Abstract
PURPOSE
The open-label, phase III EVOKE-01 study evaluated sacituzumab govitecan (SG) versus standard-of-care docetaxel in metastatic non–small cell lung cancer (mNSCLC) with progression on/after platinum-based chemotherapy, anti–PD-(L)1, and targeted treatment for actionable genomic alterations (AGAs). Primary analysis is reported.
METHODS
Patients were randomly assigned 1:1 (stratified by histology, best response to last anti–PD-(L)1–containing regimen, and AGA treatment received or not) to SG (one 10 mg/kg intravenous infusion on days 1 and 8) or docetaxel (one 75 mg/m2 intravenous infusion on day 1) in 21-day cycles. Primary end point was overall survival (OS). Key secondary end points were investigator-assessed progression-free survival (PFS), objective response rate, patient-reported symptom assessment, and safety.
RESULTS
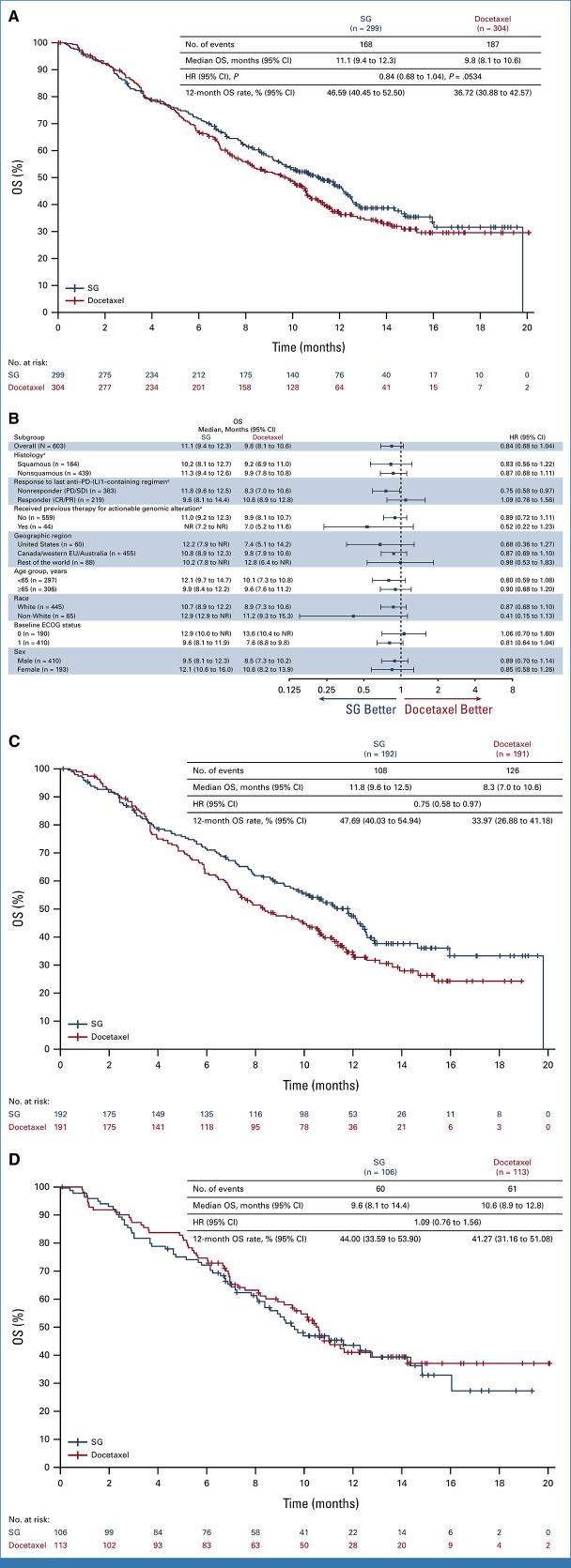
In the intention-to-treat population (SG, n = 299; docetaxel, n = 304), 55.4% had one previous line of therapy. Median follow-up was 12.7 months (range, 6.0-24.0). The primary end point was not met. There was a numerical OS improvement for SG versus docetaxel (median, 11.1 v 9.8 months; hazard ratio [HR], 0.84 [95% CI, 0.68 to 1.04]; one-sided P = .0534), consistent across squamous and nonsquamous histologies. Median PFS was 4.1 versus 3.9 months (HR, 0.92 [95% CI, 0.77 to 1.11]). An OS benefit was observed for SG (n = 192) versus docetaxel (n = 191) in mNSCLC nonresponsive to last anti–PD-(L)1–containing regimen (3.5-month median OS increase; HR, 0.75 [95% CI, 0.58 to 0.97]); this was consistent across histologies. Among patients receiving SG and docetaxel, 6.8% and 14.2% discontinued because of treatment-related adverse events (TRAEs), respectively; 1.4% and 1.0%, respectively, had TRAEs leading to death.
CONCLUSION
Although statistical significance was not met, OS numerically improved with SG versus docetaxel, which was consistent across histologies. Clinically meaningful improvement in OS was noted in mNSCLC nonresponsive to last anti–PD-(L)1–containing regimen. SG was better tolerated than docetaxel and consistent with its known safety profile, with no new safety signals.
INTRODUCTION
Treatment for advanced or metastatic non–small cell lung cancer (NSCLC) progressing after platinum-based chemotherapy and/or immunotherapy, regardless of the presence of actionable genomic alterations (AGAs) and PD-L1 status, remains limited to chemotherapy.1 However, survival outcomes are poor with current standard-of-care docetaxel and are accompanied by significant treatment-related adverse events (TRAEs) affecting patient quality of life (QoL).2-5 Although targeted therapies and immunotherapies improve outcomes when used in first-line settings, resistance mechanisms promote disease progression in most patients,6,7 and effective therapies for patients with disease progression on both immunotherapy and platinum-based chemotherapy are needed.
CONTEXT
Key Objective
Docetaxel is standard treatment for advanced/metastatic non–small cell lung cancer progressing after platinum-based chemotherapy and/or immunotherapy but is associated with modest clinical outcomes and significant toxicity. This randomized, global phase III study evaluated the trophoblast cell-surface antigen 2–directed antibody-drug conjugate (ADC) sacituzumab govitecan (SG) versus docetaxel in this setting.
Knowledge Generated
SG demonstrated a numerical overall survival (OS) benefit over docetaxel (median, 11.1 v 9.8 months; hazard ratio, 0.84; one-sided P = .0534), which was consistent across histologies and clinically meaningful in patients nonresponsive to last anti–PD-(L)1–containing regimen. SG had fewer grade ≥3 adverse events (AEs) and AE-related discontinuations versus docetaxel.
Relevance (T.E. Stinchcombe)
SG did not demonstrate a statistically significant improvement in OS, and the results of this trial may have implications for other ADCs in development.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Sacituzumab govitecan (SG) is a first-in-class, trophoblast cell-surface antigen (Trop)-2–directed antibody-drug conjugate (ADC) that selectively delivers topoisomerase I inhibitor SN-38, an active metabolite of irinotecan, to cancer cells and the surrounding tumor microenvironment.8-10 Several ADCs targeting Trop-2 are being investigated in many solid tumors, including NSCLC.11 SG is currently approved in multiple countries for the treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received ≥two previous therapies (≥one for metastatic disease) and hormone receptor–positive/human epidermal growth factor receptor 2–negative breast cancer after receipt of ≥two previous therapies, and in the United States to treat urothelial cancer after platinum-based and anti–PD-receptor-1 and/or PD-L1 (anti–PD-(L)1) therapy.
In a phase I/II basket study (IMMU-132-01), patients with heavily pretreated metastatic NSCLC derived durable clinical benefit from SG, with an objective response rate (ORR) of 16.7% (95% CI, 7.9 to 29.3) and median duration of response (DoR) of 6.0 months (95% CI, 2.5 to 21.0).12 SG was well tolerated with no new safety findings. Here, we provide the primary results of EVOKE-01 (ClinicalTrials.gov identifier: NCT05089734), a global, randomized, open-label, phase III study of SG versus docetaxel in advanced or metastatic NSCLC that progressed on/after platinum-based chemotherapy and anti–PD-(L)1–containing regimen.
METHODS
Patients
Eligible patients were age 18 years and older with pathologically documented NSCLC and measurable stage IV disease13 that progressed after platinum-based chemotherapy in combination or sequential with an anti–PD-(L)1–containing regimen. Patients with known AGAs must have received treatment with ≥one appropriate tyrosine kinase inhibitor (TKI), reflecting standard clinical practice. EGFR, ALK, and PD-L1 status were required before enrollment. Patients with stable, previously treated brain metastases (prednisone ≤10 mg maximum per day or equivalent and neurologic symptoms returned to baseline for ≥4 weeks) were also eligible. Additional details are provided in the Protocol (online only).
Study Design and Treatment
Patients were randomly assigned (1:1) with stratification by histology (squamous v nonsquamous), best response to last anti–PD-(L)1–containing regimen (investigator-assessed complete or partial response [CR/PR] v stable or progressive disease [SD/PD]), and receipt of previous therapy for AGAs (yes v no) to receive one 10 mg/kg intravenous infusion of SG on days 1 and 8 or one 75 mg/m2 intravenous infusion of docetaxel on day 1 of every 21-day cycle. Palliative and/or supportive medications, including routine prophylactic use of growth factors for both groups, were administered per investigator's discretion and were compliant with national/institutional guidelines. Treatment continued until disease progression, unacceptable toxicity, or other discontinuation criteria were met (see the Protocol for additional details).
This study was conducted in accordance with the International Council on Harmonisation Good Clinical Practice Guidelines, the Declaration of Helsinki, and any applicable local health authority and institutional review board/independent ethics committee requirements. All patients provided written informed consent. The sponsor (Gilead Sciences) designed and conducted the trial and gathered data in collaboration with the study investigators.
End Points and Assessments
The primary end point was overall survival (OS). Secondary efficacy end points were investigator-assessed progression-free survival (PFS), ORR (percentage of patients with confirmed CR or PR), DoR, disease control rate (DCR; percentage of patients with confirmed SD, CR, or PR) as determined by RECIST version 1.1, and safety. Additional secondary end points included symptoms assessed by patient-reported time to deterioration (TTD) in the NSCLC Symptom Assessment Questionnaire (SAQ) shortness-of-breath domain (decline from baseline in one point on a five-point scale) and total score (decline from baseline in two points on a 20-point scale).14
Efficacy end points were evaluated by computed tomography or magnetic resonance imaging scans in all patients at screening and every 6 weeks for the first 12 months, and then every 9 weeks thereafter. For patients with known or suspected brain or bone metastases, additional imaging assessments were performed according to the protocol. Length of study follow-up was calculated as time from random assignment to data cutoff at final analysis for each patient in the intention-to-treat (ITT) population, regardless of death. TTD was assessed on day 1 of each cycle before other treatment procedures (Data Supplement, Methods, online only). Safety/tolerability was assessed in all treated patients by Medical Dictionary for Regulatory Activities version 26.1 with severity graded using National Cancer Institute—Common Terminology Criteria for Adverse Events version 5.15
Statistical Analyses
The sample size calculation was based on treatment group comparisons with OS as the primary end point. With an anticipated number of approximately 336 death events at final analysis, the study has 90% power to detect a hazard ratio (HR) of 0.7 for SG versus docetaxel at the one-sided alpha of .025.
There was one prespecified interim analysis, and a one-sided alpha of .0075 was spent on the basis of the actual number of death events. The independent data monitoring committee recommended the study continue as planned. The study team remained blinded to the results until final analysis was completed. At final analysis, 355 death events had occurred in the ITT population, and one-sided P value boundary for statistical significance of the primary end point was ≤.0223. All reported data are based on the final analysis.
The overall type I error rate was controlled at a one-sided alpha of .025 by implementing a graphical method–based hierarchical testing procedure16; secondary end point testing occurred only if the significance boundary was reached in the primary end point, in the order of OS, PFS, ORR, and TTD in shortness-of-breath domain and total score in NSCLC-SAQ. A Lan-DeMets alpha spending function that approximates an O'Brien-Fleming approach was used to account for multiplicity introduced by including an interim analysis for OS.
OS, PFS, and TTD were analyzed using the Kaplan-Meier method, with 95% CIs determined according to the Brookmeyer-Crowley method. Between-treatment group differences were assessed by stratified log-rank test. HRs and associated 95% CIs were estimated by a stratified Cox proportional hazards model. Response rates were compared between groups by the stratified Cochran-Mantel-Haenszel method. Stratification factors used for random assignment were applied to all stratified analyses. Safety was assessed in all patients who received ≥one dose of any study drug. Safety was summarized in each group using descriptive statistics for treatment-emergent AEs (TEAEs; defined as any AEs that began or worsened on/after study drug administration through 30 days after last dose of study drug) unless otherwise specified. Efficacy and TTD were assessed in the ITT population.
RESULTS
Patients and Treatments
Between November 17, 2021, and May 30, 2023, 603 patients with stage IV disease at study entry from 177 sites in 20 countries were randomly assigned to receive SG (n = 299) or docetaxel (n = 304; Fig 1). In total, 296 patients (99.0%) in the SG group and 288 (94.7%) in the docetaxel group received study treatment. Of the 19 patients randomly assigned but not treated, 12 withdrew consent (Fig 1).
FIG 1.
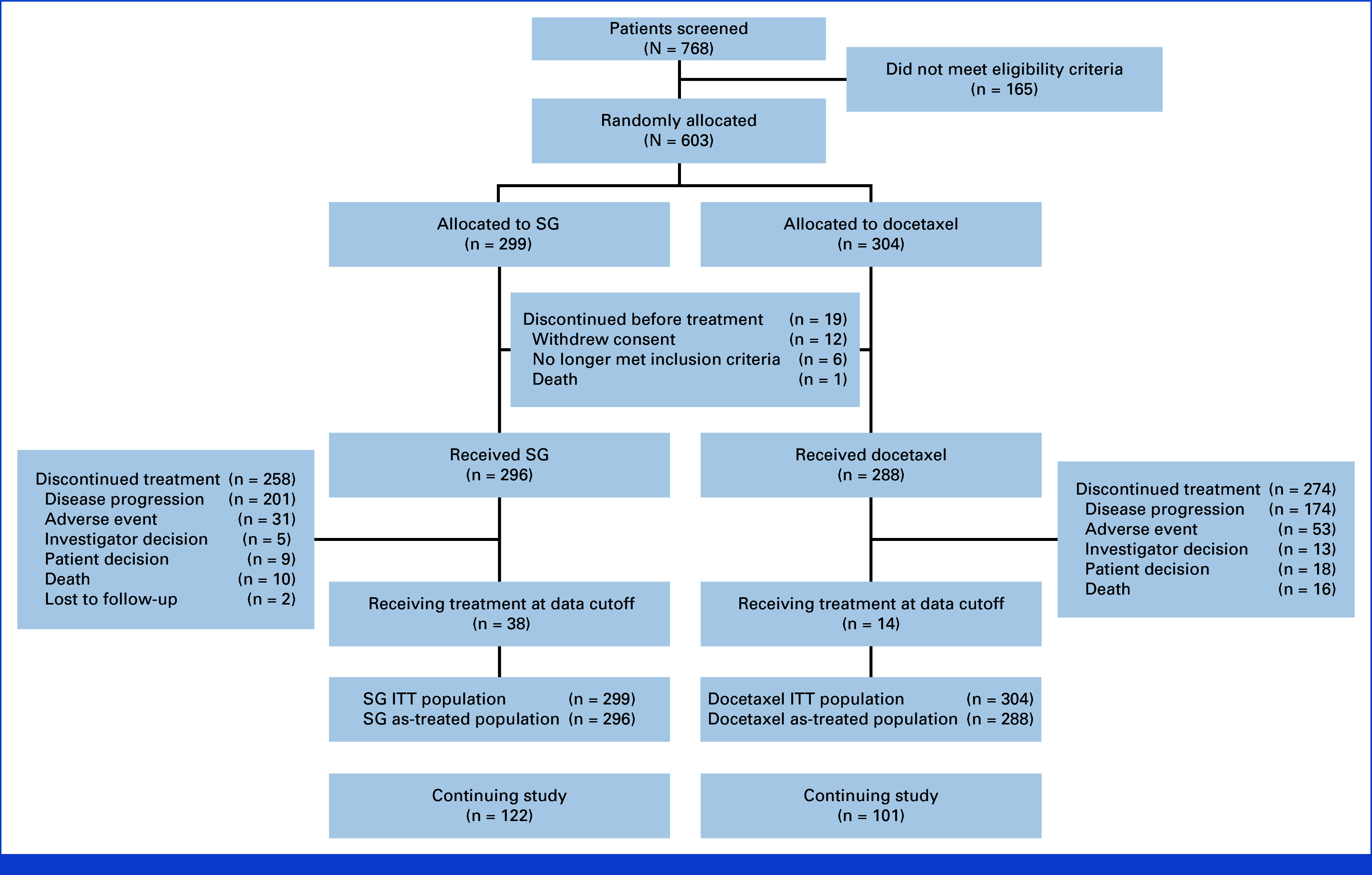
Patient disposition: CONSORT diagram. ITT, intention-to-treat; SG, sacituzumab govitecan.
Demographic and baseline disease characteristics were balanced between groups and generally consistent with this disease type (Table 1). Overall, patients in the SG and docetaxel groups had a median age of 65 years (range, 31-84), 68.0% were male, and 55.4% had received one previous line of therapy (Table 1). Most patients in both groups had nonsquamous histology (SG, 71.9%; docetaxel, 73.7%), and few (SG, 6.4%; docetaxel, 8.2%) received previous targeted therapy for an AGA because of the requirement of previous anti–PD-(L)1–containing regimen (AGAs summarized in the Data Supplement, Table S1). Patients who were nonresponsive to their last anti–PD-(L)1–containing regimen (investigator-assessed best response of SD/PD) constituted 63.5% of the study population (SG, 64.2%; docetaxel, 62.8%).
TABLE 1.
Patient Baseline Characteristics and Demographics
| Characteristic | SG (n = 299) | Docetaxel (n = 304) |
|---|---|---|
| Age, years, median (range) | 66 (31-84) | 64 (32-83) |
| <65, No. (%) | 136 (45.5) | 161 (53.0) |
| ≥65, No. (%) | 163 (54.5) | 143 (47.0) |
| Sex, No. (%) | ||
| Male | 194 (64.9) | 216 (71.1) |
| Female | 105 (35.1) | 88 (28.9) |
| Race or ethnic group, No. (%) | ||
| White | 229 (76.6) | 216 (71.1) |
| Black | 6 (2.0) | 7 (2.3) |
| Asian | 17 (5.7) | 26 (8.6) |
| Other/not specifieda | 47 (15.7) | 55 (18.1) |
| ECOG PS,b No. (%) | ||
| 0 | 101 (33.8) | 89 (29.3) |
| 1 | 198 (66.2) | 212 (69.7) |
| 2c | 0 | 1 (0.3) |
| Histology, No. (%) | ||
| Nonsquamousd | 215 (71.9) | 224 (73.7) |
| Squamous | 84 (28.1) | 80 (26.3) |
| Disease stage at diagnosis,e No. (%) | ||
| Stage I-III | 76 (25.4) | 102 (33.6) |
| Stage IV | 219 (73.2) | 202 (66.4) |
| Patients with brain metastasis, No. (%) | 35 (11.7) | 39 (12.8) |
| Previous lines of therapy, No. (%) | ||
| 1 | 167 (55.9) | 167 (54.9) |
| 2 | 103 (34.4) | 101 (33.2) |
| ≥3 | 29 (9.7) | 36 (11.8) |
| Best response to last anti–PD-(L)1–containing regimen,f No. (%) | ||
| Responder (CR/PR) | 106 (35.5) | 113 (37.2) |
| Nonresponder (SD/PD) | 192 (64.2) | 191 (62.8) |
| Not available | 1 (0.3) | 0 |
| Previous therapy for AGA, No. (%) | ||
| Yes | 19 (6.4) | 25 (8.2) |
| EGFR alterationg | 6 (2.0) | 13 (4.3) |
| ALK alterationg | 1 (0.3) | 1 (0.3) |
| No | 280 (93.6) | 279 (91.8) |
Abbreviations: AGA, actionable genomic alteration; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease; PR, partial response; SD, stable disease; SG, sacituzumab govitecan.
Other races included American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and other.
ECOG PS was missing for two patients in the docetaxel group.
ECOG PS = 2 for one patient in the docetaxel group on cycle 1, day 1.
Histologies not otherwise specified were included in the nonsquamous subgroup.
Disease stage at diagnosis was missing for four patients in the SG group.
Response to previous anti–PD-(L)1–containing regimen was per investigator assessment.
Patients with multiple types of AGA were counted once for each type; more genes shown in the Data Supplement (Table S1).
With a median study follow-up of 12.7 months (range, 6.0-24.0), the median duration of exposure was 3.45 months (range, 0.03-18.69) for SG (33.4% received SG ≥6 months) and 2.33 months (range, 0.03-19.75) for docetaxel (17.4% received docetaxel ≥6 months). Median relative dose intensity (percentage of total amount of study drug administered relative to the total amount of study drug expected to be administered while on treatment) was 98.4% for SG and 98.3% for docetaxel. At data cutoff, 122 (40.8%) and 101 (33.2%) patients in the SG and docetaxel groups, respectively, remained on study. Among the 52 patients (8.6%) who remained on study drug, more remained in the SG group (12.7% v docetaxel, 4.6%; Fig 1). The most common reason for discontinuation of study treatment was PD in both groups (SG, 201 [67.2%]; docetaxel, 174 [57.2%]; Fig 1).
Efficacy
Efficacy in the ITT Population
At the protocol-specified primary analysis, the study did not meet statistical significance for the primary end point of OS. A numerical improvement in median OS was observed with SG (11.1 months [95% CI, 9.4 to 12.3]) versus docetaxel (9.8 months [95% CI, 8.1 to 10.6]), with a 16% reduction in risk of death numerically favoring SG versus docetaxel (HR, 0.84 [95% CI, 0.68 to 1.04]; one-sided P = .0534; Fig 2A and Table 2). The 12-month OS rate was 46.59% (95% CI, 40.45 to 52.50) and 36.72% (95% CI, 30.88 to 42.57) for SG and docetaxel groups, respectively. OS results were consistent in most predefined subgroups (Fig 2B), including squamous (HR, 0.83 [95% CI, 0.56 to 1.22]) and nonsquamous (HR, 0.87 [95% CI, 0.68 to 1.11]) histologies. In the ITT population, 37.8% of patients in the SG group received subsequent anticancer therapy (most commonly docetaxel), and 31.3% in the docetaxel group received subsequent anticancer therapy (most commonly single-agent gemcitabine or vinorelbine; Data Supplement, Table S2).
FIG 2.
OS. (A) OS in the intention-to-treat population (all randomly assigned patients). The HR and 95% CIs were calculated using the Cox proportional hazards model, adjusted for randomization stratification factors of histology and best response to last anti–PD-(L)1–containing regimen received. One-sided P value derived from the stratified log-rank test. (B) Subgroup analysis of OS. HR and 95% CIs for subgroup analysis are calculated using the unstratified Cox proportional hazards model without adjustment. Not otherwise specified histology is combined with nonsquamous. (C) OS in patients nonresponsive (SD/PD) to last anti–PD-(L)1–containing regimen. (D) OS in patients responsive (CR/PR) to last previous anti–PD-(L)1–containing regimen. The HRs and 95% CIs for the subgroup analyses were calculated using the unstratified Cox proportional hazards model without any adjustment. aRandomization stratification factors. CR, complete response; ECOG, Eastern Cooperative Oncology Group; EU, European Union; HR, hazard ratio; NR, not reached; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease; SG, sacituzumab govitecan.
TABLE 2.
Summary of Treatment Efficacy
| Variable | SG (n = 299) | Docetaxel (n = 304) |
|---|---|---|
| OS, months, median (95% CI) | 11.1 (9.4 to 12.3) | 9.8 (8.1 to 10.6) |
| HR for death (95% CI), one-sided Pa | 0.84 (0.68 to 1.04), .0534 | |
| OS in patients nonresponsive (SD/PD) to last anti–PD-(L)1 regimen, months, median (95% CI) | 11.8 (9.6 to 12.5) | 8.3 (7.0 to 10.6) |
| HR for death (95% CI) | 0.75 (0.58 to 0.97) | |
| OS in patients responsive (CR/PR) to last anti–PD-(L)1 regimen, months, median (95% CI) | 9.6 (8.1 to 14.4) | 10.6 (8.9 to 12.8) |
| HR for death (95% CI) | 1.09 (0.76 to 1.56) | |
| PFS (95% CI), months, median (95% CI) | 4.1 (3.0 to 4.4) | 3.9 (3.1 to 4.2) |
| HR for disease progressionb or death (95% CI) | 0.92 (0.77 to 1.11) | |
| ORR,b,c No. (%) | 41 (13.7) | 55 (18.1) |
| CR | 0 | 3 (1.0) |
| PR | 41 (13.7) | 52 (17.1) |
| SD | 161 (53.8) | 149 (49.0) |
| PD | 66 (22.1) | 64 (21.1) |
| Not evaluable/not assessed | 31 (10.4) | 36 (11.8) |
| DCR,d No. (%) | 202 (67.6) | 204 (67.1) |
| DoR,e months, median (95% CI) | 6.7 (4.4 to 9.8) | 5.8 (4.1 to 8.3) |
| DoR rate at 6 monthse,f | 52.5 (35.6 to 66.9) | 46.5 (31.9 to 59.8) |
Abbreviations: CR, complete response; DCR, disease control rate; DoR, duration of response; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; SG, sacituzumab govitecan; v1.1, version 1.1.
On the basis of stratified log-rank test (two-sided P = .1068).
Investigator-assessed per RECIST v1.1.
Patients without tumor assessment (SG, n = 28; docetaxel, n = 35), response defined as best overall response of confirmed CR + PR; not formally tested.
Disease control defined best overall response of confirmed CR, PR, or SD as assessed by the investigator per RECIST v1.1.
Evaluated in patients with confirmed CR or PR.
On the basis of Kaplan-Meier estimates.
Median PFS was 4.1 months (95% CI, 3.0 to 4.4) with SG and 3.9 months (95% CI, 3.1 to 4.2) with docetaxel (HR, 0.92 [95% CI, 0.77 to 1.11]; Fig 3A and Table 2). Median PFS with SG was similar among patients with squamous (3.8 months [95% CI, 2.8 to 5.4]) and nonsquamous (4.1 months [95% CI, 2.9 to 5.3]) histologies (Data Supplement, Fig S1). The 6-month PFS rate was 33.71% (95% CI, 28.21 to 39.28) in the SG group and 31.39% (95% CI, 25.75 to 37.17) in the docetaxel group.
FIG 3.
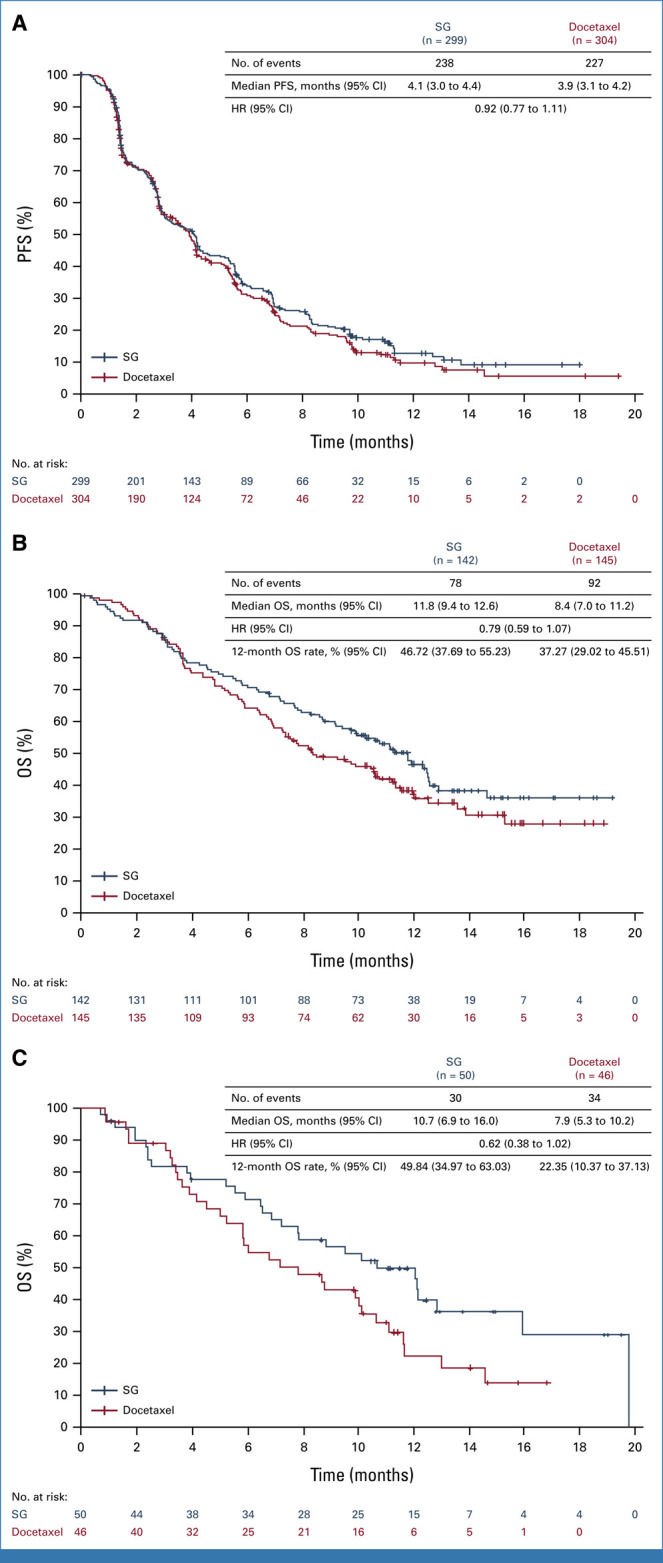
PFS in the ITT population and OS in patients with SD/PD to last anti–PD-(L)1–containing regimen by histology. (A) PFS in the ITT population (all randomly assigned patients). The HR and 95% CIs for PFS were calculated using the Cox proportional hazards model, adjusted for randomization stratification factors of histology and best response to last anti–PD-(L)1–containing regimen received. (B) OS in patients with SD/PD to last anti–PD-(L)1–containing regimen and nonsquamous NSCLC. (C) OS in patients with SD/PD to last anti–PD-(L)1–containing regimen and squamous NSCLC. The HR and 95% CIs for subgroup analyses were calculated using the unstratified Cox proportional hazards model without any adjustment. Histology not otherwise specified is combined with nonsquamous. HR, hazard ratio; ITT, intention-to-treat; NSCLC, non–small cell lung cancer; OS, overall survival; PD, progressive disease; PFS, progression-free survival; SD, stable disease; SG, sacituzumab govitecan.
ORR was 13.7% (95% CI, 10.0 to 18.1) with SG and 18.1% (95% CI, 13.9 to 22.9) with docetaxel (difference of –4.3% [95% CI, –10.1 to 1.5]; Table 2). Median DoR was 6.7 months (95% CI, 4.4 to 9.8) with SG and 5.8 months (95% CI, 4.1 to 8.3) with docetaxel (Table 2). DCR was 67.6% (95% CI, 61.9 to 72.8) in the SG group and 67.1% (95% CI, 61.5 to 72.4) in the docetaxel group (Table 2).
Efficacy on the Basis of Best Response to Last Anti–PD-(L)1–Containing Regimen
A clinically meaningful improvement in OS was observed in patients who were nonresponsive (SD/PD) to their last anti–PD-(L)1–containing regimen. Median OS was 11.8 months (95% CI, 9.6 to 12.5) with SG and 8.3 months (95% CI, 7.0 to 10.6) with docetaxel (HR, 0.75 [95% CI, 0.58 to 0.97]; Fig 2C). In patients who were responsive to previous anti–PD-(L)1–containing regimen (CR/PR), the OS HR was 1.09 (95% CI, 0.76 to 1.56; Fig 2D). Baseline demographics and characteristics and use of subsequent therapy were similar between groups (Data Supplement, Tables S2 and S3). Results were consistent across histologies (squamous: HR, 0.62 [95% CI, 0.38 to 1.02]; nonsquamous: HR, 0.79 [95% CI, 0.59 to 1.07]; Figs 3B and 3C).
Patient-Reported Disease-Related Symptoms
NSCLC-SAQ TTD in shortness-of-breath and total score were key secondary end points. The NSCLC-SAQ completion rate was ≥88.4% in SG and ≥93.7% in docetaxel through week 25 and was generally comparable across treatment groups. Median TTD was longer for SG versus docetaxel for NSCLC-SAQ shortness-of-breath domain (2.8 v 2.1 months; HR, 0.75 [95% CI, 0.61 to 0.91]; Data Supplement, Fig S2A) and total score (3.1 v 2.7 months; HR, 0.80 [95% CI, 0.66 to 0.97]; Data Supplement, Fig S2B), and this difference was sustained over the observed time period.
Safety
In the safety analysis set, any-grade TEAEs occurred in 295/296 patients (99.7%) in the SG group and 282/288 patients (97.9%) in the docetaxel group (Table 3). The incidence of grade ≥3 TEAEs was lower with SG than docetaxel (66.6% v 75.7%). TEAEs reported by a higher proportion (≥5% difference) of patients in the SG versus docetaxel group were diarrhea, alopecia, nausea, anemia, constipation, vomiting, pruritus, and abdominal pain; only diarrhea, nausea, and abdominal pain (n = 1 each) led to SG discontinuation. TEAEs reported by a higher proportion (≥5% difference) of patients in the docetaxel group were neutropenia, stomatitis, leukopenia, peripheral edema, dysgeusia, and peripheral neuropathy; neutropenia (n = 3), peripheral edema (n = 2), and peripheral neuropathy (n = 1) led to docetaxel discontinuation.
TABLE 3.
Summary of Treatment-Emergent Adverse Events in All Treated Patients
| Event | SG (n = 296), No. (%) | Docetaxel (n = 288), No. (%) | ||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| TEAEsa,b | 295 (99.7) | 197 (66.6) | 282 (97.9) | 218 (75.7) |
| TEAEs reported in ≥10% in either groupc | ||||
| Fatigue | 168 (56.8) | 37 (12.5) | 161 (55.9) | 28 (9.7) |
| Diarrhea | 156 (52.7) | 31 (10.5) | 97 (33.7) | 11 (3.8) |
| Alopecia | 128 (43.2) | 2 (0.7) | 86 (29.9) | 2 (0.7) |
| Nausea | 123 (41.6) | 5 (1.7) | 75 (26.0) | 3 (1.0) |
| Anemia | 119 (40.2) | 19 (6.4) | 89 (30.9) | 17 (5.9) |
| Neutropenia | 111 (37.5) | 73 (24.7) | 123 (42.7) | 106 (36.8) |
| Constipation | 86 (29.1) | 0 | 49 (17.0) | 1 (0.3) |
| Decreased appetite | 78 (26.4) | 7 (2.4) | 69 (24.0) | 6 (2.1) |
| Vomiting | 62 (20.9) | 7 (2.4) | 43 (14.9) | 6 (2.1) |
| Cough | 46 (15.5) | 0 | 45 (15.6) | 1 (0.3) |
| Dyspnea | 42 (14.2) | 4 (1.4) | 51 (17.7) | 13 (4.5) |
| Stomatitis | 39 (13.2) | 3 (1.0) | 58 (20.1) | 7 (2.4) |
| Leukopenia | 38 (12.8) | 15 (5.1) | 63 (21.9) | 50 (17.4) |
| Pruritus | 37 (12.5) | 1 (0.3) | 11 (3.8) | 0 |
| Pyrexia | 37 (12.5) | 2 (0.7) | 34 (11.8) | 2 (0.7) |
| Back pain | 33 (11.1) | 2 (0.7) | 19 (6.6) | 2 (0.7) |
| Abdominal pain | 31 (10.5) | 3 (1.0) | 14 (4.9) | 0 |
| Arthralgia | 30 (10.1) | 2 (0.7) | 29 (10.1) | 1 (0.3) |
| Rash | 30 (10.1) | 0 | 19 (6.6) | 0 |
| Febrile neutropenia | 23 (7.8) | 23 (7.8) | 29 (10.1) | 27 (9.4) |
| Lymphopenia | 23 (7.8) | 9 (3.0) | 31 (10.8) | 12 (4.2) |
| Peripheral edema | 16 (5.4) | 0 | 35 (12.2) | 4 (1.4) |
| Dysgeusia | 14 (4.7) | 0 | 30 (10.4) | 0 |
| Peripheral neuropathy | 11 (3.7) | 0 | 38 (13.2) | 2 (0.7) |
| Treatment-relatedc | 279 (94.3) | 156 (52.7) | 262 (91.0) | 173 (60.1) |
| TEAEs leading to discontinuation | 29 (9.8) | 48 (16.7) | ||
| Treatment-relatedc | 20 (6.8) | 41 (14.2) | ||
| TEAEs leading to death | 10 (3.4) | 13 (4.5) | ||
| Treatment-relatedd | 4 (1.4) | 3 (1.0) | ||
| TEAEs leading to dose reduction | 87 (29.4) | 112 (38.9) | ||
| TEAEs leading to treatment interruption | 171 (57.8) | 81 (28.1) | ||
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SG, sacituzumab govitecan; TEAE, treatment-emergent adverse event.
TEAE is any AE with an onset date on or after the study drug start date and no later than 30 days after last dose of study drug.
Coded according to MedDRA version 26.1 and AE severity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events. Multiple AEs were counted only once per participant for the highest severity grade for each preferred term.
Determined by investigator.
Treatment-related TEAEs that led to death, investigator-assessed, included cardiac failure, cerebrovascular accident, death, febrile neutropenia, hematemesis, ischemic stroke, myocardial ischemia, neutropenic colitis, sepsis, and septic shock (one each) in the SG group and death (n = 4), pneumonia (n = 3), cardiac failure, acute respiratory failure, cardiorespiratory arrest, intestinal obstruction, pneumonitis, and respiratory failure (one each) in the docetaxel group.
TEAEs leading to dose reductions occurred in 87 (29.4%) patients in the SG group and 112 (38.9%) in the docetaxel group. The proportion of patients with TEAEs leading to study treatment discontinuation was lower with SG (n = 29, 9.8%) than docetaxel (n = 48, 16.7%). TRAEs leading to treatment discontinuation occurred in 20 (6.8%) and 41 (14.2%) patients in the SG and docetaxel groups, respectively. There were seven deaths from TEAEs that were considered treatment-related by investigators: four SG patients (one each: febrile neutropenia, neutropenic colitis, sepsis, and septic shock) and three docetaxel patients (one each: unknown cause, pneumonia, and pneumonitis).
DISCUSSION
There is an unmet medical need for safe and effective treatment options that prolong survival in patients with metastatic NSCLC who progressed on/after platinum-based chemotherapy and combination/sequential anti–PD-(L)1–containing regimen. EVOKE-01 is one of the first ADC studies in this setting. Although the study did not meet protocol-specified statistical significance for the primary end point of OS, SG treatment resulted in a 16% reduction in risk of death versus docetaxel in the ITT population and was associated with a numerically higher OS rate at all prespecified landmark time points. OS is an objective end point less subject to assessment bias than PFS.17 There were fewer high-grade TEAEs and TEAEs leading to dose reduction and/or discontinuation with SG than docetaxel; more patients remained on SG than docetaxel at final analysis. Importantly, patients reported longer TTD in NSCLC-SAQ symptoms with SG treatment.
Despite recent advances with immune checkpoint inhibitors for earlier-line NSCLC, most patients have disease that eventually progresses. Docetaxel with/without nintedanib/ramucirumab have been investigated in this setting, but both are associated with modest efficacy and significant AEs affecting patient QoL.18-20 In the REVEL study, ramucirumab plus docetaxel improved OS versus docetaxel monotherapy in patients previously treated with platinum doublet chemotherapy but not anti–PD-(L)1.20 With the modest OS improvement from addition of ramucirumab to docetaxel (median, 10.5 months) versus docetaxel (median, 9.1 months; HR, 0.86 [95% CI, 0.75 to 0.98]; P = .023), docetaxel monotherapy is the globally accepted comparator for NSCLC studies in this setting.20 In EVOKE-01, docetaxel performed as expected. However, a delayed separation between OS Kaplan-Meier curves was observed, starting at approximately 4 months and thereafter favoring SG over docetaxel. At 12 months, the OS rate for patients with SG was approximately 10% higher than with docetaxel. Subsequent therapies were consistent with the standard of care in both treatment groups, and the OS benefit in the SG group was maintained in a sensitivity analysis censoring patients who received subsequent docetaxel. Other underlying biologic factors pertaining to this observation remain to be determined.
As response to anti–PD-(L)1 treatment has prognostic impact on OS,21 best response to last anti–PD-(L)1–containing regimen was selected as a stratification factor. Treatment of patients progressing on anti–PD-(L)1 therapy remains challenging, and multiple recent studies involving novel immunomodulatory agents, such as canakinumab, and studies with multitargeted TKIs with continued anti–PD-(L)1 therapy have failed to improve OS over docetaxel.4,22 These failures largely stem from the heterogeneity of patient populations and that the mechanism of innate and acquired resistance to anti–PD-(L)1–containing therapy is not well understood. In the first-line setting, only about 30% to 60% of patients achieve objective response to immune therapy alone or combined with chemotherapy.23-26 Herein, a clinically meaningful 3.5-month OS improvement was observed favoring SG over docetaxel, with 25% reduction in risk of death in patients who were nonresponsive to their last anti–PD-(L)1–containing regimen. Best response to previous anti–PD-(L)1–containing regimen was investigator-assessed. These patients represent a population with the highest unmet medical need, and the OS improvement observed suggests that SG is a potential treatment option in this population. Importantly, the OS difference in this subgroup was consistent across squamous and nonsquamous histologies.
Trop-2 is highly expressed in both squamous and nonsquamous NSCLC,27 making it a good target for safe and effective delivery of cytotoxic payloads. Despite an identical target, the individual constructs of Trop-2–directed ADCs, including the linker and payload, determine the therapeutic index and individual safety and efficacy of each. Recently, datopotamab deruxtecan, another Trop-2–directed ADC, demonstrated PFS improvement versus docetaxel in patients with previously treated metastatic NSCLC; however, no OS improvement has been reported to date as a final analysis has yet to be reported.5,28 The benefit of datopotamab deruxtecan seems to be limited to patients with nonsquamous histology.5 By contrast, in EVOKE-01, the numerical improvement in OS with SG over docetaxel was observed in all clinically relevant subgroups, including squamous and nonsquamous histologies. The numerical improvement in OS with SG in both histologies was more pronounced in patients who were nonresponsive to their last anti–PD-(L)1–containing regimen. Studies of Trop-2 as a possible biomarker are ongoing and will be reported.
In EVOKE-01, SG was better tolerated than docetaxel. The incidence of high-grade TEAEs and TEAEs leading to discontinuation were lower with SG than docetaxel, and median duration of exposure to SG was longer. SG demonstrated a manageable safety profile, consistent with what is known,12,29,30 with no new safety signals. The incidence of high-grade fatigue and diarrhea was higher with SG, while the incidence of high-grade neutropenia, dyspnea, stomatitis, and leukopenia was higher with docetaxel. SG-associated risks were adequately managed through monitoring and dose modification guidance per prescribing information.31 This is further supported by improvements in patient-reported TTD of NSCLC-related symptoms, which are reflective of tolerability and better disease control.
Although EVOKE-01 did not meet statistical significance for the primary end point, SG is an active therapeutic agent in metastatic NSCLC that conferred modest numerical OS benefit over docetaxel in the ITT population. A numerical OS benefit was also observed in the subgroup of patients who were nonresponsive to last anti–PD-(L)1–containing regimen. Patients with metastatic NSCLC still need novel treatment options, and these data support further investigation of SG in this patient population. A phase II study evaluating SG with pembrolizumab with/without platinum-based chemotherapy in previously untreated metastatic NSCLC lacking AGAs (EVOKE-02; ClinicalTrials.gov identifier: NCT05186974) and a phase III study evaluating SG plus pembrolizumab versus pembrolizumab monotherapy in first-line PD-L1–high metastatic NSCLC (EVOKE-03; ClinicalTrials.gov identifier: NCT05609968) are underway.32,33
ACKNOWLEDGMENT
The authors thank the patients and their caregivers and families and the study investigators for their participation and commitment to this clinical study. Medical writing assistance was provided by Miranda Bader-Goodman, PhD, Jan Nagel, PhD, and Erick T. Tatro, PhD, of Ashfield MedComms, an Inizio Company, and editorial assistance by Celia Nelson, also of Ashfield MedComms, funded by Gilead Sciences, Inc. A list of EVOKE-01 principal investigators can be found in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
List of EVOKE-01 Study Principal Investigators
| Country | Study Investigator |
|---|---|
| Australia | Elizabeth Ahern |
| Australia | Venessa Chin |
| Australia | Stephen Della-Fiorentina |
| Australia | Kevin Jasas |
| Australia | Christos Karapetis |
| Australia | Jeremy Long |
| Australia | Louise Nott |
| Australia | Kenneth O'Byrne |
| Australia | Craig Underhill |
| Austria | Richard Greil |
| Austria | Maximilian Hochmair |
| Austria | Andreas Pircher |
| Belgium | Wim Demey |
| Belgium | Paul Germonpré |
| Belgium | Elke Govaerts |
| Belgium | Thierry Pieters |
| Belgium | Reinier Wener |
| Brazil | Alan Azambuja |
| Brazil | Giuliano Borges |
| Brazil | Gilberto Castro |
| Brazil | Suellen Castro |
| Brazil | Felipe Cruz |
| Brazil | Fábio Franke |
| Brazil | Eduardo Silva |
| Canada | Parneet Cheema |
| Canada | Robert El-Maraghi |
| Canada | Swati Kulkarni |
| Canada | Rami Nassabein |
| Canada | Scott Owen |
| France | Clarisse Audigier Valette |
| France | Jaafar Bennouna |
| France | Christos Chouaid |
| France | Alexis Cortot |
| France | Sebastien Couraud |
| France | Didier Debieuvre |
| France | Fabrice Denis |
| France | Patrick Dumont |
| France | Radj Gervais |
| France | Nicolas Girard |
| France | Etienne Giroux Leprieur |
| France | Florian Guisier |
| France | Sandrine Hiret |
| France | Henri Janicot |
| France | Corinne Lamour |
| France | Jeannick Madelaine |
| France | Marie Marcq |
| France | Emilie Pluquet |
| France | Jean Louis Pujol |
| France | Magali Ravoire |
| France | Marielle Sabatini |
| France | Alain Vergnenegre |
| Germany | Sabine Bohnet |
| Germany | Martin Faehling |
| Germany | Melanie Janning |
| Germany | Eckart Laack |
| Germany | Niels Reinmuth |
| Germany | Achim Rittmeyer |
| Germany | Claas Wesseler |
| Greece | Sofia Baka |
| Greece | George Fountzilas |
| Greece | Panagiotis Katsaounis |
| Greece | Athanasios Kotsakis |
| Greece | Ioannis (Giannis) Mountzios |
| Greece | Konstantinos Syrigos |
| Israel | Julia Dudnik |
| Israel | Carmel Fink |
| Israel | Ofer Merimsky |
| Israel | Hovav Nechushtan |
| Israel | Julia Sobolev |
| Italy | Franceso Agustoni |
| Italy | Anna Cecilia Bettini |
| Italy | Matteo Brighenti |
| Italy | Giulio Cerea |
| Italy | Filippo De Marinis |
| Italy | Salvatore Grisanti |
| Italy | Francesco Grossi |
| Italy | Andrea Luciani |
| Italy | Evaristo Maiello |
| Italy | Hector Soto Parra |
| Italy | Pierfrancesco Tassone |
| Italy | Giuseppe Tonini |
| Japan | Yoko Agemi |
| Japan | Koichi Azuma |
| Japan | Tomohisa Baba |
| Japan | Yuka Fujita |
| Japan | Eiki Ichihara |
| Japan | Takashi Kasai |
| Japan | Terufumi Kato |
| Japan | Haruki Kobayashi |
| Japan | Shoichi Kuyama |
| Japan | Kazumi Nishino |
| Japan | Masahide Oki |
| Japan | Kyoichi Okishio |
| Japan | Tomohiro Sakamoto |
| Japan | Yuki Sato |
| Japan | Yuki Shinno |
| Japan | Takayuki Takahama |
| Japan | Koichi Takayama |
| Japan | Yasutaka Watanabe |
| Japan | Noriko Yanagitani |
| Japan | Yoshitaka Zenke |
| Mexico | Daniel Motola Kuba |
| Mexico | Tomas Daniel Pineda Razo |
| The Netherlands | Robin Cornelissen |
| The Netherlands | Lizza Hendriks |
| The Netherlands | Jeroen Kloover |
| The Netherlands | Klaar Maas |
| The Netherlands | Cor van der Leest |
| Poland | Lubomir Bodnar |
| Poland | Boguslawa Karaszewska |
| Poland | Andrzej Mruk |
| Portugal | Paula Alves |
| Portugal | Antonio Araujo |
| Portugal | Isabel Domingues |
| Portugal | Ana da Conceicao Fernandes Rodrigues |
| Portugal | Nuno Gil |
| Portugal | Helena Magalhaes |
| Portugal | Barbara Parente |
| Puerto Rico | Marcia Correa |
| Puerto Rico | Hector Velez-Cortes |
| Spain | Andres Aguilar Hernandez |
| Spain | Ruben Alonso Calderon |
| Spain | Joaquim Barrera |
| Spain | David Baz |
| Spain | Antonio Calles |
| Spain | Maria Campelo |
| Spain | Francisco Carpeno |
| Spain | Enric Costa |
| Spain | Manuel Dols |
| Spain | Manuel Domine Gomez |
| Spain | Miguel Fernandez de Sanmamed |
| Spain | Enriqueta Felip Font |
| Spain | Jose Fuentes |
| Spain | Maria Pilar Garrido Lopez |
| Spain | Oscar Juan Vidal |
| Spain | Jose Larriba |
| Spain | Laia Martinez |
| Spain | Ramon Palmero Sanchez |
| Spain | Luis Paz-Ares |
| Spain | Silverio Ros Martinez |
| Spain | Jon Zugazagoitia Fraile |
| Turkey | Ahmet Bilici |
| Turkey | Irfan Cicin |
| Turkey | Umut Demirci |
| Turkey | Yesim Eralp |
| Turkey | Mahmut Gumus |
| Turkey | Ozan Yazici |
| The United Kingdom | Shobhit Baijal |
| The United Kingdom | Katy Clarke |
| The United Kingdom | Farah Lim |
| The United Kingdom | Dakshinamoorthy Muthukumar |
| The United Kingdom | Nicola Steele |
| The United Kingdom | Yvonne Summers |
| The United States of America | Ahmed Abdelaziz |
| The United States of America | Eric Avery |
| The United States of America | Firas Badin |
| The United States of America | Charles Connor |
| The United States of America | Davey Daniel |
| The United States of America | Amir Faridi |
| The United States of America | January Fields-Meehan |
| The United States of America | Marina Garassino |
| The United States of America | Todd Gersten |
| The United States of America | Daniel Haggstrom |
| The United States of America | David Hakimian |
| The United States of America | William Houck |
| The United States of America | Henrik Illum |
| The United States of America | Roger Keresztes |
| The United States of America | Charles Kurkul |
| The United States of America | Charles Kuzma |
| The United States of America | Rachel Lerner |
| The United States of America | Steven Liu |
| The United States of America | Smitha Menon |
| The United States of America | Rami Owera |
| The United States of America | Vipul Patel |
| The United States of America | Ivor Percent |
| The United States of America | Andrew Piper |
| The United States of America | Sucharu Prakash |
| The United States of America | Douglas Reznick |
| The United States of America | Jorge Rios-Perez |
| The United States of America | Jun Sun |
| The United States of America | Restituto Tibayan |
| The United States of America | Anne Traynor |
| The United States of America | William Walsh |
| The United States of America | Donald Wender |
Luis G. Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati Therapeutics, GlaxoSmithKline, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck
Oscar Juan-Vidal
Honoraria: AstraZeneca/MedImmune, Takeda, Janssen, Amgen
Consulting or Advisory Role: Lilly, Takeda, AstraZeneca Spain, Janssen Oncology, Roche/Genentech
Research Funding: AstraZeneca Spain (Inst)
Travel, Accommodations, Expenses: Takeda, AstraZeneca/MedImmune, Pfizer, Roche/Genentech
Giannis S. Mountzios
Honoraria: Roche, Boehringer Ingelheim, AstraZeneca, MSD, BMS GmbH & Co KG, Novartis, Amgen, Takeda
Consulting or Advisory Role: Roche, AstraZeneca/Greece, MSD, Amgen, Novartis, BMS GmbH & Co KG, Takeda
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Ipsen, MSD, Roche, Novartis, BMS GmbH & Co KG, Demo Pharmaceutical
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, PeerVoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee-Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, ETOP IBCSG Partners Member of the Scientific Committee
Niels Reinmuth
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer Ingelheim, MSD Oncology, Takeda, Amgen
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme, Pfizer, Boehringer Ingelheim, Takeda, Amgen, Lilly, AbbVie, Merck KGaA, Sanofi Aventis GmbH, Janssen Oncology
Travel, Accommodations, Expenses: Janssen
Filippo de Marinis
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bristol Myers Squibb, Roche/Genentech, Pfizer, Novartis, Takeda, Daichii Sankyo, Merck Serono
Nicolas Girard
Employment: AstraZeneca
Consulting or Advisory Role: Roche, Lilly, AstraZeneca, Novartis, Pfizer, Bristol Myers Squibb, MSD, Takeda, Janssen, Sanofi, Amgen, Gilead Sciences, BeiGene, AbbVie, Daiichi Sankyo/Astra Zeneca, LEO Pharma, Ipsen
Research Funding: Roche (Inst), AstraZeneca (Inst), BMS (Inst), MSDavenir (Inst)
Travel, Accommodations, Expenses: Roche, Janssen Oncology
Takayuki Takahama
Speakers' Bureau: AstraZeneca Japan, Chugai Pharmaceutical Co Ltd, Roche Diagnostics Solutions, MSD K.K
Research Funding: Pfizer, Takeda
Scott P. Owen
Honoraria: AstraZeneca, Bristol Myers Squibb, Roche Canada, Takeda, Merck, Novocure
Consulting or Advisory Role: Merck, AstraZeneca, Roche Canada, Takeda, Bristol Myers Squibb, Bayer
Firas B. Badin
Speakers' Bureau: Bristol Myers Squibb/Pfizer, Jazz Pharmaceuticals, Merck Serono, Merck, Lilly, GE Healthcare, Regeneron
Irfan Cicin
Consulting or Advisory Role: Pfizer (Inst), MSD Oncology (Inst), Roche (Inst), Novartis/Ipsen (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), Servier (Inst), Abdi Ibrahim (Inst), Nobelpharma (Inst), AbbVie (Inst), Teva, Janssen Oncology (Inst), Takeda (Inst), Gen (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Abdi Ibrahim (Inst), Astellas Pharma, MSD (Inst), Gen (Inst), Teva (Inst)
Sabeen Mekan
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Riddhi Patel
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences
Eric Zhang
Employment: Gilead Sciences
Stock and Other Ownership Interests: Merck, BeiGene
Divyadeep Karumanchi
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Marina Chiara Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb, Daiichi Sankyo/Astra Zeneca, Regeneron, Pfizer, Blueprint Pharmaceutic, Novartis, Sanofi/Aventis, Medscape, Oncohost, Revolution Medicines
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Sanofi, Celgene, Daiichi Sankyo, Pfizer, Seagen, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron, AbbVie, Mirati Therapeutics, Merck, Boehringer Ingelheim, Abion, Gilead Sciences
Speakers' Bureau: AstraZeneca, MSD Oncology, Merck, Mirati Therapeutics, Daiichi Sankyo/Astra Zeneca
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca, Merck
Uncompensated Relationships: Merck
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 2839
PRIOR PRESENTATION
Presented in part as an oral presentation at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31-June 4, 2024.
SUPPORT
Supported by Gilead Sciences, Inc. L.G.P.-A. was funded by the ISCIII (PMPTA22/00167; PMP21/00107; SPLEC2200C009241XV0; PI20/00870; AC20/0070) and CIBERONC (CD16/12/00442), Comunidad de Madrid, CAM (P2022/BMD7437), AECC (TRNSC18004PAZ), and Fundación CRIS contra el cáncer (Unidad Integral CRIS de Inmuno-oncología), and cofunded by FEDER from Regional Development European Funds (European Union).
CLINICAL TRIAL INFORMATION
NCT05089734 (EVOKE-01)
DATA SHARING STATEMENT
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting no conflict of interest. The requested proposal must also include a statistician. Approval of such requests is at Gilead Sciences' discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
AUTHOR CONTRIBUTIONS
Conception and design: Luis G. Paz-Ares, Nicolas Girard, Vipul M. Patel, Firas B. Badin, Sabeen Mekan, Riddhi Patel, Marina Chiara Garassino
Administrative support: Douglas M. Reznick
Provision of study materials or patients: Luis G. Paz-Ares, Oscar Juan-Vidal, Giannis S. Mountzios, Enriqueta Felip, Niels Reinmuth, Filippo de Marinis, Nicolas Girard, Takayuki Takahama, Douglas M. Reznick, Sabeen Mekan, Marina Chiara Garassino
Collection and assembly of data: Luis G. Paz-Ares, Oscar Juan-Vidal, Giannis S. Mountzios, Enriqueta Felip, Filippo de Marinis, Nicolas Girard, Takayuki Takahama, Scott P. Owen, Douglas M. Reznick, Sabeen Mekan, Riddhi Patel, Divyadeep Karumanchi
Data analysis and interpretation: Luis G. Paz-Ares, Oscar Juan-Vidal, Giannis S. Mountzios, Enriqueta Felip, Niels Reinmuth, Filippo de Marinis, Nicolas Girard, Takayuki Takahama, Scott P. Owen, Firas B. Badin, Irfan Cicin, Sabeen Mekan, Riddhi Patel, Eric Zhang, Divyadeep Karumanchi, Marina Chiara Garassino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Luis G. Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati Therapeutics, GlaxoSmithKline, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck
Oscar Juan-Vidal
Honoraria: AstraZeneca/MedImmune, Takeda, Janssen, Amgen
Consulting or Advisory Role: Lilly, Takeda, AstraZeneca Spain, Janssen Oncology, Roche/Genentech
Research Funding: AstraZeneca Spain (Inst)
Travel, Accommodations, Expenses: Takeda, AstraZeneca/MedImmune, Pfizer, Roche/Genentech
Giannis S. Mountzios
Honoraria: Roche, Boehringer Ingelheim, AstraZeneca, MSD, BMS GmbH & Co KG, Novartis, Amgen, Takeda
Consulting or Advisory Role: Roche, AstraZeneca/Greece, MSD, Amgen, Novartis, BMS GmbH & Co KG, Takeda
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Ipsen, MSD, Roche, Novartis, BMS GmbH & Co KG, Demo Pharmaceutical
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, PeerVoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee-Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, ETOP IBCSG Partners Member of the Scientific Committee
Niels Reinmuth
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer Ingelheim, MSD Oncology, Takeda, Amgen
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme, Pfizer, Boehringer Ingelheim, Takeda, Amgen, Lilly, AbbVie, Merck KGaA, Sanofi Aventis GmbH, Janssen Oncology
Travel, Accommodations, Expenses: Janssen
Filippo de Marinis
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Bristol Myers Squibb, Roche/Genentech, Pfizer, Novartis, Takeda, Daichii Sankyo, Merck Serono
Nicolas Girard
Employment: AstraZeneca
Consulting or Advisory Role: Roche, Lilly, AstraZeneca, Novartis, Pfizer, Bristol Myers Squibb, MSD, Takeda, Janssen, Sanofi, Amgen, Gilead Sciences, BeiGene, AbbVie, Daiichi Sankyo/Astra Zeneca, LEO Pharma, Ipsen
Research Funding: Roche (Inst), AstraZeneca (Inst), BMS (Inst), MSDavenir (Inst)
Travel, Accommodations, Expenses: Roche, Janssen Oncology
Takayuki Takahama
Speakers' Bureau: AstraZeneca Japan, Chugai Pharmaceutical Co Ltd, Roche Diagnostics Solutions, MSD K.K
Research Funding: Pfizer, Takeda
Scott P. Owen
Honoraria: AstraZeneca, Bristol Myers Squibb, Roche Canada, Takeda, Merck, Novocure
Consulting or Advisory Role: Merck, AstraZeneca, Roche Canada, Takeda, Bristol Myers Squibb, Bayer
Firas B. Badin
Speakers' Bureau: Bristol Myers Squibb/Pfizer, Jazz Pharmaceuticals, Merck Serono, Merck, Lilly, GE Healthcare, Regeneron
Irfan Cicin
Consulting or Advisory Role: Pfizer (Inst), MSD Oncology (Inst), Roche (Inst), Novartis/Ipsen (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), Servier (Inst), Abdi Ibrahim (Inst), Nobelpharma (Inst), AbbVie (Inst), Teva, Janssen Oncology (Inst), Takeda (Inst), Gen (Inst), Astellas Pharma (Inst), Gilead Sciences (Inst)
Speakers' Bureau: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Abdi Ibrahim (Inst), Astellas Pharma, MSD (Inst), Gen (Inst), Teva (Inst)
Sabeen Mekan
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Riddhi Patel
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences
Eric Zhang
Employment: Gilead Sciences
Stock and Other Ownership Interests: Merck, BeiGene
Divyadeep Karumanchi
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Marina Chiara Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb, Daiichi Sankyo/Astra Zeneca, Regeneron, Pfizer, Blueprint Pharmaceutic, Novartis, Sanofi/Aventis, Medscape, Oncohost, Revolution Medicines
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Sanofi, Celgene, Daiichi Sankyo, Pfizer, Seagen, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen, Regeneron, AbbVie, Mirati Therapeutics, Merck, Boehringer Ingelheim, Abion, Gilead Sciences
Speakers' Bureau: AstraZeneca, MSD Oncology, Merck, Mirati Therapeutics, Daiichi Sankyo/Astra Zeneca
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Incyte (Inst), Takeda (Inst), Spectrum Pharmaceuticals (Inst), Blueprint Medicines (Inst), Lilly (Inst), Ipsen (Inst), Janssen (Inst), Exelixis (Inst), MedImmune (Inst), Sanofi (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca, Merck
Uncompensated Relationships: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ettinger DS, Wood DE, Aisner DL, et al. : NCCN Guidelines® insights: Non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw 21:340-350, 2023 [DOI] [PubMed] [Google Scholar]
- 2.Borghaei H, de Marinis F, Dumoulin D, et al. : SAPPHIRE: Phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Ann Oncol 35:66-76, 2024 [DOI] [PubMed] [Google Scholar]
- 3.Neal J, Pavlakis N, Kim S-W, et al. : CONTACT-01: Efficacy and safety from a phase III study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy. J Thorac Oncol 41:S39-S40, 2023 [Google Scholar]
- 4.Paz-Ares L, Goto Y, Lim WDT, et al. : 1194MO canakinumab (CAN) + docetaxel (DTX) for the second- or third-line (2/3L) treatment of advanced non-small cell lung cancer (NSCLC): CANOPY-2 phase III results. Ann Oncol 32:S953-S954, 2021 [Google Scholar]
- 5.Ahn M, Lisberg AE, Paz-Ares L, et al. : Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): Results of the randomized phase III study TROPION-Lung01. Ann Oncol 34:S1661-S1706, 2023 [Google Scholar]
- 6.Insa A, Martin-Martorell P, Di Liello R, et al. : Which treatment after first line therapy in NSCLC patients without genetic alterations in the era of immunotherapy? Crit Rev Oncol Hematol 169:103538, 2022 [DOI] [PubMed] [Google Scholar]
- 7.Majeed U, Manochakian R, Zhao Y, et al. : Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J Hematol Oncol 14:108, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg DM, Cardillo TM, Govindan SV, et al. : Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6:22496-22512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg DM, Sharkey RM: Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 20:871-885, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Bardia A, Mayer IA, Diamond JR, et al. : Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol 35:2141-2148, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson BE, Meric-Bernstam F: Leveraging TROP2 antibody-drug conjugates in solid tumors. Annu Rev Med 75:31-48, 2024 [DOI] [PubMed] [Google Scholar]
- 12.Bardia A, Messersmith WA, Kio EA, et al. : Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 32:746-756, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Greene FL, Edge SB, et al. : The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67:93-99, 2017 [DOI] [PubMed] [Google Scholar]
- 14.PRO Consortium Critical Path Institute : Non-Small Cell Lung Cancer Symptom Assessment Questionnaire (NSCLC-SAQ) (v 1.0). 2021. https://www.c-pathcoas.org/nsclc-saq
- 15.US Department of Health and Human Services : Common Terminology Criteria for Adverse Events (CTCAE) (v 5.0). 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 16.Maurer W, Bretz F: Multiple testing in group sequential trials using graphical approaches. Stat Biopharm Res 5:311-320, 2013 [Google Scholar]
- 17.US Food and Drug Administration : Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics Guidance for Industry. Silver Spring, MD, 2018 [Google Scholar]
- 18.Capelletto E, Migliorino MR, Morabito A, et al. : Final results of the SENECA (SEcond line NintEdanib in non-small cell lung CAncer) trial. Lung Cancer 134:210-217, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Westeel V, Schuette W, Urban T, et al. : Safety and tolerability of weekly docetaxel plus nintedanib: A phase I trial after first-line chemotherapy failure in NSCLC. PLoS One 18:e0292307, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garon EB, Ciuleanu TE, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Tian T, Yu M, Yu Y, et al. : Immune checkpoint inhibitor (ICI)-based treatment beyond progression with prior immunotherapy in patients with stage IV non-small cell lung cancer: A retrospective study. Transl Lung Cancer Res 11:1027-1037, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaraju C, Vaidya G, Jain AS, et al. : Overall survival prediction of docetaxel-based second-line treatment for advanced non-small cell lung cancer: A systematic review and meta-analysis. Oman Med J 37:e419, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Abreu D, Powell SF, Hochmair MJ, et al. : Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32:881-895, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Inamura K, Yokouchi Y, Kobayashi M, et al. : Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 8:28725-28735, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Study of DS-1062a versus docetaxel in previously treated advanced or metastatic non-small cell lung cancer with or without actionable genomic alterations (TROPION-LUNG01), 2024 . https://clinicaltrials.gov/study/NCT04656652
- 29.Rugo HS, Bardia A, Marme F, et al. : Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 40:3365-3376, 2022 [DOI] [PubMed] [Google Scholar]
- 30.Tagawa ST, Balar AV, Petrylak DP, et al. : TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 39:2474-2485, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilead Sciences, Inc : TRODELVY® Prescribing Information. Foster City, CA, Gilead Sciences, 2023 [Google Scholar]
- 32.Cho BC, Dols MC, Reyes Cabanillas R, et al. : OA05.04 sacituzumab govitecan + pembrolizumab in 1L metastatic non-small cell lung cancer: Preliminary results of the EVOKE-02 study. J Thorac Oncol 18:S54, 2023 [Google Scholar]
- 33.Moskovitz M, Okamoto I, Chen P, et al. : Abstract CT067: Pembrolizumab with and without sacituzumab govitecan as first-line treatment for metastatic non-small-cell lung cancer (NSCLC) with PD-L1 TPS ≥50%: Phase 3 KEYNOTE-D46/EVOKE-03 study. Cancer Res 83, 2023. (suppl 8; abstr CT067) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting no conflict of interest. The requested proposal must also include a statistician. Approval of such requests is at Gilead Sciences' discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.