at these immunodominant epitopes targeted by neutralizing antibodies (Figure 1c).18–20 However, some neutralizing antibodies can target epitopes within the stem or stalk regions, which are relatively conserved between viral variants, are less susceptible to immune escape, and can be broadly protective.19,21,22 Non-neutralizing antibodies that target subdominant epitopes or regions in the stem or stalk play an important role in recruiting innate and cellular antiviral immunity, and for some viral pathogens, this can occur through the effector functions of Fc-receptors.23,24 The accumulation of mutations, largely detected in the RBD of recent SARS-CoV-2 variants, further demonstrates the immunodominance of this domain and its significant role in humoral responses. This suggests that SARS-CoV-2 antigen evolution is strongly influenced by the selective pressures exerted by neutralizing responses, similar to influenza’s antigenic drift.19,20
Since the 1950s, the term OAS has been used interchangeably with other terminologies such as antigen imprinting, immunological imprinting, and immune imprinting to describe the lifelong biased reactivity toward the original antigen. Importantly, the intended terminology stemmed from epidemiological observations of differential antibody reactivity shaped by both exposure history and age, as highlighted in several excellent reviews.25–29 Several additional concepts are complementary and peripheral to immune imprinting. For instance, antigenic seniority denotes the quantitative hierarchal nature of antibody induction, wherein the highest titers are observed against antigens encountered earlier in life with progressively lower titers against subsequent exposures to antigenically related viruses (Figure 1c). Similarly, the term back-boosting describes an increase or “bosting” of cross-reactive recall responses to conserved epitopes between the ancestral and latest viral exposure, rather than the induction of de novo antibody synthesis toward variant-specific epitopes (Figure 1c). Conversely, back-boosting reflects the immune system’s propensity for inducing cross-reactive antibodies with each infection. Indeed, recall responses can be favored over de novo antibody production, as MBCs can be activated with significantly lower antigen concentrations compared to naïve B cells, despite the latter having higher affinity for the immunizing antigen, underlying the basis of immune imprinting effects.30–33 Furthermore, antibody feedback mechanisms can steer the antibody response and restrict the generation of de novo responses with similar specificities. This can occur through antigen sequestering (clearance) by established cellular or humoral responses following exposure to closely related strains.30,32,34–39 One example is epitope masking, where existing antibodies bind to conserved but non-neutralizing epitopes, promoting antigen clearance. This mechanism restricts novel antigen interactions with memory or naïve B cells and hinders the induction of de novo antibody synthesis against similar epitopes.34–36,40–42
While much attention for deciphering the mechanisms that drive immune imprinting has focused on humoral immunity, T cell-mediated immunity also plays a crucial role. Indeed, recent evidence highlights the role of T cells in shaping antibody responses. Richards et al. show that reduced CD4+ T cell responses in individuals with recurrent exposures to influenza can lead to diminished protective antibody responses.37 Regulatory T cells induced by the initial exposure can reduce the amount of available antigen for presentation to APCs, thereby suppressing activation of naïve B cells and the synthesis of variant-specific de novo antibodies.38 Moreover, Schiepers et al. show that memory T cells assist B cells in targeting subdominant epitopes to access secondary germinal centers (GCs) following boosting with a homologous antigen, even in the presence of preexisting antibodies.39 Thus, this evidence highlights the interplay between humoral and cellular compartments in favoring imprinting responses, which can be further exacerbated by limiting the amount of antigen available following recurrent exposures to antigenically similar variants.
Immune imprinting in recurrent exposures to influenza
The skewing of memory recall responses to a restricted range of epitopes from the earliest infections has been shown to suppress the induction of variant-specific de novo responses, thereby increasing an individual’s susceptibility to new infection with antigenically distant viruses.43,44 This phenomenon of immune imprinting has been described in detail for influenza.25–29 Multiple studies have shown that the immune response is biased toward influenza strains encountered earlier in life and that during ensuing exposures to antigenically related strains, antibody production toward the ancestral strain predominates. Lessler et al. observed antigenic seniority for influenza in a cross-sectional study where individuals had higher antibody titers against strains encountered earlier in life rather than against strains from recent infections.44 Back-boosting of neutralizing antibodies against preceding influenza strains following subsequent influenza exposures was observed in a longitudinal analysis of the Framingham Heart Study data.43 Other studies have also confirmed these findings, reporting an increase in antibodies against previously encountered antigenically related strains following both influenza infection and vaccination.19,30,43,45–50
In immune imprinting, back-boosting coincides with a reduction in the de novo response to newer strains, with much of the recent evidence arising after the 2009 H1N1 pandemic. Choi et al. determined that vaccination for the 2009 pandemic H1N1 influenza virus induced relatively lower antibody titers among individuals who were vaccinated with the seasonal H1N1 vaccine three months prior.51 Recalled cross-reactive antibodies against seasonal H1N1 strains were shown by Andrews et al. to block the development of broadly neutralizing antibodies against the HA stalk following vaccination against the 2009 pandemic strain through epitope masking.19 Imprinting was shown to decrease protection against antigenically related influenza strains and worsen disease outcomes. During the 1918 Spanish flu and 2009 H1N1 pandemics, the mortality rate was higher for those exposed to discordant subtypes in early childhood.26 Mortality rates varied similarly according to age (and therefore exposure profile) in the 1957 H2N2 pandemic.52 Similarly, vaccine effectiveness against H1N1 was impaired for middle-aged and older adults during the 2015–2016 flu season when the circulating H1N1 influenza strain acquired a new glycosylation site.53 Furthermore, reduced protection from vaccination in the 2018/2019 H3N2 epidemic was associated with age and prior exposure to a mutated H3N2 strain during the 1968 pandemic.54
Although much attention has been focused on the detrimental impacts of imprinting, it does not solely impair immune function.25 Indeed, older individuals may benefit from increased protection and lower mortality rates compared to immunologically naive individuals due to immune imprinting.55 These beneficial impacts of immune imprinting, acquired from infections encountered even several decades earlier, can persist throughout an individual’s lifetime. Birth cohort studies reporting increased titers against influenza strains encountered earlier in life suggest that imprinting reinforces immune memory against earlier strains upon exposure to antigenically related strains throughout life.26 A multi-country study by Gostic et al. found that individuals initially infected with influenza A group 1 (H1, H2, and H5 seasonal subtypes) or group 2 (H3 and H7 seasonal subtypes) had a relatively low risk of mortality and severe illness from subsequent infections with the corresponding HA subtypes.25,26,46 This protection likely results from antigenic seniority and the back-boosting of cross-reactive antibodies targeting conserved regions between the initial and infecting virus of the same HA subtype. In accordance with this evidence, epidemiological studies have reported lower mortality rates in the 2009 H1N1pandemic for older generations exposed to the 1918 H1N1 Spanish flu compared to younger generations.56 For example, in China, 92% of hospitalizations and 77% of deaths resulting from 2009 H1N1 infections occurred in individuals under the age of 50. Similarly, during the 1918 pandemic, older individuals had lower mortality rates relative to younger individuals.46 These observations indicate that imprinting induces a strong recall response toward the initial viral exposures and the induction of cross-reactive antibodies through back-boosting. This may confer improved protection for older individuals as they are exposed to antigenically similar viruses across their lifetime, thereby strengthening immunity against the initial virus.
Effect of previous HCoV infections on the induction of SARS-CoV-2 humoral responses
Early in the SARS-CoV-2 pandemic, some studies suggested that prior exposure to human seasonal coronaviruses (HCoVs) 229E, HKU-1, NL63, or OC43 could negatively impact the development of humoral responses specific to SARS-CoV-257–60. The shared sequence homology between HCoVs and SARS-CoV-2 hinted at the potential for immune imprinting. Indeed, back-boosting of antibodies cross-reactive to spike proteins of HCoVs has been observed following SARS-CoV-2 infection and vaccination.57,58,60,61 For example, Yin et al. determined that COVID-19 vaccination was associated with increased titers against OC43 and HKU-1. Other studies report that SARS-CoV-2 infection also stimulated the production of antibodies against OC43 and HKU-1.60,62,63 However, the literature on the clinical implications of immune imprinting from prior exposure to HCoVs on SARS-CoV-2 immunity is nuanced. Some evidence indicates that such imprinting may worsen clinical outcomes of SARS-CoV-2 infection.57–60 Indeed, Aguilar-Bretones et al. reported an expansion of MBCs specific for HCoV-OC43 with limited cross-reactivity to SARS-CoV-2 in severely ill SARS-CoV-2 patients, but not in mildly ill patients.57 Furthermore, they showed relatively low IgG antibody reactivity and neutralization to SARS-CoV-2 in these patients with severe COVID-19.57 In contrast, Guo et al. observed that disease severity correlated with a greater induction of back-boosting antibodies against HCoV-OC43, which were also cross-reactive with SARS-CoV-2.58
Additionally, some researchers have demonstrated a link between immune imprinting and post-acute sequelae, also called post-COVID-19 condition (PCC).59 In convalescent SARS-CoV-2 patients with underlying rheumatic disease, those who developed PCC had reduced antibody reactivity to SARS-CoV-2 and increased reactivity to HCoV-OC43 compared to those who did not develop PCC.59 In turn, Galipeau et al. suggest that the relative ratio of prior antibody responses against all four HCoVs, rather than the absolute titer against a specific HCoV, correlated with increased SARS-CoV-2 neutralization.64 However, some studies did not observe a correlation between back-boosting of HCoV antibodies upon infection with SARS-CoV-2 and more severe disease.60,61 This contrasts with some influenza studies that reported the potential detrimental effect of immune imprinting.25,26,43,44,51 In particular, Anderson et al. and Aydillo et al. report back-boosting of antibodies against HCoVs following SARS-CoV-2 infection in hospitalized adult patients, but this had no bearing on disease severity.60,61 However, Aydillo et al. noted a delay in de novo antibody production against SARS-CoV-2 in severely ill patients.60 Although these findings reveal conflicting perspectives on the impact of prior seasonal HCoV infections on disease severity following SARS-CoV-2 infection, they suggest the involvement of complex immune imprinting influences through the induction of back-boosting antibodies to these HCoVs.
The dynamics of serum antibody responses and the antigenic distance hypothesis
The extent of immune imprinting and antibody reactivity toward subsequent infections appears to be fundamentally dependent on the antigenic relatedness between the primary and subsequent antigens, as first postulated by Smith et al.65 Antigenic distance between two antigenically similar viruses can either enhance or hinder immune protection against disease.65 When the level of relatedness between the primary and secondary strains is high, significant back-boosting of antibodies can occur (Figure 1b). Although this could still protect from infection when back-boosted antibodies still effectively neutralized the circulating variant strain. Indeed, Hensley et al. eloquently demonstrated that mice heterologously boosted with an antigenically related influenza variant, which differed by 13 amino acid substitutions relative to the priming variant, preferentially induced recall antibody responses.66 Although approximately 55% of isolated monoclonal antibodies were specific to the priming antigen, adoptive transfer of serum antibodies following boosting still protected mice from infectious challenge with the secondary strain.66 In contrast, when circulating variants acquire immune escape mutations but are still sufficiently antigenically similar to the primary exposure, recall responses are favored at the expense of de novo antibody synthesis and to the detriment of protection as discussed above.19,30,43,45–50 However, immune imprinting can be overcome by boosting with more distantly related viral antigens, as shown in several mouse studies.41,67–69
The proportion of de novo serum antibodies synthesized relative to those produced through recall responses, and the compounding effect of subsequent antigen re-exposures have been challenging to study (Figure 1c). Recently, Schiepers and colleagues employed an eloquent molecular fate-mapping technique to decipher antibody kinetics and their cellular origins following repeated antigen exposures.41 In transgenic mice primed either by infection or vaccination with messenger RNA-Lipid Nanoparticle (mRNA-LNP), they distinguished antibodies generated from initially primed MBCs and those from de novo synthesis. In a prime vaccination followed by a two-dose boost regimen with the ancestral SARS-CoV-2 spike vaccine (homologous boosting), Schiepers et al. observed a 55-fold suppression of de novo B cell responses as a result of dominant recall responses. However, a heterologous boost with the Omicron BA.1 spike mRNA vaccine, Omicron BA.1 being a variant of SARS-CoV-2 that shares 91% sequence in the RBD with the ancestral strain, resulted in a partial alleviation of immune imprinting. In particular, they observed only a 3.6-fold suppression of de novo antibody responses with the heterologous boost. Following the heterologous boost, Schiepers et al. detected new BA.1-specific B cell clones that were not cross-reactive with the ancestral strain and that showed greater neutralization against the BA.1 variant specifically. Furthermore, by varying the amino acid sequence of influenza boosting antigens, they experimentally demonstrated that immune imprinting is a function of antigenic distance between the primary and subsequently encountered antigens.41,67,68 Collectively, these findings suggest that immune imprinting can be alleviated when the boosting antigen is sufficiently distinct from the priming antigen.
B cell activation outcomes and the dynamics of germinal center reactions
Given the role of GC reactions in shaping the diversity of the humoral response, understanding B cell clonal selection and expansion following primary or repeated antigen exposures is critical for dissecting the fundamentals of immune imprinting (Figure 2). In GCs, activated B cells will undergo iterative rounds of somatic hypermutation, where only the clones expressing the highest affinity receptors to the antigen are selected for clonal expansion by receiving survival signals from T follicular helper cells.70 Some of these affinity-matured clones make their way into circulation, serving as a source of antibodies or as MBCs (Figure 2). However, a portion of MBC clones will undergo further affinity maturation, leading to inter-clonal competition and a progressive loss of MBC clonal diversity given that selection favors clones with the highest antigen affinity to immunodominant epitopes (Figure 2). Immunodominant epitopes for SARS-CoV-2 and influenza viruses are frequently detected in the RBD and RBS, compared to the relatively immuno-subdominant stem or stalk, respectively (Figure 1b). This immunogenicity could result from their increased epitope exposure compared to the more sheltered regions of the stem or stalk, repetitive epitope display, or specific amino acid sequences leading to improved B cell receptor cross-linking.19,20,71 Nevertheless, GC reactions concurrently lead to the breadth of the humoral response. Through lineage tracing experiments, Hagglof et al. demonstrated that ongoing GCs continuously recruit activated B cells and up to 30% of late-stage GCs can consist of clones with limited rounds of mutations and low antigen affinity.72 Their findings suggest that as the GC reactions develop, antibody-mediated feedback can favor the entry and expansion of B cells specific for non-dominant epitopes, thereby contributing to the maintenance of antibody diversity. Indeed, following primary COVID-19 mRNA vaccination in humans, Weber et al. detected early exported MBCs with limited clonal affinity maturation.73 These MBCs exhibited broad neutralization against currently circulating viral variants, emphasizing the diversity of the early humoral repertoire.
Figure 2.
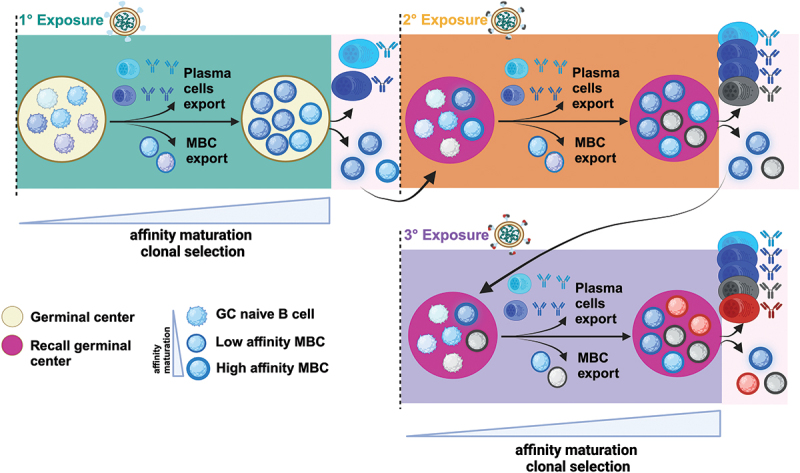
B cell activation outcomes and dynamics of germinal center (GC) reactions following primary and secondary infections with antigenically distant viruses. the schematic illustration depicts the dynamics of GC reactions following primary and secondary infections with antigenically distant viruses. Activated B cells form GCs where they undergo isotype switching, somatic hypermutation, and affinity maturation. Only clones expressing the highest affinity receptors to the antigen are selected for clonal expansion by receiving survival signals from T follicular helper cells. Some of these affinity-matured clones make their way into circulation, serving as a source of antibodies or as low-affinity MBCs. However, a portion of MBC clones will undergo further affinity maturation, leading to a progressive loss of clonal diversity as selection favors clones with the highest antigen affinity to immunodominant epitopes. These high-affinity B cells can differentiate into plasma cells or into MBCs. Following re-exposure through subsequent infection or vaccine boosting, recall GCs are mostly composed of activated B cells without prior GC experience (gc-naïve B cells) and a limited subset of dominant affinity-matured MBCs.
Recent evidence highlights that mRNA-LNP vaccines induce potent GC responses in both mice and humans, which have important implications for vaccine design.15,74–82 However, the mechanisms by which the distinct mRNA and LNP components of mRNA-LNP vaccines promote robust GC reactions remain largely undefined. Recent findings by Bettini et al. and others suggest that both components promote the differentiation of T follicular helper cells and dendritic cells, thereby enhancing the overall magnitude of GC reactions compared to protein-subunit vaccines adjuvanted with LNP.74,80,81,83 Moreover, studies from several groups suggest that prolonged antigen expression and availability following mRNA-LNP vaccination may contribute to enhanced and sustained GC reactions.77,80,82,84–87 Indeed, comparisons between mRNA-LNP vaccines and protein subunit vaccines adjuvanted with LNP or other adjuvants highlight the potent adjuvant properties of LNP, which likely significantly contribute to the impressive immunogenicity of the mRNA-LNP vaccine platform.74,79–81,83,86,88,89 Overall these findings suggest that the LNP component of vaccines has intrinsic adjuvant activity and is a major driver in promoting durable and protective antibodies through the combined induction of T follicular helper cells, long-lived plasma cells, MBC and prolonged GC reactions.15,74–82 Although ongoing GCs have been detected over varying periods following SARS-CoV-2 exposure from natural infection or vaccination, these GC reactions are to shown drive clonal diversification through antibody-mediated feedback to produce antibodies with sufficient breadth to neutralize a range of antigenic variants.36,77,87,90,91
Two alternative models exist to explain GC dynamics following re-exposure through subsequent infection or boosting. First, in the conventional model, affinity-matured MBCs can readily enter secondary or recall GCs for further affinity maturation to finetune their affinity for antigenically drifted viruses.15 However, recent evidence highlights another potential model of GC dynamics and demonstrates a bottleneck in the recruitment of the diverse, affinity-matured MBCs into recall GCs, restricting entry to only a small portion of high-affinity immunodominant clones in mice and humans34,39,92 (Figure 2). Mesin et al. demonstrate that recall GCs are mostly composed of activated B cells without prior GC experience (GC-naïve B cells) and a limited subset of dominant affinity-matured MBCs regardless of re-infection or boosting in mouse models. Their findings suggest that antibody-mediated feedback suppresses the recruitment of these affinity-matured MBCs, promoting the recruitment and diversification of GC-naïve B cells in recall GCs, thereby circumventing immune imprinting (Figure 2). Similarly, human lymph node analysis by Turner et al. showed substantial recruitment of GC-naïve B cells to recall GCs following vaccination with influenza despite prior exposures.93 However, their results suggest a higher propensity for humans, relative to mice, for MBC entry into recall GCs, highlighting a greater potential for immune imprinting to occur. Yet, despite the large diversity of MBCs generated by each exposure, recall antibodies seem to consistently derive from a subset of dominant MBCs generated by the original exposure (Figure 2),8,41,73,94 In a longitudinal analysis of the B cell repertoire in humans following both vaccination and subsequent Omicron infection, Weber et al. found that a substantial proportion of humoral responses stemmed from recall of the original cohort of MBCs produced following the primary vaccination series.73 Thus, understanding dynamics of germinal center reactions and their role in the humoral response can have important implications for alleviating immune imprinting in the context of recurrent exposures to antigenically distinct viruses.
Influence of the vaccination platform in promoting or overriding immune imprinting
Secondary viral infection and vaccination can result in varying intensities of immune imprinting. The immune system often favors recall responses over of de novo synthesis given that MBCs can be activated with much lower antigen concentrations than naïve B cells. Indeed, these effects can be further exacerbated when the amount of antigen available during the secondary exposure is limited, favoring recall responses.30,32,34–39 Several studies demonstrate this phenomenon by reducing the dose of protein subunit vaccines or infectious viruses. In the case of the latter, the humoral and cellular memory responses established during the initial exposure can suppress viral replication, thereby limiting the amount of viral antigens available to stimulate new responses.30–33,84,85 In the case of protein subunit vaccination, the amount of administered antigen may be more susceptible to antibody feedback mechanisms, potentially exacerbating the detrimental effects of immune imprinting. In contrast, vaccine platforms that are self-replicating, such as some DNA or mRNA vaccines, may bypass preexisting antibodies and effectively overcome the constraints of immune imprinting mechanisms.27,68
Indeed, intramuscular administration of mRNA-LNP vaccines leads to uptake and synthesis of encoded antigens primarily by local cells at the injection site and the draining lymph node, with limited spread to other tissues.82,95,96 APCs, such as monocytes, macrophages, dendritic cells, play a critical role in mRNA-LNP uptake, antigen production, and subsequent antigen presentation for robust induction of humoral and cellular responses.74,82,86,95,97 Indeed, monocytes, macrophages and dendritic cells can directly uptake and efficiently translate the mRNA of encoded antigens, thereby driving the immune response through presentation of processed antigen on MHC class I and MHC class II molecules to produce both cellular and humoral responses, respectively. Muscle cells can also uptake and produce antigens and depending on the mRNA design, the synthesized antigen will either be membrane-anchored or secreted.98,99 Additionally, mRNA-LNP can be passively transported to draining lymph nodes, although this seems to depend on particle size and composition and needs further investigation.86 While B cells have the potential to directly uptake and synthesize the encoded antigens, this appears to be less common compared to the direct uptake by macrophages, monocytes and dendritic cells.74,95,97,100 Instead, B cells will likely interact with either secreted soluble antigens or processed antigens presented on the surface of APCs, which may influence the strength of receptor induced signaling, based on the degree of cross-linking.14,15,101 In fact, membrane-bound antigens have been shown to be more efficiently recognized compared to soluble antigens.14,102–104
Anderson et al. demonstrate through absorption assays, that a first SARS-CoV-2 exposure through natural infection induced back-boosted antibodies with high cross-reactivity to the stem region of sCoV spike protein, contrasting with mRNA-LNP vaccination, which predominantly produced antibodies with higher affinity to the RBD of SARS-CoV-262. Furthermore, studies by England et al. and Willis et al. demonstrated that mRNA-LNP vaccines against SARS-CoV-2 or influenza effectively overcame maternal antibody inhibition of de novo humoral responses in vaccinated mouse pups.79,83 Although Willis et al. showed limited de novo responses following a live attenuated influenza vaccine (LAIV) or inactivated vaccine immunization in presence of maternal antibodies, England et al. demonstrated that adjuvanted protein subunit vaccines effectively evoked de novo responses despite the presence of the maternal antibodies, with mRNA-LNP vaccines inducing the highest neutralizing titers.79,83 Indeed, the inclusion of adjuvants during initial or secondary vaccination has been shown to alleviate the effects of immune imprinting. Kim et al. showed that adjuvants which preferentially stimulate dendritic cells, such as CpG ODN, Bordetella pertussis toxin, or squalene-based oil-in-water, elicit strain-specific de novo total and neutralizing antibody responses.68
In contrast, viruses and VLPs can bind to B cells with high affinity and avidity by crosslinking BCRs with repetitive antigens displayed on their surfaces, thereby enhancing B cell responses and retention in the lymph nodes.100,105 Cohen et al., as well as others groups, have demonstrated that immunization with heterotypic VLPs, which simultaneously display RBDs from eight different SARS-like beta coronaviruses (sarbecovirus), promote crosslinking interactions with high avidity to subdominant, conserved regions of the adjacent RBD antigens.106–110 This leads to the induction of broadly cross-reactive and neutralizing responses compared to homotypic VLPs that display only one type of RBD antigen. Furthermore, through fate mapping experiments discussed above, Cohen et al. demonstrate that boosting with heterotypic VLPs induces de novo antibody synthesis in mice and non-human primates previously primed with two to four doses of DNA, mRNA, or viral vectored SARS-CoV-2 vaccines.107 Additionally, serum antibody epitope mapping using deep mutational scanning revealed that boosting with heterotypic VLPs, relative to the homotypic VLPs, elicit antibodies with preferential binding to conserved epitopes of the RBD. These epitopes exhibit low variability among sarbecoviruses and SARS-CoV-2 variants. In contrast, antibodies induced by homotypic VLPs vaccination primarily targeted epitopes on the apex of the antigen. Although more accessible, these epitopes of the RBD are more variable likely due to greater selective pressures, consistent with findings by several groups.107,108,111–114 This highlights the potential of immunization with heterotypic VLPs or “universal vaccines” as a strategy to mitigate the effects of immune imprinting following recurrent infections with antigenically distinct variants.
Immune imprinting following recurrent exposures to SARS-CoV-2 antigens through natural infection or vaccination
The extensive characterization of population immunity following recurrent exposures to SARS-CoV-2 through booster vaccinations or breakthrough infections has provided immunologists with a unique opportunity to dissect the effects of immune imprinting in the context of SARS-CoV-2. The continuous circulation and transmission of SARS-CoV-2 has led to the emergence of variants of concern (VOC), characterized by substantial antigenic variation, evolved fitness advantages and distinguished from other viral variants by their increased risk of causing severe COVID-19 disease. These changes are primarily driven by selective pressures arising from recurrent vaccinations and infections.19,20,115,116 Notably, the efficacy of ancestral SARS-CoV-2 mRNA-LNP and viral vector vaccines was reduced against the Beta variant, showing more than a ten-fold reduction in neutralizing titers in sera from vaccinated donors.117–119 Breakthrough infections with the Alpha or Delta variants resulted in a greater increase in antibody titers against the ancestral strain compared to the VOC strain in individuals vaccinated with three doses of ancestral mRNA-LNP, highlighting the effects of immune imprinting.87,120 Meanwhile Zar et al. show antibody back-boosting to ancestral, Beta, Delta or Omicron variants without a clear hierarchical order.121 Efforts to enhance vaccine efficacy by updating vaccines have led to improved VOC neutralization. However, individuals previously vaccinated with the ancestral mRNA vaccines showed dominant recall antibody responses following monovalent Beta or Delta boosters, or bivalent ancestral and Beta/Delta boosters.8,122,123 In contrast, boosting with a bivalent ancestral and Beta mRNA vaccine, relative to boosting with the ancestral vaccine, led to a reduction in symptomatic infection from 10.7% to 3.4%.123
Following the emergence of Omicron and its sub-variants, which possess a greater number of immune escape mutations over earlier variants, varying effects of immune imprinting have been observed.115 Omicron breakthrough infections predominantly promoted recall responses, leading to reduced neutralization of Omicron variants. Although some de novo antibody synthesis has been observed, it remains at a low level.8,124,125 Vaccination with an Omicron-specific mRNA-LNP booster has been shown to produce de novo antibody responses and superior neutralization against Omicron variants compared to individuals boosted with the ancestral vaccine, which demonstrates the benefits of updating the vaccine to match circulating variants.123,126–129 However, conflicting evidence reported from some studies indicate no significant differences in neutralizing antibody titers between those boosted with the Omicron bivalent vaccine or the ancestral vaccine, highlighting potential effects of immune imprinting.130–134 Whereas, Alsoussi et al. demonstrated that in individuals previously vaccinated with the ancestral SARS-CoV-2 mRNA vaccine, variant-specific de novo B cell responses were only induced with Omicron-specific boosting, and not with the ancestral or bivalent Beta/Delta variant boosters (which shared 98.7% and 99.1% sequence conservation with the ancestral RBD, respectively)8. Although being of low frequency, these clones targeting BA.1 variant-specific epitopes were derived from GC-naïve B cells recruited into recall GCs of the draining lymph nodes following heterologous boosting. Yisimayi et al. demonstrate that boosting with two doses of Omicron protein subunit or mRNA vaccines induced robust variant-specific de novo humoral responses in both mice and individuals previously immunize with ancestral SARS-CoV-2 viral vector vaccines.67 These findings suggest that priming with inactivated viral vector vaccines may lead to a reduced degree of immune imprinting compared to mRNA vaccines, potentially due the stronger immune response elicited by initial mRNA vaccination. Interestingly, breakthrough infections in individuals with diverse exposure histories from vaccination and infection, have been shown to increase the breadth of neutralizing antibody responses against previous and circulating VOCs, as well as elicit de novo responses.120,121,124,125 In fact, Hoffman et al. demonstrated that boosting with ancestral and BA4.5 bivalent vaccines produced enhanced neutralizing responses against circulating Omicron variant in individuals who had previously received two doses of ancestral mRNA-LNP vaccines and subsequently experienced a breakthrough infection. However, the highest neutralizing titers were observed against the ancestral strain, highlighting the effects of immune imprinting.124 These findings collectively suggest that Omicron-specific booster vaccinations may partially alleviate the effects of immune imprinting caused by repeated exposures to early SARS-CoV-2 variants. However, their findings also highlight that maintaining the ancestral antigen in the bivalent booster vaccine may favor immune imprinting, supporting the collective decision to proceed with monovalent SARS-CoV-2 variant specific booster roll-out. Indeed, monovalent XBB.1.5 boosters administered to individuals with a prior vaccination history markedly increased serum neutralizing antibody titers against the XBB.1.5 variant, as well as against the more recently emerged EG.5.1 and JN.1 variants.135,136
However, these studies emphasize the persistence of immune imprinting, given that the highest neutralizing responses were observed against the ancestral variant. Notably, Tortorici et al. showed that depletion of antibodies that are cross-reactive with the ancestral antigen abrogated the observed neutralization of XBB1.5, suggesting that mRNA vaccination with the XBB1.5 booster primarily induces recall responses.137 However, Johnston et al. demonstrated the development of variant-specific de novo humoral responses, albeit at a low frequency, following boosting with the XBB1.5 monovalent mRNA vaccine in individuals who were previously vaccinated with ancestral mRNA vaccines and experienced breakthrough infections.135 Indeed, these studies evaluating neutralizing responses following vaccination with updated booster vaccines highlight the persistence of immune imprinting, which can be reduced when the boosting antigen is sufficiently distinct from ancestral SARS-CoV-2. Therefore, comprehensive evaluations of antigenic distance scores between primary and subsequent viral variants, while comparing various vaccine platforms, may help predict their ability to promote recall or de novo antibody responses. When interpreting these studies, it is crucial to consider variables such as 1) differences in antigen presentation by different vaccine platforms (and therefore their recognition by the immune system), 2) antigenic distance between primary and subsequent antigens, and 3) the nature of primary exposure and subsequent re-exposures (natural infection or vaccination).
While several comparisons between influenza and SARS-CoV-2 studies regarding immune imprinting have been discussed, it is important to approach these interpretations with caution due to inherent differences in their virology, epidemiology, and vaccination approaches. Furthermore, this review highlights the importance of investigating whether different vaccine platforms induce varying degrees of immune imprinting following repeated exposures. Collectively, these findings suggest that boosting with a significantly distinct antigen is paramount in steering the humoral immune response away from favoring recall and toward de novo responses that target variant-specific epitopes.
Conclusion
Given the role of immune imprinting in shaping humoral responses following recurrent exposures to antigenically distant viruses, its impact should be thoroughly considered when designing vaccination strategies. The evolving landscape of SARS-CoV-2 and the emergence of variants with immune escape mutations have prompted multiple updates to COVID-19 vaccines. These updates aim to improve vaccine efficacy against circulating variants, similar to seasonal updates for influenza vaccines. Recent studies on SARS-CoV-2 and influenza have shown that boosting with sufficiently antigenically distinct vaccine promotes the induction of variant-specific de novo responses that are less cross-reactive with previously encountered antigens. Indeed, some of the next-generation vaccine strategies focus on developing universal vaccines, which induce both variant-specific and broadly protective cross-reactive responses by simultaneously expressing multiple surface glycoproteins from distinct influenza subtypes or coronaviruses.107,138 Ultimately, the effectiveness of these vaccines will depend on their capacity to overcome the constraints of immune imprinting and address age-related differences in disease risk, which are ultimately shaped by individual’s distinct infection histories. A comprehensive understanding of immune imprinting is essential for guiding vaccine design and maximizing vaccine effectiveness against both current and future viral infections.
Acknowledgments
M.M. holds an Ontario Graduate Scholarship. M.-A.L. holds a Faculty of Medicine Chair of Excellence in Pandemic Viruses and Preparedness Research. M.-A.L. and C.C. are members of and have received research funding from the Coronavirus Variants Rapid Response Network (CoVaRR-Net). Figures were created with BioRender.com.
Biography
Marc-André Langlois is a molecular virologist specializing in microbiology and immunology, with a research program dedicated to unraveling the intricate dynamics between viruses and host immune defenses. A Full Professor at the University of Ottawa, Dr. Langlois also holds the position of Executive Director for the national Coronavirus Variants Rapid Response Network (CoVaRR-Net). Additionally, Dr. Langlois is an esteemed member of the College of the Royal Society of Canada, and holds the Faculty of Medicine Chair of Excellence in Pandemic Viruses and Preparedness Research.
Funding Statement
The work was supported by the Canadian Institutes of Health Research [VR2-172722]; Canadian Institutes of Health Research [ARR-175622].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
M.M., A.K., C.C., and M.-A.L. designed the study. M.M. and M.-A.L. designed the figures. M.M., A.K., and M.A.L wrote the manuscript. All authors edited the final version of the manuscript.
References
- 1.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–14. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen KE, Davenport FM, Hennessy AV, Francis T Jr. Characterization of influenza antibodies by serum absorption. J Exp Med. 1956;104:199–209. doi: 10.1084/jem.104.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport FM, Hennessy AV, Francis T Jr. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J Exp Med. 1953;98:641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport FM, Hennessy AV. A serologic recapitulation of past experiences with influenza A; antibody response to monovalent vaccine. J Exp Med. 1956;104:85–97. doi: 10.1084/jem.104.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis T. On the doctrine of original antigenic sin. Proc Am Phil Soc. 1960;104(6):572–578. [Google Scholar]
- 6.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 8.Alsoussi WB, Malladi SK, Zhou JQ, Liu Z, Ying B, Kim W, Schmitz AJ, Lei T, Horvath SC, Sturtz AJ, et al. SARS-CoV-2 omicron boosting induces de novo B cell response in humans. Nature. 2023;617(7961):592–598. doi: 10.1038/s41586-023-06025-4. [DOI] [PubMed] [Google Scholar]
- 9.Underwood AP, Gupta M, Wu BR, Eltahla AA, Boo I, Wang JJ, Agapiou D, Abayasingam A, Reynaldi A, Keoshkerian E, Zhao Y. B-cell characteristics define HCV reinfection outcome. J Hepatol. 2024; doi: 10.1016/j.jhep.2024.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Lindesmith LC, Boshier FAT, Brewer-Jensen PD, Roy S, Costantini V, Mallory ML, Zweigart M, May SR, Conrad H, O’Reilly KM, et al. Immune imprinting drives human norovirus potential for global spread. mBio. 2022;13(5):e0186122. doi: 10.1128/mbio.01861-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol. 2010;84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwon P, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol. 2011;85(1):410–421. doi: 10.1128/JVI.01826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce SK, Liu W. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat Rev Immunol. 2010;10(11):767–777. doi: 10.1038/nri2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 15.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 16.Khoury DS, Schlub TE, Cromer D, Steain M, Fong Y, Gilbert PB, Subbarao K, Triccas JA, Kent SJ, Davenport MP, Correlates of protection, thresholds of protection, and immunobridging among persons with SARS-CoV-2 infection. Emerg Infect Dis. 2023;29:381–388. doi: 10.3201/eid2902.221422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett BJ, Grove J, MacLean OA, Wilkie C, De Lorenzo G, Furnon W, Cantoni D, Scott S, Logan N, Ashraf S, et al. SARS-CoV-2 omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022;7(8):1161–1179. doi: 10.1038/s41564-022-01143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Dunand CJ, Taylor WM, Lim S, Huang M, Qu X. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med. 2015;7:316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han AX, de Jong SPJ, Russell CA. Co-evolution of immunity and seasonal influenza viruses. Nat Rev Microbiol. 2023;21(12):805–817. doi: 10.1038/s41579-023-00945-8. [DOI] [PubMed] [Google Scholar]
- 21.Ng KW, Faulkner N, Finsterbusch K, Wu M, Harvey R, Hussain S, Greco M, Liu Y, Kjaer S, Swanton C, Gandhi S. SARS-CoV-2 S2-targeted vaccination elicits broadly neutralizing antibodies. Sci Transl Med. 2022;14:eabn3715. doi: 10.1126/scitranslmed.abn3715. [DOI] [PubMed] [Google Scholar]
- 22.Maltseva M, Galipeau Y, Renner TM, Deschatelets L, Durocher Y, Akache B, Langlois MA. Characterization of systemic and mucosal humoral immune responses to an adjuvanted intranasal SARS-CoV-2 protein subunit vaccine candidate in mice. Vaccines. 2022;11(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe A, McCarthy KR, Kuraoka M, Schmidt AG, Adachi Y, Onodera T, Tonouchi K, Caradonna TM, Bajic G, Song S, McGee CE. Antibodies to a conserved influenza head interface epitope protect by an IgG subtype-dependent mechanism. Cell. 2019;177:1124–1135 e1116. doi: 10.1016/j.cell.2019.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He, W., Tan, G.S., Mullarkey, C.E., Lee, A.J., Lam, M.M.W., Krammer, F., Henry, C., Wilson, P.C., Ashkar, A.A., Palese, P. and Miller, M.S. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza a virus. Proc Natl Acad Sci USA. 2016;113:11931–11936. doi: 10.1073/pnas.1609316113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koutsakos M, Ellebedy AH. Immunological imprinting: understanding COVID-19. Immunity. 2023;56(5):909–913. doi: 10.1016/j.immuni.2023.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J Immunol. 2019;202:335–340. doi: 10.4049/jimmunol.1801149. [DOI] [PubMed] [Google Scholar]
- 27.Yewdell JW, Santos JJS. Original antigenic sin: how original? How sinful? Cold Spring Harb Perspect Med. 2021;11(5):a038786. doi: 10.1101/cshperspect.a038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CQ, Vishwanath S, Carnell GW, Chan ACY, Heeney JL. Immune imprinting and next-generation coronavirus vaccines. Nat Microbiol. 2023;8(11):1971–1985. doi: 10.1038/s41564-023-01505-9. [DOI] [PubMed] [Google Scholar]
- 29.Gouma S, Anderson EM, Hensley SE. Challenges of making effective influenza vaccines. Annu Rev Virol. 2020;7:495–512. doi: 10.1146/annurev-virology-010320-044746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Skountzou I, Compans R, Jacob J. Original antigenic sin responses to influenza viruses. J Immunol. 2009;183:3294–3301. doi: 10.4049/jimmunol.0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster RG, Kasel JA, Couch RB, Laver WG. Influenza virus subunit vaccines. II. Immunogenicity and original antigenic sin in humans. J Infect Dis. 1976;134:48–58. doi: 10.1093/infdis/134.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Fazekas de St G, Webster RG. Disquisitions on original antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braciale TJ, Gerhard W, Klinman NR. Analysis of the humoral immune response to influenza virus in vitro. J Immunol. 1976;116:827–834. [PubMed] [Google Scholar]
- 34.Tas JM, Koo JH., Lin, YC., Xie, Z., Steichen, JM., Jackson, AM., Hauser, BM., Wang, X., Cottrell, CA., Torres, JL. et al., Antibodies from primary humoral responses modulate the recruitment of naive B cells during secondary responses. Immunity. 2022;55:1856–1871 e1856. doi: 10.1016/j.immuni.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Y., Meyer-Hermann, M., George, L.A., Figge, M.T., Khan, M., Goodall, M., Young, S.P., Reynolds, A., Falciani, F., Waisman, A. et al., Germinal center B cells govern their own fate via antibody feedback. J Exp Med. 2013;210:457–464. doi: 10.1084/jem.20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue, T., Shinnakasu, R., Kawai, C., Yamamoto, H., Sakakibara, S., Ono, C., Itoh, Y., Terooatea, T., Yamashita, K., Okamoto, T. and Hashii, N. Antibody feedback contributes to facilitating the development of omicron-reactive memory B cells in SARS-CoV-2 mRNA vaccinees. J Exp Med. 2023;220. doi: 10.1084/jem.20221786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards, K.A., Shannon, I., Treanor, J.J., Yang, H., Nayak, J.L. and Sant, A.J. Evidence that blunted CD4 T-Cell responses underlie deficient protective antibody responses to influenza vaccines in repeatedly vaccinated human subjects. J Infect Dis. 2020;222:273–277. doi: 10.1093/infdis/jiz433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndifon W. A simple mechanistic explanation for original antigenic sin and its alleviation by adjuvants. J R Soc Interface. 2015;12. doi: 10.1098/rsif.2015.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiepers A, van ‘t Wout MFL, Hobbs A, Mesin L, Victora GD. Opposing effects of pre-existing antibody and memory T cell help on the dynamics of recall germinal centers. bioRxiv. 2023; doi: 10.1101/2023.12.15.571936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer-Babajew D, Wang Z, Muecksch F, Cho A, Loewe M, Cipolla M, Raspe R, Johnson B, Canis M, DaSilva J, et al. Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination. Nature. 2023;613(7945):735–742. doi: 10.1038/s41586-022-05609-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiepers A, van ’t Wout MFL, Greaney AJ, Zang T, Muramatsu H, Lin PJC, Tam YK, Mesin L, Starr TN, Bieniasz PD, et al. Molecular fate-mapping of serum antibody responses to repeat immunization. Nature. 2023;615(7952):482–489. doi: 10.1038/s41586-023-05715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarnitsyna, V.I., Ellebedy, A.H., Davis, C., Jacob, J., Ahmed, R. and Antia, R. Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza. Philos Trans R Soc Lond B Biol Sci. 2015;370. doi: 10.1098/rstb.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, M.S., Gardner, T.J., Krammer, F., Aguado, L.C., Tortorella, D., Basler, C.F. and Palese, P. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med. 2013;5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJD, Guan Y, Jiang CQ, Cummings DAT, et al. Evidence for antigenic seniority in influenza a (H3N2) antibody responses in southern China. PLOS Pathog. 2012;8(7):e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunez IA, Carlock MA, Allen JD, Owino SO, Moehling KK, Nowalk P, Susick M, Diagle K, Sweeney K, Mundle S, et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza a strains. PLOS One. 2017;12(11):e0185666. doi: 10.1371/journal.pone.0185666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science. 2016;354(6313):722–726. doi: 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NMH, Pham QT, et al. Antibody landscapes after influenza virus infection or vaccination. Science. 2014;346(6212):996–1000. doi: 10.1126/science.1256427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter, D.M., Lu, H.R., Bloom, C.E., Crevar, C.J., Cherry, J.L., Lipman, D.J. and Ross, T.M. Complex patterns of human antisera reactivity to novel 2009 H1N1 and historical H1N1 influenza strains. PLOS One. 2012;7:e39435. doi: 10.1371/journal.pone.0039435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J, Boutz DR, Chromikova V, Joyce MG, Vollmers C, Leung K, Horton AP, DeKosky BJ, Lee C-H, Lavinder JJ, et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med. 2016;22(12):1456–1464. doi: 10.1038/nm.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skowronski, D.M., Hottes, T.S., McElhaney, J.E., Janjua, N.Z., Sabaiduc, S., Chan, T., Gentleman, B., Purych, D., Gardy, J., Patrick, D.M. and Brunham, R.C. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis. 2011;203:158–167. doi: 10.1093/infdis/jiq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi YS, Baek YH, Kang W, Nam SJ, Lee J, You S, Chang D-Y, Youn J-C, Choi YK, Shin E-C, et al. Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin Vaccine Immunol. 2011;18(9):1519–1523. doi: 10.1128/CVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gagnon A, Acosta E, Hallman S, Bourbeau R, Dillon LY, Ouellette N, Earn DJD, Herring DA, Inwood K, Madrenas J, et al. Pandemic paradox: early life H2N2 pandemic influenza infection enhanced susceptibility to death during the 2009 H1N1 pandemic. mBio. 2018;9(1). doi: 10.1128/mBio.02091-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewnard JA, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines (Basel). 2018;6(2):28. doi: 10.3390/vaccines6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skowronski, D.M., Sabaiduc, S., Leir, S., Rose, C., Zou, M., Murti, M., Dickinson, J.A., Olsha, R., Gubbay, J.B., Croxen, M.A. and Charest, H. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV). Euro Surveill. 2019;24. doi: 10.2807/1560-7917.ES.2019.24.46.1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma J, Dushoff J, Earn DJ. Age-specific mortality risk from pandemic influenza. J Theor Biol. 2011;288:29–34. doi: 10.1016/j.jtbi.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, et al. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLOS Med. 2013;10(11):e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilar-Bretones, M., Westerhuis, B.M., Raadsen, M.P., de Bruin, E., Chandler, F.D., Okba, N.M., Haagmans, B.L., Langerak, T., Endeman, H., van den Akker, J.P. and Gommers, D.A. Seasonal coronavirus-specific B cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J Clin Invest. 2021;131. doi: 10.1172/JCI150613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo, L., Wang, Y., Kang, L., Hu, Y., Wang, L., Zhong, J., Chen, H., Ren, L., Gu, X., Wang, G. and Wang, C. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect. 2021;10:664–676. doi: 10.1080/22221751.2021.1905488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydillo T, Rombauts A, Stadlbauer D, Aslam S, Abelenda-Alonso G, Escalera A, Amanat F, Jiang K, Krammer F, Carratala J, et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat Commun. 2021;12(1):3781. doi: 10.1038/s41467-021-23977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson, E.M., Goodwin, E.C., Verma, A., Arevalo, C.P., Bolton, M.J., Weirick, M.E., Gouma, S., McAllister, C.M., Christensen, S.R., Weaver, J. and Hicks, P. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin, D., Han, Z., Lang, B., Li, Y., Mai, G., Chen, H., Feng, L., Chen, Y.Q., Luo, H., Xiong, Y. and Jing, L. Effect of seasonal coronavirus immune imprinting on the immunogenicity of inactivated COVID-19 vaccination. Front Immunol. 2023;14:1195533. doi: 10.3389/fimmu.2023.1195533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson, E.M., Li, S.H., Awofolaju, M., Eilola, T., Goodwin, E., Bolton, M.J., Gouma, S., Manzoni, T.B., Hicks, P., Goel, R.R. and Painter, M.M. SARS-CoV-2 infections elicit higher levels of original antigenic sin antibodies compared with SARS-CoV-2 mRNA vaccinations. Cell Rep. 2022;41:111496. doi: 10.1016/j.celrep.2022.111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herman, J.D., Atyeo, C., Zur, Y., Cook, C.E., Patel, N.J., Vanni, K.M., Kowalski, E.N., Qian, G., Srivatsan, S., Shadick, N.A. and Rao, D.A. Humoral immunity to an endemic coronavirus is associated with postacute sequelae of COVID-19 in individuals with rheumatic diseases. Sci Transl Med. 2023;15:eadf6598. doi: 10.1126/scitranslmed.adf6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galipeau Y, Siragam V, Laroche G, Marion E, Greig M, McGuinty M, Booth RA, Durocher Y, Cuperlovic-Culf M, Bennett SAL, et al. Relative ratios of human seasonal coronavirus antibodies predict the efficiency of cross-neutralization of SARS-CoV-2 spike binding to ACE2. EBioMedicine. 2021;74:103700. doi: 10.1016/j.ebiom.2021.103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci USA. 1999;96:14001–14006. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linderman SL, Hensley SE, Sant AJ. Antibodies with ‘original antigenic sin’ properties are valuable components of secondary immune responses to influenza viruses. PLOS Pathog. 2016;12(8):e1005806. doi: 10.1371/journal.ppat.1005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yisimayi A, Song W, Wang J, Jian F, Yu Y, Chen X, Xu Y, Yang S, Niu X, Xiao T, et al. Repeated omicron exposures override ancestral SARS-CoV-2 immune imprinting. Nature. 2024;625(7993):148–156. doi: 10.1038/s41586-023-06753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JH, Davis WG, Sambhara S, Jacob J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc Natl Acad Sci USA. 2012;109:13751–13756. doi: 10.1073/pnas.0912458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramsay, L.C., Buchan, S.A., Stirling, R.G., Cowling, B.J., Feng, S., Kwong, J.C. and Warshawsky, B.F. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17:9. doi: 10.1186/s12916-018-1239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Victora GD, Wilson PC. Germinal center selection and the antibody response to influenza. Cell. 2015;163(3):545–548. doi: 10.1016/j.cell.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hägglöf, T., Cipolla, M., Loewe, M., Chen, S.T., Mesin, L., Hartweger, H., ElTanbouly, M.A., Cho, A., Gazumyan, A., Ramos, V. and Stamatatos, L. Continuous germinal center invasion contributes to the diversity of the immune response. Cell. 2023;186:147–161 e115. doi: 10.1016/j.cell.2022.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber, T., Dähling, S., Rose, S., Affeldt, P., Vanshylla, K., Ullrich, L., Gieselmann, L., Teipel, F., Gruell, H., Di Cristanziano, V. and Kim, D.S. Enhanced SARS-CoV-2 humoral immunity following breakthrough infection builds upon the preexisting memory B cell pool. Sci Immunol. 2023;8:eadk5845. doi: 10.1126/sciimmunol.adk5845. [DOI] [PubMed] [Google Scholar]
- 74.Bettini, E., Chudnovskiy, A., Protti, G., Nakadakari-Higa, S., Ceglia, S., Castano, D., Chiu, J., Muramatsu, H., Mdluli, T., Abraham, E. and Lipinszki, Z. Distinct components of nucleoside-modified messenger RNA vaccines cooperate to instruct efficient germinal center responses. bioRxiv. 2024; doi: 10.1101/2024.05.17.594726. [DOI] [Google Scholar]
- 75.Lederer, K., Bettini, E., Parvathaneni, K., Painter, M.M., Agarwal, D., Lundgreen, K.A., Weirick, M., Muralidharan, K., Castaño, D., Goel, R.R. and Xu, X. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell. 2022;185:1008–1024. doi: 10.1016/j.cell.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan HX, Juno JA, Lee WS, Barber-Axthelm I, Kelly HG, Wragg KM, Esterbauer R, Amarasena T, Mordant FL, Subbarao K, et al. Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques. Nat Commun. 2021;12(1):1403. doi: 10.1038/s41467-021-21665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, Lei T, Thapa M, Chen RE, Case JB, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lederer, K., Castaño, D., Atria, D.G., Oguin, T.H., Wang, S., Manzoni, T.B., Muramatsu, H., Hogan, M.J., Amanat, F., Cherubin, P. and Lundgreen, K.A. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willis, E., Pardi, N., Parkhouse, K., Mui, B.L., Tam, Y.K., Weissman, D. and Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci Transl Med. 2020;12. doi: 10.1126/scitranslmed.aav5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pardi, N., Hogan, M.J., Naradikian, M.S., Parkhouse, K., Cain, D.W., Jones, L., Moody, M.A., Verkerke, H.P., Myles, A., Willis, E. and LaBranche, C.C. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022;55(6):1136–1138. doi: 10.1016/j.immuni.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pardi, N., Tuyishime, S., Muramatsu, H., Kariko, K., Mui, B.L., Tam, Y.K., Madden, T.D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.England, R.N., Drapeau, E.M., Alameh, M.G., Hosseinzadeh, R., Weissman, D. and Hensley, S.E. Evaluation of mRNA-lnp and adjuvanted protein SARS-CoV-2 vaccines in a maternal antibody mouse model. NPJ Vaccines. 2024;9:110. doi: 10.1038/s41541-024-00901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cirelli KM, Crotty S. Germinal center enhancement by extended antigen availability. Curr Opin Immunol. 2017;47:64–69. doi: 10.1016/j.coi.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tam, H.H., Melo, M.B., Kang, M., Pelet, J.M., Ruda, V.M., Foley, M.H., Hu, J.K., Kumari, S., Crampton, J., Baldeon, A.D. and Sanders, R.W. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA. 2016;113:6639–6648. doi: 10.1073/pnas.1606050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbeke R, Hogan MJ, Lore K, Pardi N. Innate immune mechanisms of mRNA vaccines. Immunity. 2022;55(11):1993–2005. doi: 10.1016/j.immuni.2022.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Röltgen, K., Nielsen, S.C., Silva, O., Younes, S.F., Zaslavsky, M., Costales, C., Yang, F., Wirz, O.F., Solis, D., Hoh, R.A. and Wang, A. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shirai, S., Kawai, A., Shibuya, M., Munakata, L., Omata, D., Suzuki, R. and Yoshioka, Y. Lipid nanoparticle acts as a potential adjuvant for influenza split vaccine without inducing inflammatory responses. Vaccines. 2020;8. doi: 10.3390/vaccines8030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Awasthi S, Hook LM, Swaminathan G, Cairns TM, Brooks B, Smith JS, Ditto NT, Gindy ME, Bett AJ, Espeseth AS, et al. Antibody responses to crucial functional epitopes as a novel approach to assess immunogenicity of vaccine adjuvants. Vaccine. 2019;37(29):3770–3778. doi: 10.1016/j.vaccine.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, Agudelo M, Barnes CO, Gazumyan A, Finkin S, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, Hoffmann HH, Barnes CO, Cipolla M, Ramos V, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mesin, L., Schiepers, A., Ersching, J., Barbulescu, A., Cavazzoni, C.B., Angelini, A., Okada, T., Kurosaki, T. and Victora, G.D. Restricted clonality and limited germinal center reentry characterize memory B cell reactivation by boosting. Cell. 2020;180:92–106. doi: 10.1016/j.cell.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner JS, Zhou JQ, Han J, Schmitz AJ, Rizk AA, Alsoussi WB, Lei T, Amor M, McIntire KM, Meade P, et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature. 2020;586(7827):127–132. doi: 10.1038/s41586-020-2711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carreno JM, Singh G, Simon V, Krammer F, group, P. V. I. s . Bivalent COVID-19 booster vaccines and the absence of BA.5-specific antibodies. Lancet Microbe. 2023; 4: e569. doi: 10.1016/S2666-5247(23)00118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, Wimmers F, Sanyal M, Weidenbacher PA, Feng Y, et al. Mechanisms of innate and adaptive immunity to the pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543–555. doi: 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hassett, K.J., Benenato, K.E., Jacquinet, E., Lee, A., Woods, A., Yuzhakov, O., Himansu, S., Deterling, J., Geilich, B.M., Ketova, T. and Mihai, C. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang, F., Lindgren, G., Lin, A., Thompson, E.A., Ols, S., Röhss, J., John, S., Hassett, K., Yuzhakov, O., Bahl, K. and Brito, L.A. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25:2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lazzaro S, Giovani C, Mangiavacchi S, Magini D, Maione D, Baudner B, Geall AJ, De Gregorio E, D’Oro U, Buonsanti C, et al. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology. 2015;146(2):312–326. doi: 10.1111/imm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McNamara MA, Nair SK, Holl EK. RNA-Based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10(11):787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 101.Heesters BA, van der Poel CE, Das A, Carroll MC. Antigen presentation to B cells. Trends Immunol. 2016;37:844–854. doi: 10.1016/j.it.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30(1):44–55. doi: 10.1016/j.immuni.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Depoil, D., Fleire, S., Treanor, B.L., Weber, M., Harwood, N.E., Marchbank, K.L., Tybulewicz, V.L. and Batista, F.D. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 104.Carrasco YR, Batista FD. B cell recognition of membrane-bound antigen: an exquisite way of sensing ligands. Curr Opin Immunol. 2006;18:286–291. doi: 10.1016/j.coi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 105.Lainscek D, Fink T, Forstnerič V, Hafner-Bratkovič I, Orehek S, Strmšek Ž, Manček-Keber M, Pečan P, Esih H, Malenšek Š, et al. A nanoscaffolded spike-rbd vaccine provides protection against SARS-CoV-2 with minimal anti-scaffold response. Vaccines. 2021;9(5):431. doi: 10.3390/vaccines9050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen AA, Gnanapragasam PNP, Lee YE, Hoffman PR, Ou S, Kakutani LM, Keeffe JR, Wu H-J, Howarth M, West AP, et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science. 2021;371(6530):735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen, A.A., Keeffe, J.R., Schiepers, A., Dross, S.E., Greaney, A.J., Rorick, A.V., Gao, H., Gnanapragasam, P.N., Fan, C., West, A.P. and Ramsingh, A.I. Mosaic sarbecovirus vaccination elicits cross-reactive responses in pre-immunized animals. bioRxiv. 2024; doi: 10.1101/2024.02.08.576722. [DOI] [Google Scholar]
- 108.Cohen, A.A., van Doremalen, N., Greaney, A.J., Andersen, H., Sharma, A., Starr, T.N., Keeffe, J.R., Fan, C., Schulz, J.E., Gnanapragasam, P.N. and Kakutani, L.M. Mosaic RBD nanoparticles protect against multiple sarbecovirus challenges in animal models. bioRxiv. 2022; doi: 10.1101/2022.03.25.485875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burnett, D.L., Jackson, K.J., Langley, D.B., Aggarwal, A., Stella, A.O.Johansen, M.D., Balachandran, H., Lenthall, H., Rouet, R., Walker, G. and Saunders, B.M. Immunizations with diverse sarbecovirus receptor-binding domains elicit SARS-CoV-2 neutralizing antibodies against a conserved site of vulnerability. Immunity. 2021;54:2908–2921. doi: 10.1016/j.immuni.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walls, A.C., Miranda, M.C., Schäfer, A., Pham, M.N., Greaney, A., Arunachalam, P.S., Navarro, M.J., Tortorici, M.A., Rogers, K., O’Connor, M.A. and Shirreff, L. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell. 2021;184:5432–5447. doi: 10.1016/j.cell.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greaney, A.J., Starr, T.N., Gilchuk, P., Zost, S.J., Binshtein, E., Loes, A.N., Hilton, S.K., Huddleston, J., Eguia, R., Crawford, K.H. and Dingens, A.S. Complete mapping of mutations to the SARS-CoV-2 Spike receptor-binding Domain that escape antibody recognition. Cell Host Microbe. 2021;29:44–57. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barnes CO, Jette CA, Abernathy ME, Dam KMA, Esswein SR, Gristick HB, Malyutin AG, Sharaf NG, Huey-Tubman KE, Lee YE, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greaney AJ, Starr TN, Barnes CO, Weisblum Y, Schmidt F, Caskey M, Gaebler C, Cho A, Agudelo M, Finkin S, et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat Commun. 2021;12(1):4196. doi: 10.1038/s41467-021-24435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piccoli, L., Park, Y.J., Tortorici, M.A., Czudnochowski, N., Walls, A.C., Beltramello, M., Silacci-Fregni, C., Pinto, D., Rosen, L.E., Bowen, J.E. and Acton, O.J. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 Spike receptor-binding Domain by structure-guided high-resolution serology. Cell. 2020;183:1024–1042. doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, de Silva TI, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Telenti A, Arvin A, Corey L, Corti D, Diamond MS, García-Sastre A, Garry RF, Holmes EC, Pang PS, Virgin HW, et al. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596(7873):495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 117.Li T, Han X, Gu C, Guo H, Zhang H, Wang Y, Hu C, Wang K, Liu F, Luo F, et al. Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants. Nat Commun. 2021;12(1):6304. doi: 10.1038/s41467-021-26539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, Caspi I, Levy R, Leshchinsky M, Ken Dror S, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27(8):1379–1384. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goel, R.R., Painter, M.M., Lundgreen, K.A., Apostolidis, S.A., Baxter, A.E., Giles, J.R., Mathew, D., Pattekar, A., Reynaldi, A., Khoury, D.S. and Gouma, S. Efficient recall of omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185:1875–1887. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zar HJ, MacGinty R, Workman L, Botha M, Johnson M, Hunt A, Burd T, Nicol MP, Flasche S, Quilty BJ, et al. Natural and hybrid immunity following four COVID-19 waves: a prospective cohort study of mothers in South Africa. EClinicalMedicine. 2022;53:101655. doi: 10.1016/j.eclinm.2022.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chu L, Vrbicky K, Montefiori D, Huang W, Nestorova B, Chang Y, Carfi A, Edwards DK, Oestreicher J, Legault H, et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med. 2022;28(5):1042–1049. doi: 10.1038/s41591-022-01739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, McGhee N, Tomassini JE, Chen X, Chang Y, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med. 2022;387(14):1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoffmann, M., Behrens, G.M., Arora, P., Kempf, A., Nehlmeier, I., Cossmann, A., Manthey, L., Dopfer-Jablonka, A. and Pöhlmann, S. Effect of hybrid immunity and bivalent booster vaccination on omicron sublineage neutralisation. Lancet Infect Dis. 2023;23:25–28. doi: 10.1016/S1473-3099(22)00792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaku, C.I., Bergeron, A.J., Ahlm, C., Normark, J., Sakharkar, M., Forsell, M.N. and Walker, L.M. Recall of preexisting cross-reactive B cell memory after omicron BA.1 breakthrough infection. Sci Immunol. 2022;7:eabq3511. doi: 10.1126/sciimmunol.abq3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chalkias S, Whatley JL, Eder F, Essink B, Khetan S, Bradley P, Brosz A, McGhee N, Tomassini JE, Chen X, et al. Original SARS-CoV-2 monovalent and omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: phase 2/3 trial interim results. Nat Med. 2023;29(9):2325–2333. doi: 10.1038/s41591-023-02517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kurhade, C., Zou, J., Xia, H., Liu, M., Chang, H.C., Ren, P., Xie, X. and Shi, P.Y. Low neutralization of SARS-CoV-2 omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. 2023;29:344–347. doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]
- 128.Davis-Gardner ME, Lai L, Wali B, Samaha H, Solis D, Lee M, Porter-Morrison A, Hentenaar IT, Yamamoto F, Godbole S, et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA bivalent booster. N Engl J Med. 2023;388(2):183–185. doi: 10.1056/NEJMc2214293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou, J., Kurhade, C., Patel, S., Kitchin, N., Tompkins, K., Cutler, M., Cooper, D., Yang, Q., Cai, H., Muik, A. and Zhang, Y. Neutralization of BA.4-BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with bivalent vaccine. N Engl J Med. 2023;388:854–857. doi: 10.1056/NEJMc2214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Collier, A.R.Y., Miller, J., Hachmann, N.P., McMahan, K., Liu, J., Bondzie, E.A., Gallup, L., Rowe, M., Schonberg, E., Thai, S. and Barrett, J. Immunogenicity of BA.5 bivalent mRNA vaccine boosters. N Engl J Med. 2023;388:565–567. doi: 10.1056/NEJMc2213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang, Q., Bowen, A., Valdez, R., Gherasim, C., Gordon, A., Liu, L. and Ho, D.D. Antibody response to omicron BA.4-BA.5 bivalent booster. N Engl J Med. 2023;388:567–569. doi: 10.1056/NEJMc2213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang, Q., Guo, Y., Tam, A.R., Valdez, R., Gordon, A., Liu, L. and Ho, D.D. Deep immunological imprinting due to the ancestral spike in the current bivalent COVID-19 vaccine. Cell Rep Med. 2023;4:101258. doi: 10.1016/j.xcrm.2023.101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang, Q., Bowen, A., Tam, A.R., Valdez, R., Stoneman, E., Mellis, I.A., Gordon, A., Liu, L. and Ho, D.D. SARS-CoV-2 neutralising antibodies after bivalent versus monovalent booster. Lancet Infect Dis. 2023;23:527–528. doi: 10.1016/S1473-3099(23)00181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang, Q., Bowen, A., Ho, J., Zhang, R.M., Valdez, R., Stoneman, E., Gordon, A., Liu, L. and Ho, D.D. SARS-CoV-2 neutralising antibodies after a second BA.5 bivalent booster. Lancet. 2023;402:1827–1828. doi: 10.1016/S0140-6736(23)02278-X. [DOI] [PubMed] [Google Scholar]
- 135.Johnston, T.S., Li, S.H., Painter, M.M., Atkinson, R.K., Douek, N.R., Reeg, D.B., Douek, D.C., Wherry, E.J. and Hensley, S.E. Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants. Immunity. 2024;57:912–925. doi: 10.1016/j.immuni.2024.02.017. [DOI] [PubMed] [Google Scholar]
- 136.Wang, Q., Guo, Y., Bowen, A., Mellis, I.A., Valdez, R., Gherasim, C., Gordon, A., Liu, L. and Ho, D.D. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe. 2024;32:315–321 e313. doi: 10.1016/j.chom.2024.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tortorici, M.A., Addetia, A., Seo, A.J., Brown, J., Sprouse, K., Logue, J., Clark, E., Franko, N., Chu, H. and Veesler, D. Persistent immune imprinting occurs after vaccination with the COVID-19 XBB.1.5 mRNA booster in humans. Immunity. 2024;57:904–911 e904. doi: 10.1016/j.immuni.2024.02.016. [DOI] [PubMed] [Google Scholar]
- 138.Arevalo CP, Bolton MJ, Le Sage V, Ye N, Furey C, Muramatsu H, Alameh M-G, Pardi N, Drapeau EM, Parkhouse K, et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science. 2022;378(6622):899–904. doi: 10.1126/science.abm0271. [DOI] [PMC free article] [PubMed] [Google Scholar]


