Abstract
PURPOSE
Socioeconomic status (SES) influences the survival outcomes of patients with early breast cancer (EBC). However, limited research investigates social inequalities in their quality of life (QoL). This study examines the socioeconomic inequalities in QoL after an EBC diagnosis and their time trends.
PATIENTS AND METHODS
We used data from the French prospective multicentric CANTO cohort (ClinicalTrials.gov identifier: NCT01993498), including women with EBC enrolled between 2012 and 2018. QoL was assessed using the European Organisation for Research and Treatment of Cancer QoL Core 30 questionnaire (QLQ-C30). summary score at diagnosis and 1 and 2 years postdiagnosis. We considered three indicators of SES separately: self-reported financial difficulties, household income, and educational level. We first analyzed the trajectories of the QLQ-C30 summary score by SES group. Then, social inequalities in QLQ-C30 summary score and their time trends were quantified using the regression-based slope index of inequality (SII), representing the absolute change in the outcome along socioeconomic gradient extremes. The analyses were adjusted for age at diagnosis, Charlson Comorbidity Index, disease stage, and type of local and systemic treatment.
RESULTS
Among the 5,915 included patients with data on QoL at diagnosis and at the 2-year follow-up, social inequalities in QLQ-C30 summary score at baseline were statistically significant for all SES indicators (SIIfinancial difficulties = –7.6 [–8.9; –6.2], SIIincome = –4.0 [–5.2; –2.8]), SIIeducation = –1.9 [–3.1; –0.7]). These inequalities significantly increased (interaction P < .05) in year 1 and year 2 postdiagnosis, irrespective of prediagnosis health, tumor characteristics, and treatment. Similar results were observed in subgroups defined by menopausal status and type of adjuvant systemic treatment.
CONCLUSION
The magnitude of preexisting inequalities in QoL increased over time after EBC diagnosis, emphasizing the importance of considering social determinants of health during comprehensive cancer care planning.
INTRODUCTION
Organized programs have improved breast cancer screening uptake, leading to more diagnoses, particularly early breast cancer (EBC).1-3 Advances in diagnosis and treatment of EBC during the past decades have significantly transformed patient outcomes and contributed to excellent long-term survival rates.4,5 Consequently, the absolute number of breast cancer survivors has greatly increased, leading to a raised interest in patient outcomes beyond survival rates, for instance, in patient quality of life (QoL).
CONTEXT
Key Objective
To examine the socioeconomic status-related inequalities in quality of life (QoL) of patients with early breast cancer (EBC) using data from a large multicentric French patient cohort.
Generated Knowledge
In the context of universal health care and a strong welfare state, this study identified and quantified significant socioeconomic QoL inequalities after the diagnosis and treatment of EBC. These inequalities increased over the first and second years postdiagnosis and were independent of patient age, EBC stage, and treatment.
Relevance (I. Cheng)
With the strengths of a prospective study design in assessing the temporal relationships between socioeconomic factors and QoL among women diagnosed with EBC, this study identified an increase over time in the magnitude of socioeconomic inequalities on symptoms experienced by women with EBC in a setting of universal healthcare coverage and a strong welfare state. These findings point to the importance of investigating structural, community, and individual level solutions to address these breast cancer inequalities.*
*Relevance section written by JCO Associate Editor Iona Cheng, PhD, MPH.
Breast cancer is characterized not only by heterogeneity at the molecular level6,7 but also at the demographic level, affecting women of different ages.8 This heterogeneity leads to complex multidisciplinary management, often involving combined surgical, radiotherapy, and systemic therapy approaches.9 The treatment complexity results in many associated adverse events that can significantly affect patient QoL.10,11
While health care is key in determining health outcomes, socioeconomic determinants of health are increasingly recognized, with health inequalities identified between and within countries or communities.12-14 Addressing inequalities is a moral and public health imperative, often requiring measures beyond the health care sector. To be tackled, inequalities must first be identified, quantified, and contextualized.
While social inequalities in breast cancer diagnosis and survival outcomes are widely documented,15-18 less is known about social inequalities in the QoL of survivors of breast cancer. These inequalities were described in cross-sectional studies19-21 that assessed the QoL of patients with breast cancer with different lengths of survivorship and different disease stages (advanced and EBC). Such study designs did not use repeated measurements, and QoL trajectories were not assessed. Moreover, limited sample size and incomplete data on menopausal status and cancer treatment often hindered evaluating the impact of these factors on identified inequalities.
This study assesses and quantifies socioeconomic inequalities in patient QoL during the 2 years after an EBC diagnosis.
PATIENTS AND METHODS
Data Source
We used data from the CANTO study cohort22 (ClinicalTrials.gov identifier: NCT01993498), which collects data from women diagnosed with EBC (stage I, II or III, according to ESMO guidelines)23 treated in 26 French cancer centers (complete list in Appendix Table A1, online only). CANTO's network includes comprehensive cancer centers and public nonteaching, private, and university hospitals. Eligible consecutive patients were invited to participate, and clinical data were obtained directly from their electronic health records.
Besides clinicopathologic outcomes, CANTO participants' data includes an extensive list of patient-reported QoL outcomes (PROs), including the European Organisation for Research and Treatment of Cancer (EORTC) QoL Core 30 questionnaire (QLQ-C30).24,25 The QLQ-C30 (version 4.0) has 30 items grouped into nine multi-item scales and six single-item scales assessing several QOL domains. Furthermore, a comprehensive questionnaire allows a complete sociodemographic characterization. All sociodemographic and QoL questionnaires were self-administered.
Data used in this study concerns patients enrolled between 2012 and 2018, collected at diagnosis (baseline, ie, before the start of cancer treatment) and approximately 1 (year 1) and 2 years (year 2) after diagnosis.
All patients provided written informed consent, and the relevant ethics committee approved the study.
Variables of Interest
Outcome Variables
The outcome variable was the summary score of the QLQ-C30 (henceforth QLQ-C30 score).26 The QLQ-C30 score was calculated according to the EORTC guidelines by averaging 13 of the 15 QLQ-C30 scales, with higher scores reflecting a better QoL.27 The individual symptom scales of the QLQ-C30 (pain, fatigue, insomnia, dyspnea, constipation, diarrhea, appetite, and nausea) and the breast cancer-specific EORTC QLQ-BR23 questionnaire (breast symptoms, arm symptoms, and adverse events of systemic treatment)26 were used for symptom analysis.
Exposure Variables
We assessed socioeconomic status (SES) using self-reported financial difficulties, income (household income adjusted for consumption unit at diagnosis), and educational level. Financial difficulties were categorized into low, medium, and high groups on the basis of a Likert scale. The high group comprised women facing difficulty with monthly household resources, the medium group managed adequately but was on the limit, and the low group reported financial comfort.
Monthly household income was self-reported as a 12-category variable from below 500€ to above 8,000€ (1€ approximately $1.07 US dollars in September 2023). Household income per consumption unit was estimated using the square root method described by the Organization for Economic Co-operation and Development.28 For ease of visualization, income terciles were used when describing the QoL trajectories and quintiles when social inequalities were quantified. Educational level was categorized as high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Covariates
These included age at diagnosis, menopausal status (premenopausal/postmenopausal), Charlson Comorbidity Index, stage, participation in a clinical trial (yes/no), endocrine therapy (tamoxifen ± ovarian function suppression [OFS], aromatase inhibitors ± OFS, both ± OFS, none or OFS only), use and type of chemotherapy (none, adjuvant, neoadjuvant or both), surgery type (none, mastectomy or breast-conserving surgery), use of radiotherapy (yes/no), and type of lymph node management (sentinel lymph node biopsy or none, axillary dissection or other, including supraclavicular and internal mammary lymph node dissection). Trastuzumab use was not included in the models as previous studies did not consistently associate it with patient QoL.29
Study Cohort
The CANTO study cohort includes 11,400 patients, 13.0% (n = 1,476) having elected to exit the study before the end of follow-up and 4.4% (n = 500) lost to follow-up. This study analyzed patients with data for 27 of 30 QLQ-C30 questions, enabling summary score calculation.
Over time, data missingness increased, with 18.4% (n = 2,098) at baseline, 31.2% (n = 3,552) at Year 1, and 41.1% (n = 4,686) at Year 2. We included participants with QLQ-C30 data at baseline and Year 2 (n = 5,915, 51.9% of all CANTO participants), with Year 1 data missing for 15% (n = 888) of included patients. Demographic and clinicopathologic characteristics showed no major differences between included and excluded patients, although there was a tendency toward lower SES among those not included (Appendix Table A2).
Statistical Analysis
Analyses were conducted separately for each SES indicator. First, we described the trajectories of the QLQ-C30 score from diagnosis to Year 1 and Year 2. This involved calculating the median and interquartile ranges of the QLQ-C30 score for each period (diagnosis, Year 1, and Year 2) within each SES group. Subsequently, changes in QLQ-C30 score from diagnosis to Year 1 and Year 2, according to SES group, were assessed using multivariate generalized estimating equations with an independent correlation structure.30 The mean least square differences between baseline and Year 1 and Year 2 were reported.
Second, we sought to detect and quantify social inequalities in the QLQ-C30 score. Therefore, we calculated the slope index of inequality (SII) at each time point and SES indicator. This summary measure of inequality is a regression-based estimation of the absolute difference in a continuous outcome variable between the two extremes of a socioeconomic explanatory variable, taking into consideration the intermediate groups and their relative size.31 For instance, a SII = 5 for a particular QLQ-C30 score would mean an average difference of 5 units between the two extremes of the used socioeconomic variable. To estimate the SII, we calculated the ridit scores of each SES variable, representing the average cumulative frequency of each group of an ordinal categorical variable.32,33 For instance, if 20% of the participants had a low-income level, the ridit score is 0.2/2 = 0.10. If those with a medium-income level represented 50% of the participants, the ridit score for this income group would be 0.2 + (0.5/2) = 0.45. The ridit scores of each group were then included in multivariate linear regression models as explanatory variables to estimate the SII. If treatment had no impact on social inequalities in QoL or if, after the first period, they returned to what they were before treatment, we expected them to resemble those at baseline. To determine how social inequalities changed over time, we introduced interaction terms between the ridit score and the analyzed time points (Year 1 and Year 2) into the multivariable linear regression models, using the baseline as the reference period.
Owing to breast cancer heterogeneity, the incidence of different subtypes and therapeutic regimens may vary according to menopausal status. Furthermore, the type of systemic treatment (endocrine therapy and chemotherapy) is a main determinant of patient QoL and is associated with different QoL trajectories.34 As such, analyses were first performed for all women and then stratified by menopausal status or systemic treatment regimen (chemotherapy and endocrine therapy). Since clinical trial participation could affect received care and follow-up, inequality analyses were also performed in this patient subgroup. Similar inequality analyses were performed for the individual symptoms scales of the QLQ-C30 and QLQ-BR23 questionnaires.
All models were adjusted for age at diagnosis, Charlson Comorbidity Index, stage, type of lymph node management, use and type of chemotherapy, use and type of endocrine therapy, type of primary tumor surgery, and use of radiotherapy. Finally, we did not perform any formal adjustment for multiplicity because of the study's observational nature.
Ethics Approval
The study was approved by the national regulatory authorities and ethics committee (ID-RCB:2011-A01095-36, 312 11-039). CANcer TOxicities is a trial research involving humans benefiting from authorization from the French National Agency for the Safety of Medicines and Health Products obtained on 14 September 2011 (number B111158-20) and from the Ile-de-France VII Committee for the Protection of Individuals obtained on 14 October 2011 (number 11-039).
Informed consent was obtained from all participants involved in this study.
RESULTS
Cohort Characteristics
Of the 5,915 included study participants, 1.7% (n = 102), 9.4% (n = 558), and 2.5% (n = 147) had missing data for educational level, income, and financial difficulties, respectively. Concerning financial difficulties at baseline, 12.3% (n = 708), 21.7% (n = 1,255), and 66% (n = 3,805) reported high, medium, and low levels of financial difficulty, respectively. Those reporting a high level of financial difficulty were younger, most often premenopausal, and had comorbidities compared with those with low financial difficulties. Not surprisingly, their educational and income levels were lower. Moreover, a higher proportion was diagnosed with stage III EBC (Table 1). There were no differences between the financial difficulty-defined groups regarding breast cancer histology, grade, and immunohistochemistry-defined subtype (Appendix Table A3). Women with high financial difficulties underwent mastectomy and axillary lymph node dissection more frequently than those with low financial difficulties. Radiotherapy utilization was consistent across groups. Neoadjuvant and/or adjuvant chemotherapy was more prevalent in women facing high financial difficulties. Owing to age differences, adjuvant endocrine therapy with tamoxifen was more frequent in women with high financial difficulties compared with those with low financial difficulties (Table 1).
TABLE 1.
Patient Characteristics According to Level of Financial Difficulties
| Characteristic | High | Medium | Low | P |
|---|---|---|---|---|
| No. (%) | 708 (12.3) | 1,255 (21.7) | 3,805 (66) | |
| Age at diagnosis, median (IQR) | 52.1 (45.5-60.6) | 53.2 (45.6-62.6) | 56.5 (48.7-64.3) | <.001 |
| Age at diagnosis, No. (%) | <.001 | |||
| <40 | 63 (8.9) | 143 (11.4) | 237 (6.2) | |
| 40-49 | 236 (33.3) | 340 (27.1) | 891 (23.4) | |
| 50-59 | 219 (30.9) | 369 (29.4) | 1,191 (31.3) | |
| 60-69 | 140 (19.8) | 300 (23.9) | 1,161 (30.5) | |
| ≥70 | 50 (7.1) | 103 (8.2) | 325 (8.5) | |
| Menopausal status, No. (%) | <.001 | |||
| Premenopausal | 342 (49.3) | 580 (47.1) | 1,447 (38.7) | |
| Postmenopausal | 352 (50.7) | 652 (52.9) | 2,295 (61.3) | |
| Charlson Comorbidity Index, No. (%) | <.001 | |||
| 0 | 507 (77.1) | 947 (82.5) | 2,968 (84.3) | |
| ≥1 | 151 (22.9) | 201 (17.5) | 554 (15.7) | |
| Breast cancer stage, No. (%) | <.001 | |||
| I | 330 (46.9) | 572 (46.1) | 1,983 (52.6) | |
| II | 296 (42.0) | 530 (42.7) | 1,451 (38.4) | |
| III | 78 (11.1) | 139 (11.2) | 341 (9.0) | |
| Breast cancer surgery type, No. (%) | .028 | |||
| None | 0 (0.0) | 1 (0.1) | 1 (<0.1) | |
| Mastectomy | 214 (30.2) | 341 (27.2) | 947 (24.9) | |
| BCS | 494 (69.8) | 911 (72.7) | 2,852 (75.1) | |
| Lymph node management, No. (%) | <.001 | |||
| SNLB only or none | 418 (59.0) | 743 (59.2) | 2,515 (66.1) | |
| Axillary dissection or other | 290 (41.0) | 512 (40.8) | 1,290 (33.9) | |
| Radiotherapy, No. (%) | .97 | |||
| No | 57 (8.1) | 98 (7.8) | 296 (7.8) | |
| Yes | 651 (91.9) | 1,157 (92.2) | 3,508 (92.2) | |
| Chemotherapy type, No. (%) | <.001 | |||
| None | 294 (41.5) | 520 (41.4) | 1,861 (48.9) | |
| Adjuvant | 324 (45.8) | 539 (42.9) | 1,530 (40.2) | |
| Neoadjuvant or both | 90 (12.7) | 196 (15.7) | 413 (10.9) | |
| Endocrine therapy, No. (%) | <.001 | |||
| TAM ± OFS | 220 (31.1) | 348 (27.8) | 945 (24.8) | |
| AI ± OFS | 268 (37.9) | 492 (39.2) | 1,746 (45.9) | |
| AI + TAM ± OFS | 104 (14.7) | 186 (14.8) | 467 (12.3) | |
| None or OFS only | 116 (16.4) | 228 (18.2) | 645 (17.0) | |
| Household adjusted income €, median (IQR) | 1,237 (750-1,591) | 1,591 (1,237-2,021) | 2,475 (1,750-3,182) | <.001 |
| Income, No. (%) | <.001 | |||
| Low (lowest tertile) | 510 (77.5) | 553 (47.5) | 697 (20.0) | |
| Medium | 113 (17.2) | 474 (40.7) | 1,186 (34.1) | |
| High (highest tertile) | 35 (5.3) | 137 (11.8) | 1,598 (45.9) | |
| Educational level, No. (%) | <.001 | |||
| Low | 393 (56.5) | 530 (42.8) | 1,199 (31.8) | |
| Medium | 225 (32.4) | 481 (38.9) | 1,413 (37.5) | |
| High | 77 (11.1) | 226 (18.3) | 1,154 (30.6) |
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: AI, aromatase inhibitor; BCS, breast-conserving surgery; OFS, ovarian function suppression; SNLB, sentinel lymph node biopsy; TAM, tamoxifen.
Participants in different income groups showed similar cohort characteristics (Appendix Table A4). Nevertheless, individuals with higher education levels were younger, and their EBC treatment characteristics reflected this younger age (Appendix Table A5).
Concerning QoL, overall, the higher the SES, as defined by financial difficulties, income, or educational level, the higher the median QLQ-C30 score at diagnosis, Year 1, and Year 2 (Fig 1A).
FIG 1.
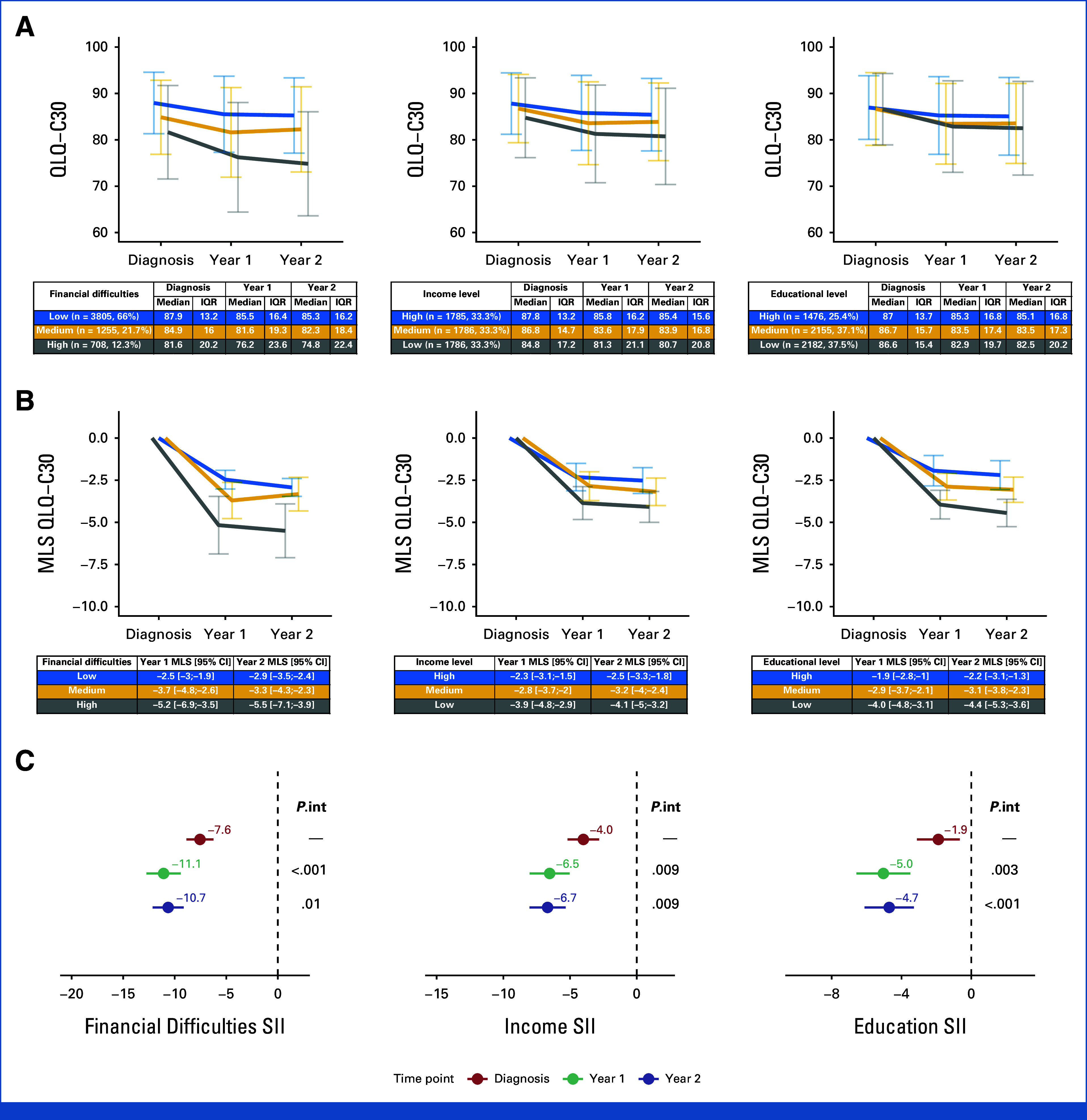
(A) QLQ-C30 summary scores according to SES indicator at baseline, Year 1, and Year 2. (B) Changes in QLQ-C30 summary scores relative to baseline and according to SES indicator. (C) Inequalities in QLQ-C30 summary scores for each of the studied time points and SES indicators. Models in (B) and (C) are adjusted for age, comorbidities, breast cancer stage at diagnosis, type of lymph node management, surgery type, type of systemic treatment, and use of radiotherapy. Reported P values are for interaction by time points (P.int). MLS, mean least square differences; QLQ–C30, QoL Core 30 questionnaire; SES, socioeconomic status; SII, slope index of inequality.
Changes in QoL Compared With Baseline
In adjusted regression models, there was a reduction in QLQ-C30 score compared with baseline in all levels of financial difficulty (all with P < .001 compared with baseline; Fig 1B). The same trends in QLQ-C30 score were observed for income and educational levels (Fig 1B).
Social inequalities in QLQ-C30 score at baseline were statistically significant for all SES indicators (eg, SIIfinancial difficulties = –7.6 [–8.9; –6.2]). Compared with baseline, social inequalities significantly increased (interaction P < .05) in Year 1 (eg, SIIfinancial difficulties = –11.1 [–12.8; –9.4]) and Year 2 (eg, SIIfinancial difficulties = –10.7 [–12.2; –9.1]; Fig 1C).
Social Inequalities in Menopausal Status and Treatment-Related Subgroups
A decrease in QLQ-C30 score between baseline and Years 1 and 2 was observed in all subgroups defined by SES and menopausal status or treatment type (chemotherapy, endocrine therapy). Furthermore, the lower the SES, the more pronounced the decrease in QLQ-C30 score in all menopausal status and treatment subgroups (Appendix Table A6).
As such, when calculating the indexes of inequality, significant (P < .001) inequalities in QLQ-C30 score were observed in all subgroups, time points, and SES indicators except for educational level-related inequalities at baseline in premenopausal women and those treated with chemotherapy (Fig 2).
FIG 2.
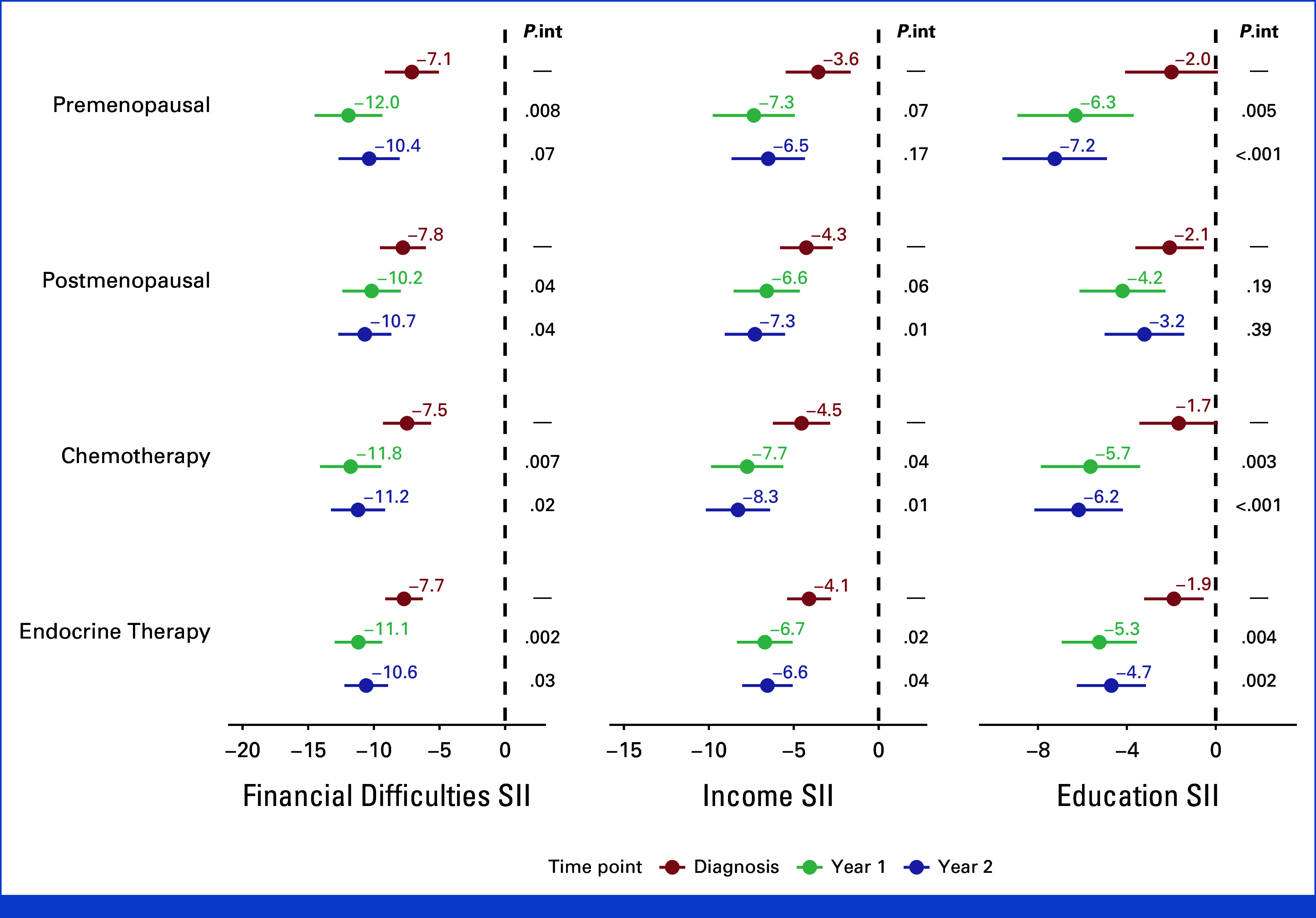
Inequalities in QLQ-C30 summary scores for menopausal status and treatment subgroups for each studied time point and SES indicator. Models are adjusted for age, comorbidities, breast cancer stage at diagnosis, type of lymph node management, surgery type, type of systemic treatment, and use of radiotherapy. Reported P values are for interaction by time points (P.int). QLQ-Q30, QoL Core 30 questionnaire; SES, socioeconomic status; SII, slope index of inequality.
We observed an overall numerical increase in QLQ-C30 score inequalities in Year 1 and Year 2, compared with the baseline in all subgroups. However, statistically significant increases were not universal (Fig 2).
Similar inequalities and trends were observed in women who participated in clinical trials (n = 1,471, 24.9%, Appendix Fig A1).
Social Inequalities in QLQ-C30 and QLQ-BR23 Symptom Scales
We observed that, with few exceptions, there was an increase in the magnitude of inequalities for individual symptoms after diagnosis and treatment of EBC. However, not all these increases were statistically significant. Of note, a significant increase in inequalities was observed in symptoms related to adverse events of systemic treatment and nausea in Year 1 and arm symptoms in Year 2, independently of which indicator was used to establish SES groups (Appendix Fig A2).
DISCUSSION
In this cohort study, we observed social inequalities in QoL after a diagnosis of EBC, as measured by the QLQ-C30 summary score. Using a large individual-level sample, we confirm that inequalities in cancer care extend to survivors' QoL, with women with a lower SES being unequally affected by diagnosis and treatment. In addition, inequalities in QoL are already observed at diagnosis, as previously described,35 and the differential impact of disease further increases them, with incremental impact over time. Furthermore, the observed inequalities and their dynamics, besides age and comorbidities, were independent of other clinicopathologic and treatment-related variables. The increase in preexisting inequalities was still observed in analyses stratified by menopausal status and type of treatment, suggesting that addressing them could benefit a large proportion of women with EBC. Similar results were observed in clinical trial participants despite them theoretically having a more consistent and close follow-up, potentially leveling the playing field for access to care.
The QLQ-C30 summary score inequalities, particularly those related to financial difficulties and income, were above a 5-unit difference. No specific threshold for a minimal clinically important difference has been calculated for the QLQ-C30 summary score, and the EORTC recognizes that no single minimal important difference can be applied to all disease settings.36 However, a 5-unit difference was identified as a potential minimal important difference in patients with advanced or metastatic cancer,36,37 and arguably, a lower threshold should be applied to detect an important QoL deterioration in an early disease setting.38
We observed an overall increase in inequalities of the symptom scales of QLQ-C30 and QLQ-BR23 with different inequality patterns between baseline, Year 1, and Year 2. The differences between Years 1 and 2 could be related to some symptoms being experienced early in breast cancer treatment (eg, nausea and other side effects of systemic treatment) while others have a chronic nature (eg, arm symptoms). However, the overall trend of increasing inequalities in all symptoms suggests that all contribute to the identified increased inequalities in QLQ-C30 summary score.
It is worth noting that this study was performed in France, a country with a strong welfare state and a health care system marked by easy access, universal coverage, and excellent health outcomes.39 However, as highlighted previously by the WHO, inequalities in health occur even in countries with a strong welfare state.40 Moreover, health equity has long been identified as the objective of several highly performant European health systems.41 Yet, inequalities persist, further confirming the need for a trans-sectoral approach beyond the health sector to improve patient outcomes.
Additionally, using a summary measure of inequality, rather than just comparing the two extremes, allowed us to uncover an inequality gradient throughout the socioeconomic distribution. As noted by Marmot et al,12,42 poor health affects not only the poor but also those in the middle whose health outcomes, despite being better than those of the poor, are still not at par with those at the top of the socioeconomic distribution. The observation of a similar social gradient in the QoL of survivors of EBC suggests that measures that go beyond supporting those worse off are needed to reduce the inequality gap.
The identified inequalities were independent of prediagnosis health conditions, disease stage, and treatment, suggesting that their origin may lie beyond the health care sector. Several elements not captured by detailed clinical and socioeconomic surveys may affect patient well-being (eg, traveling distance to the treating center, degree of support from partner, the need for enhanced childcare, the household built environment, etc). In addition, access to evidence-based approaches for managing side effects of EBC treatment11 may differ according to SES, contributing to the observed inequalities in QoL.
Moreover, impaired QoL may affect patients' social and working lives and delay their return to work after EBC treatment.43 Thus, it is essential to tackle social inequalities in QoL up-front to avoid the QoL burden of EBC disproportionally affecting the most socially deprived patients.
It is thus apparent that not only do the origins of social inequalities in QoL (among other health outcomes) lie beyond the health care sector but so do their repercussions. The precedence of survival outcomes over QoL outcomes is reflected in the vast number of studies available on inequalities of the former versus the latter. Nevertheless, the burden of a disease is not accurately measured if not considering quality besides the length of life. The most current generic measures of disease burden, quality-adjusted life year, and disable-adjusted life year consider QoL and are widely viewed as better representations of a disease burden than measures that focus solely on mortality indicators.44,45 The QoL implications of the disease were factored in an economic analysis that estimated the monetary value related to health inequities in the European Union to represent 9.4% of the gross domestic product, approximately 15% of social security benefits, and 20% of the total health care costs.46 As the most common cancer and with a high proportion of survivors, it is not surprising that a considerable swath of the estimated costs is related to the economic consequences of QoL in breast cancer survivors. In addition, we observed that the increase in social inequalities occurs already during the first analyzed period and persists thereafter, with a further analysis of long-term outcomes needed to determine whether this increase is permanent or temporary.
To the best of our knowledge, this is the largest cohort study quantifying socioeconomic inequalities in QoL among patients with EBC from diagnosis to the second year after diagnosis. Its longitudinal design enables the assessment of how these inequalities evolved during the studied periods. In addition, the use of three socioeconomic indicators reinforces the hypothesis of a social inequality gradient. This suggests that inequalities are not limited to comparisons between the extremes of the social status scale, such as those with and without financial difficulties. Finally, detailed clinicopathologic patient data allowed us to determine the independence of the identified social inequalities from disease stage or type of EBC treatment.
This study has several limitations. First, it was only restricted to the second year after EBC diagnosis. Follow-up for up to 6 years is ongoing for the whole cohort, and future studies will highlight the long-term trends of inequalities in this population. Second, since patients were included between 2012 and 2018, the standard of care may have changed (for instance, the use of immunotherapy and CDK 4/6 inhibitors), potentially affecting the observed inequalities. Third, no information was available about immigration origin, and non-French speaking patients were excluded from the CANTO study. While approximately 80% of immigrants in France originate from Africa and Europe, the majority are French speakers,47 yet a very small subset of patients with low SES and specific health care needs may not be represented in this study. Fourth, no data were available concerning traveling time to treatment centers and the availability of local support systems. Fifth, despite being multicentric, this was not an international study, as such results may not be generalizable to other contexts.
In conclusion, this study identified and quantified social inequalities in the QoL of patients with EBC in a French population. Furthermore, the inequalities increased after EBC diagnosis, and this increase was not related to differential disease stages or treatments between groups with different SESs.
Clinicians and society should be aware that the price of socioeconomic inequalities in health is also reflected in suboptimal QoL patient outcomes. The widening of social inequalities in patient QoL after EBC in a country with a strong welfare state and universal health care coverage highlights the importance of considering the patients' socioeconomic environment and the wider set of forces and systems shaping the conditions of daily life when developing strategies to improve patient outcomes.
ACKNOWLEDGMENT
We thank all patients who have kindly participated in the CANTO study.
APPENDIX
FIG A1.
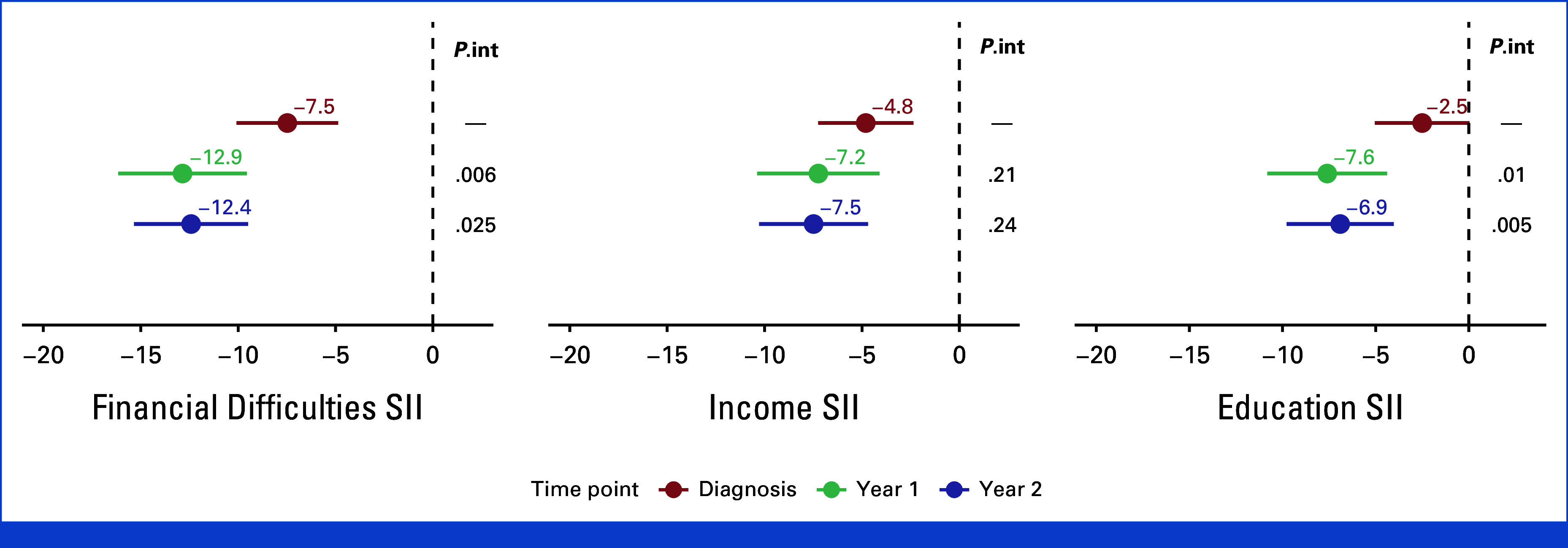
Inequalities in QLQ-C30 summary scores in patients that participated in clinical trials for each studied time point and SES indicator. Models are adjusted for age, comorbidities, breast cancer stage at diagnosis, type of lymph node management, surgery type, type of systemic treatment, and use of radiotherapy. Reported P values are for interaction by time points (P.int). QLQ-C30, QoL Core 30 questionnaire; SES, socioeconomic status; SII, slope index of inequality.
FIG A2.
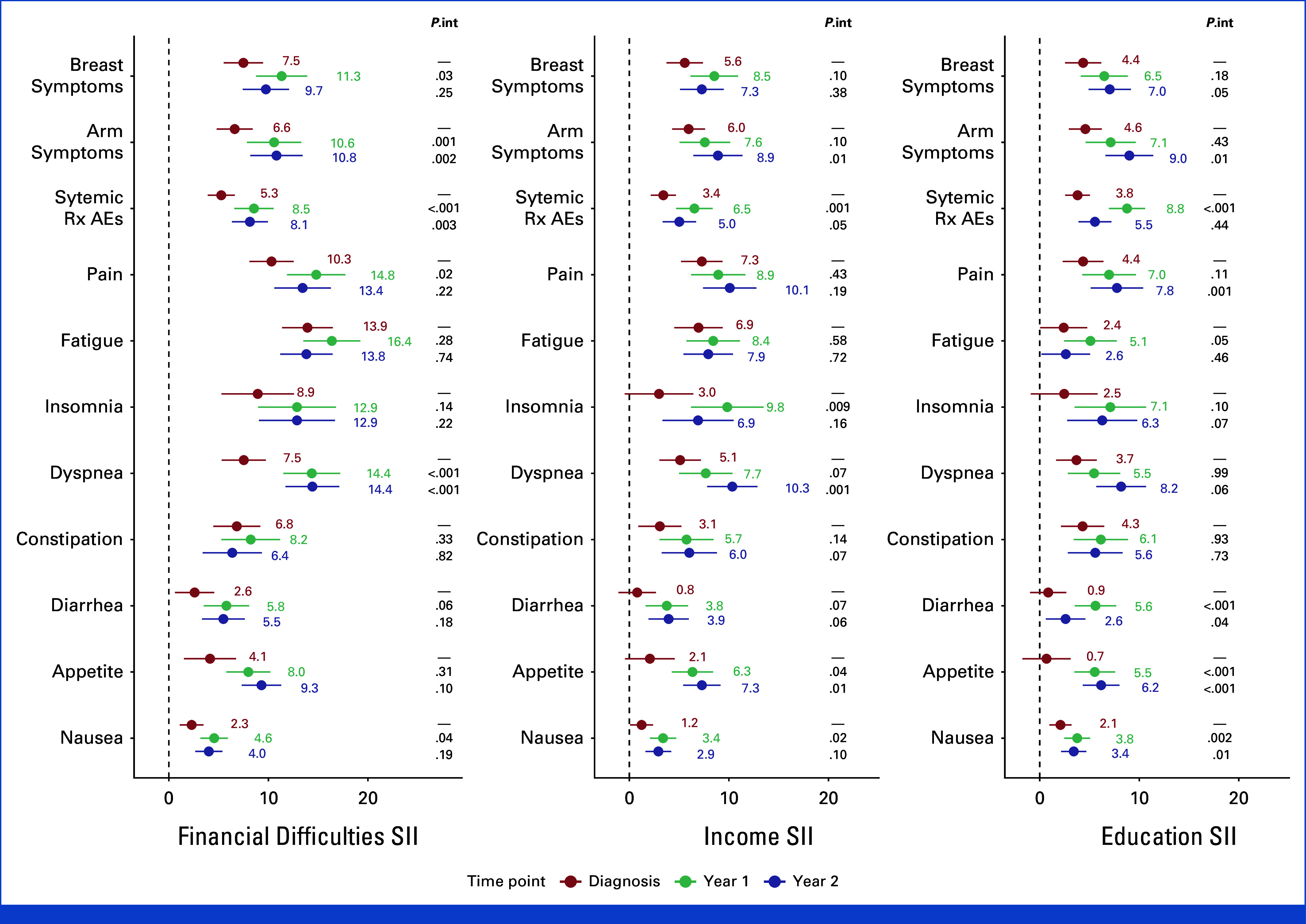
Inequalities in QLQ-C30 and QLQ-BR23 symptom scales scores for each studied time point and SES indicator. Models are adjusted for age, comorbidities, breast cancer stage at diagnosis, type of lymph node management, surgery type, type of systemic treatment, and use of radiotherapy. Reported p values are for interaction by time points (P.int). AEs, adverse events; QLQ-C30, QoL Core 30 questionnaire; Rx, treatment; SES, socioeconomic status; SII, slope index of inequality.
TABLE A1.
List of Participating Centers in the CANTO Study
| Number | Center | Location |
|---|---|---|
| 1 | Institut Gustave Roussy | Villejuif |
| 2 | Institut Jean Godinot | Reims |
| 3 | Institut Bergonié | Bordeaux |
| 4 | ICM | Montpellier |
| 5 | Centre Oscar Lambret | Lille |
| 6 | Centre GF Leclerc | Dijon |
| 7 | Centre Alexis Vautrin | Nancy |
| 8 | ICO Angers | Angers |
| 9 | Centre Léon Bérard | Lyon |
| 10 | Institut Curie Paris | Paris |
| 11 | Centre François Baclesse | Caen |
| 12 | ICO Nantes | Nantes |
| 13 | Institut Paoli Calmettes | Marseille |
| 14 | Institut Curie St Cloud | St Cloud |
| 15 | Centre Jean Perrin | Clermont |
| 16 | Centre Henri Becquerel | Rouen |
| 17 | Centre Antoine Lacassagne | Nice |
| 18 | Centre Paul Strauss | Strasbourg |
| 19 | Centre Eugène Marquis | Rennes |
| 20 | Clinique Sainte Catherine | Avignon |
| 21 | Hôpital de la Source | Orléans |
| 22 | AP-HP St Louis | Paris |
| 23 | Institut Claudius Régaud | Toulouse |
| 24 | AP-HP La Pitié Salpétrière | Paris |
| 25 | CH Blois | Blois |
| 26 | Clinique du Parc | Dijon |
TABLE A2.
Patient Characteristics According to Inclusion Status
| Characteristic | Included | Not Included |
|---|---|---|
| No. (%) | 5,915 (51.9) | 5,485 (48.1) |
| Age at diagnosis, median (IQR) | 55.3 (47.5-63.5) | 57.8 (48.8-66.6) |
| Age at diagnosis, No. (%) | ||
| <40 | 449 (7.6) | 366 (6.7) |
| 40-49 | 1,502 (25.4) | 1,188 (21.7) |
| 50-59 | 1,827 (30.9) | 1,519 (27.7) |
| 60-69 | 1,642 (27.8) | 1,576 (28.7) |
| ≥70 | 495 (8.4) | 836 (15.2) |
| Menopausal status, No. (%) | ||
| Premenopausal | 2,425 (41.7) | 1,873 (34.7) |
| Postmenopausal | 3,388 (58.3) | 3,528 (65.3) |
| Charlson Comorbidity Index, No. (%) | ||
| 0 | 4,520 (82.8) | 3,734 (77.7) |
| ≥1 | 942 (17.2) | 1,071 (22.3) |
| Breast cancer stage, No. (%) | ||
| I | 2,954 (50.4) | 2,527 (47.0) |
| II | 2,339 (39.9) | 2,340 (43.5) |
| III | 572 (9.8) | 511 (9.5) |
| Histology, No. (%) | ||
| Invasive ca. NST | 4,580 (77.5) | 4,257 (77.9) |
| Invasive lobular ca | 760 (12.9) | 689 (12.6) |
| Mixed NST/lobular | 566 (9.6) | 522 (9.5) |
| Grade, No. (%) | ||
| 1 | 1,048 (17.9) | 946 (17.5) |
| 2 | 3,137 (53.6) | 2,872 (53.1) |
| 3 | 1,671 (28.5) | 1,594 (29.5) |
| IHC-defined subtypes, No. (%) | ||
| Hormone receptor+/HER2+ | 624 (10.6) | 549 (10.1) |
| Hormone receptor–/HER2+ | 228 (3.9) | 209 (3.9) |
| Hormone receptor+/HER2– | 4,493 (76.4) | 4,108 (75.8) |
| Hormone receptor–/HER2– | 533 (9.1) | 555 (10.2) |
| Breast cancer surgery type, No. (%) | ||
| None | 2 (<1) | 19 (0.3) |
| Mastectomy | 1,544 (26.1) | 1,518 (27.9) |
| BCS | 4,362 (73.8) | 3,900 (71.7) |
| Lymph node management, No. (%) | ||
| SNLB only or none | 3,764 (63.6) | 3,372 (62.0) |
| Axillary dissection or other | 2,151 (36.4) | 2,071 (38.0) |
| No. (%) | 5,915 (51.9) | 5,485 (48.1) |
| Radiotherapy, No. (%) | ||
| No | 467 (7.9) | 511 (9.4) |
| Yes | 5,447 (92.1) | 4,909 (90.6) |
| Chemotherapy type, No. (%) | ||
| None | 2,740 (46.3) | 2,594 (47.7) |
| Adjuvant | 2,453 (41.5) | 2,045 (37.6) |
| Neoadjuvant or both | 721 (12.2) | 795 (14.7) |
| Endocrine therapy, No. (%) | ||
| TAM ± OFS | 1,551 (26.2) | 1,263 (23.7) |
| AI ± OFS | 2,566 (43.4) | 2,596 (48.7) |
| AI + TAM ± OFS | 772 (13.1) | 410 (7.7) |
| None or OFS only | 1,023 (17.3) | 1,066 (20.0) |
| Household adjusted income €, median (IQR) | 2,013 (1,375-2,750) | 1,750 (1,237-2,475) |
| Financial difficulties, No. (%) | ||
| High | 708 (12.3) | 802 (18.3) |
| Medium | 1,255 (21.8) | 1,051 (23.9) |
| Low | 3,805 (66.0) | 2,538 (57.8) |
| Income level, No. (%) | ||
| Low (lowest tertile) | 1,562 (29.2) | 1,538 (39.0) |
| Medium | 1,795 (33.5) | 1,305 (33.1) |
| High (highest tertile) | 2,000 (37.3) | 1,099 (27.9) |
| Educational level, No. (%) | ||
| Low | 2,182 (37.5) | 2,126 (47.7) |
| Medium | 2,155 (37.1) | 1,419 (31.8) |
| High | 1,476 (25.4) | 916 (20.5) |
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: AI, aromatase inhibitor; BCS, breast-conserving surgery; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; NST, no special type; OFS, ovarian function suppression; SNLB, sentinel lymph node biopsy; TAM, tamoxifen.
TABLE A3.
Histopathological Characteristics According to Socioeconomic Status
| Characteristic | Financial Difficulties | Income | Educational Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Medium | Low | P | Low (lowest tertile) | Medium | High (highest tertile) | P | Low | Medium | High | P | |
| Histology, No. (%) | .85 | .062 | .38 | |||||||||
| Invasive ca. NST | 560 (79.1) | 964 (76.9) | 2,935 (77.3) | 1,417 (79.4) | 1,385 (77.7) | 1,349 (75.7) | 1,689 (77.5) | 1,682 (78.2) | 1,123 (76.3) | |||
| Invasive lobular ca | 85 (12.0) | 165 (13.2) | 492 (13.0) | 210 (11.8) | 240 (13.5) | 244 (13.7) | 279 (12.8) | 280 (13.0) | 189 (12.8) | |||
| Mixed NST/lobular | 63 (8.9) | 124 (9.9) | 371 (9.8) | 157 (8.8) | 157 (8.8) | 190 (10.7) | 212 (9.7) | 190 (8.8) | 160 (10.9) | |||
| Grade, No. (%) | .18 | .005 | .37 | |||||||||
| 1 | 119 (17.0) | 206 (16.6) | 701 (18.6) | 294 (16.6) | 333 (18.9) | 326 (18.4) | 401 (18.5) | 368 (17.3) | 261 (17.9) | |||
| 2 | 377 (53.8) | 656 (52.8) | 2,032 (53.9) | 907 (51.3) | 943 (53.5) | 972 (54.9) | 1,176 (54.3) | 1,132 (53.1) | 768 (52.6) | |||
| 3 | 205 (29.2) | 380 (30.6) | 1,034 (27.4) | 566 (32.0) | 488 (27.7) | 473 (26.7) | 587 (27.1) | 630 (29.6) | 431 (29.5) | |||
| IHC-defined subtypes, No. (%) | .10 | .062 | .097 | |||||||||
| Hormone receptor+/HER2+ | 77 (10.9) | 156 (12.5) | 373 (9.9) | 201 (11.3) | 184 (10.4) | 183 (10.3) | 215 (9.9) | 230 (10.8) | 169 (11.6) | |||
| Hormone receptor–/HER2+ | 23 (3.3) | 52 (4.2) | 147 (3.9) | 76 (4.3) | 48 (2.7) | 82 (4.6) | 68 (3.1) | 94 (4.4) | 63 (4.3) | |||
| Hormone receptor+/HER2– | 548 (77.7) | 918 (73.6) | 2,921 (77.3) | 1,333 (74.9) | 1,380 (78.0) | 1,351 (76.2) | 1,700 (78.2) | 1,606 (75.1) | 1,106 (75.6) | |||
| Hormone receptor–/HER2– | 57 (8.1) | 122 (9.8) | 337 (8.9) | 169 (9.5) | 158 (8.9) | 158 (8.9) | 192 (8.8) | 208 (9.7) | 125 (8.5) | |||
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: IHC, immunohistochemistry; HER2, human epidermal growth factor receptor 2; NST, no special type.
TABLE A4.
Patient Characteristics According to Income
| Characterisitic | Low (lowest tertile) | Medium | High (highest tertile) | P |
|---|---|---|---|---|
| No. (%) | 1,786 (33.3) | 1,786 (33.3) | 1,785 (33.3) | |
| Age at diagnosis, median (IQR) | 52.5 (45.3-62.3) | 55.7 (47.3-63.6) | 56.2 (49.3-63.2) | <.001 |
| Age at diagnosis, No. (%) | <.001 | |||
| <40 | 182 (10.2) | 154 (8.6) | 87 (4.9) | |
| 40-49 | 543 (30.4) | 452 (25.3) | 411 (23.0) | |
| 50-59 | 503 (28.2) | 524 (29.3) | 644 (36.1) | |
| 60-69 | 408 (22.8) | 516 (28.9) | 516 (28.9) | |
| ≥70 | 150 (8.4) | 140 (7.8) | 127 (7.1) | |
| Menopausal status, No. (%) | <.001 | |||
| Premenopausal | 856 (48.8) | 741 (42.2) | 671 (38.3) | |
| Postmenopausal | 897 (51.2) | 1,017 (57.8) | 1,082 (61.7) | |
| Charlson Comorbidity Index, No. (%) | <.001 | |||
| 0 | 1,314 (79.8) | 1,364 (82.7) | 1,431 (86.7) | |
| ≥1 | 333 (20.2) | 285 (17.3) | 220 (13.3) | |
| Breast cancer stage, No. (%) | <.001 | |||
| I | 800 (45.2) | 899 (50.7) | 940 (53.2) | |
| II | 763 (43.1) | 712 (40.1) | 672 (38.0) | |
| III | 206 (11.6) | 163 (9.2) | 155 (8.8) | |
| Breast cancer surgery type, No. (%) | .002 | |||
| None | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Mastectomy | 524 (29.4) | 427 (23.9) | 437 (24.5) | |
| BCS | 1,260 (70.6) | 1,356 (76.0) | 1,344 (75.5) | |
| Lymph node management, No. (%) | <.001 | |||
| SNLB only or none | 1,056 (59.1) | 1,142 (63.9) | 1,190 (66.7) | |
| Axillary dissection or other | 730 (40.9) | 644 (36.1) | 595 (33.3) | |
| Radiotherapy, No. (%) | .75 | |||
| No | 145 (8.1) | 133 (7.4) | 141 (7.9) | |
| Yes | 1,640 (91.9) | 1,653 (92.6) | 1,644 (92.1) | |
| Chemotherapy type, No. (%) | <.001 | |||
| None | 739 (41.4) | 838 (46.9) | 865 (48.5) | |
| Adjuvant | 800 (44.8) | 745 (41.7) | 704 (39.5) | |
| Neoadjuvant or both | 247 (13.8) | 203 (11.4) | 215 (12.1) | |
| Endocrine therapy, No. (%) | <.001 | |||
| TAM ± OFS | 549 (30.8) | 480 (26.9) | 421 (23.6) | |
| AI ± OFS | 693 (38.8) | 763 (42.7) | 820 (46.0) | |
| AI + TAM ± OFS | 241 (13.5) | 239 (13.4) | 228 (12.8) | |
| None or OFS only | 301 (16.9) | 304 (17.1) | 315 (17.7) | |
| Household adjusted income €, median (IQR) | 1,237 (884-1,375) | 2,013 (1,750-2,250) | 3,250 (2,750-4,000) | <.001 |
| Educational level, No. (%) | <.001 | |||
| Low | 984 (56.1) | 653 (36.9) | 285 (16.0) | |
| Medium | 581 (33.1) | 740 (41.8) | 691 (38.9) | |
| High | 190 (10.8) | 376 (21.3) | 801 (45.1) | |
| Financial difficulties, No. (%) | <.001 | |||
| High | 510 (29.0) | 113 (6.4) | 35 (2.0) | |
| Medium | 553 (31.4) | 474 (26.7) | 137 (7.7) | |
| Low | 697 (39.6) | 1,186 (66.9) | 1,598 (90.3) |
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: AI, aromatase inhibitor; BCS, breast-conserving surgery; OFS, ovarian function suppression; SNLB, sentinel lymph node biopsy; TAM, tamoxifen.
TABLE A5.
Patient Characteristics According to Educational Level
| Characteristic | Low | Medium | High | P |
|---|---|---|---|---|
| No. (%) | 2,182 (37.5) | 2,155 (37.1) | 1,476 (25.4) | |
| Age at diagnosis, median (IQR) | 59.4 (51.3-66.2) | 52.9 (45.8-61.9) | 51.3 (44.6-60.3) | <.001 |
| Age at diagnosis, No. (%) | <.001 | |||
| <40 | 49 (2.2) | 210 (9.7) | 186 (12.6) | |
| 40-49 | 394 (18.1) | 619 (28.7) | 475 (32.2) | |
| 50-59 | 691 (31.7) | 680 (31.6) | 431 (29.2) | |
| 60-69 | 782 (35.8) | 500 (23.2) | 313 (21.2) | |
| ≥70 | 266 (12.2) | 146 (6.8) | 71 (4.8) | |
| Menopausal status, No. (%) | <.001 | |||
| Premenopausal | 599 (27.9) | 1,029 (48.6) | 772 (53.5) | |
| Postmenopausal | 1,550 (72.1) | 1,090 (51.4) | 671 (46.5) | |
| Charlson Comorbidity Index, No. (%) | <.001 | |||
| 0 | 1,542 (77.0) | 1,696 (84.8) | 1,207 (88.6) | |
| ≥1 | 460 (23.0) | 305 (15.2) | 155 (11.4) | |
| Breast cancer stage, No. (%) | .32 | |||
| I | 1,106 (50.9) | 1,091 (51.0) | 708 (48.7) | |
| II | 843 (38.8) | 856 (40.0) | 596 (41.0) | |
| III | 224 (10.3) | 191 (8.9) | 149 (10.3) | |
| Breast cancer surgery type, No. (%) | .081 | |||
| None | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Mastectomy | 541 (24.8) | 554 (25.7) | 425 (28.8) | |
| BCS | 1,638 (75.1) | 1,597 (74.2) | 1,049 (71.2) | |
| Lymph node management, No. (%) | .04 | |||
| SNLB only or none | 1,432 (65.6) | 1,363 (63.2) | 910 (61.7) | |
| Axillary dissection or other | 750 (34.4) | 792 (36.8) | 566 (38.3) | |
| Radiotherapy, No. (%) | .79 | |||
| No | 175 (8.0) | 163 (7.6) | 120 (8.1) | |
| Yes | 2,007 (92.0) | 1,991 (92.4) | 1,356 (91.9) | |
| Chemotherapy type, No. (%) | <.001 | |||
| None | 1,074 (49.2) | 965 (44.8) | 644 (43.6) | |
| Adjuvant | 910 (41.7) | 904 (42.0) | 606 (41.1) | |
| Neoadjuvant or both | 198 (9.0) | 285 (13.3) | 226 (15.3) | |
| Endocrine therapy, No. (%) | <.001 | |||
| TAM ± OFS | 358 (16.4) | 672 (31.2) | 506 (34.3) | |
| AI ± OFS | 1,182 (54.2) | 798 (37.0) | 522 (35.4) | |
| AI + TAM ± OFSs | 283 (13.0) | 289 (13.4) | 194 (13.2) | |
| OFS only | 4 (0.2) | 3 (0.1) | 0 (0.0) | |
| None | 354 (16.2) | 392 (18.2) | 253 (17.2) | |
| Household adjusted income €, median (IQR) | 1,591 (1,237-2,021) | 2,021 (1,588-2,750) | 2,750 (2,013-3,753) | <.001 |
| Income level, No. (%) | <.001 | |||
| Low (lowest tertile) | 984 (51.2) | 581 (28.9) | 190 (13.9) | |
| Medium | 653 (34.0) | 740 (36.8) | 376 (27.5) | |
| High (highest tertile) | 285 (14.8) | 691 (34.3) | 801 (58.6) | |
| Financial difficulties, No. (%) | <.001 | |||
| High | 393 (18.5) | 225 (10.6) | 77 (5.3) | |
| Medium | 530 (25.0) | 481 (22.7) | 226 (15.5) | |
| Low | 1,199 (56.5) | 1,413 (66.7) | 1,154 (79.2) |
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: AI, aromatase inhibitor; BCS, breast-conserving surgery; OFS, ovarian function suppression; SNLB, sentinel lymph node biopsy; TAM, tamoxifen.
TABLE A6.
Change in the QLQ-C30 Summary Score Over Time According to SES, Menopausal Status and Treatment Regimen
| SES Variable | Premenopausal | Postmenopausal | Chemotherapy | Endocrine Therapy | ||||
|---|---|---|---|---|---|---|---|---|
| Year 1 MLS (95% CI) | Year 2 MLS (95% CI) | Year 1 MLS (95% CI) | Year 2 MLS (95% CI) | Year 1 MLS (95% CI) | Year 2 MLS (95% CI) | Year 1 MLS (95% CI) | Year 2 MLS (95% CI) | |
| Financial difficulties | ||||||||
| Low | –2.5 (–3.1 to –1.9) | –3.3 (–3.9 to –2.8) | –2.6 (–3.3 to –1.9) | –3.4 (–4.1 to –2.8) | –3 (–3.8 to –2.2) | –2.7 (–3.5 to –2) | –2.5 (–3.1 to –1.9) | –3.3 (–3.9 to –2.8) |
| Medium | –3.6 (–4.7 to –2.4) | –3.7 (–4.8 to –2.6) | –4.1 (–5.6 to –2.6) | –4.5 (–5.9 to –3.1) | –4.7 (–6.1 to –3.3) | –3.5 (–4.8 to –2.2) | –3.6 (–4.7 to –2.4) | –3.7 (–4.8 to –2.6) |
| High | –5.2 (–7.1 to –3.4) | –5.7 (–7.4 to –4) | –4.6 (–7.1 to –2.2) | –5.6 (–7.9 to –3.3) | –6.1 (–8.4 to –3.8) | –5.5 (–7.6 to –3.4) | –5.2 (–7.1 to –3.4) | –5.7 (–7.4 to –4) |
| Income | ||||||||
| High | –2.1 (–3.6 to –0.7) | –1.7 (–3 to –0.3) | –2.4 (–3.4 to –1.4) | –3 (–3.9 to –2.1) | –2.9 (–4.1 to –1.8) | –2.2 (–3.2 to –1.1) | –2.4 (–3.2 to –1.5) | –3 (–3.8 to –2.2) |
| Medium | –2.7 (–4 to –1.3) | –2.4 (–3.7 to –1.1) | –3 (–4.1 to –1.9) | –3.8 (–4.9 to –2.7) | –3.4 (–4.6 to –2.2) | –3.3 (–4.4 to –2.2) | –2.9 (–3.9 to –2) | –3.7 (–4.6 to –2.8) |
| Low | –3.6 (–5 to –2.3) | –3.2 (–4.6 to –1.9) | –4 (–5.4 to –2.6) | –4.8 (–6.1 to –3.5) | –4.8 (–6.1 to –3.5) | –4.1 (–5.3 to –2.9) | –3.8 (–4.8 to –2.7) | –4.2 (–5.2 to –3.2) |
| Educational level | ||||||||
| High | –1.6 (–2.8 to –0.3) | –1.2 (–2.4 to –0.1) | –2.5 (–3.8 to –1.3) | –3.3 (–4.6 to –2.1) | –2.5 (–3.7 to –1.3) | –1.9 (–3.1 to –0.8) | –1.9 (–2.8 to –0.9) | –2.6 (–3.6 to –1.7) |
| Medium | –3 (–4.1 to –1.8) | –2.1 (–3.2 to –1) | –2.8 (–3.9 to –1.8) | –4 (–5 to –3) | –3.3 (–4.5 to –2.2) | –2.6 (–3.7 to –1.6) | –3 (–3.8 to –2.1) | –3.5 (–4.4 to –2.7) |
| Low | –4.8 (–6.4 to –3.1) | –5.1 (–6.7 to –3.5) | –3.5 (–4.5 to –2.6) | –4.1 (–5 to –3.2) | –5.2 (–6.5 to –4) | –5.1 (–6.2 to –3.9) | –4 (–4.9 to –3) | –4.7 (–5.6 to –3.8) |
NOTE. Income refers to tertiles of household income per consumption unit. Financial difficulties defined according to how monthly household resources allowed participants to live: high (with difficulty), medium (adequately but on the limit), or low (financially comfortable). Educational level: high (high school and three or more years of higher education), medium (secondary school ± 2 years of professional training), and low (no education or primary education).
Abbreviations: MLS, mean least square differences; QLQ-C30, QoL Core 30 questionnaire; SES, socioeconomic status.
José Luis Sandoval
Travel, Accommodations, Expenses: Pfizer
Maria Alice Franzoi
Speakers' Bureau: Novartis (Inst)
Research Funding: Resilience Care (Inst)
Antonio di Meglio
Expert Testimony: Kephren, Techspert.io
Arlindo R. Ferreira
Employment: Resilience Care
Honoraria: Roche, Novartis, Gilead Sciences, Merck Sharp & Dohme, Seagen
Travel, Accommodations, Expenses: Daiichi Sankyo Europe GmbH, Lilly
Alessandro Viansone
Honoraria: Seagen
Consulting or Advisory Role: Seagen
Speakers' Bureau: AstraZeneca/Daiichi Sankyo
Research Funding: Pfizer
Expert Testimony: Seagen
Travel, Accommodations, Expenses: Eisai Europe
Fabrice André
Consulting or Advisory Role: Guardant Health (Inst), AstraZeneca (Inst), Lilly, Daiichi Sankyo (Inst), Roche (Inst), Lilly (Inst), Pfizer (Inst), Owkin (Inst), Novartis (Inst), N-Power Medicine (Inst), Servier (Inst), Gilead Sciences (Inst), Boston Pharmaceuticals (Inst)
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst), Owkin (Inst), Guardant Health (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Christelle Jouannaud
Honoraria: Pfizer, Daiichi Sankyo/Astra Zeneca, Gilead Sciences
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca
Travel, Accommodations, Expenses: MSD Oncology, Viatris
Paul Cottu
Honoraria: Pfizer, Novartis (Inst), Roche, NanoString Technologies (Inst), Lilly, Daiichi Sankyo, AstraZeneca, Gilead Sciences
Consulting or Advisory Role: Pfizer, Lilly
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Lilly
Christelle Levy
Consulting or Advisory Role: Seagen, Gilead Sciences, Lilly
Travel, Accommodations, Expenses: Gilead Sciences, Pfizer
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Novartis (Inst), Sandoz
Research Funding: Resilience Care (Inst)
Travel, Accommodations, Expenses: Novartis
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the San Antonio Breast Cancer Conference 2023, San Antonio, TX, December 5-9, 2023.
SUPPORT
Supported by the French Government under the Investment for the Future program managed by the National Research Agency (ANR), grant no ANR-10-COHO-0004; and Fondation ARC (Grand Prix Oberling-Haguenau Fondation ARC). J.L.S. is supported by the Swiss National Science Foundation (P500PM_214231).
AUTHOR CONTRIBUTIONS
Conception and design: José Luis Sandoval, Antonio di Meglio, Arlindo R. Ferreira, Alessandro Viansone, Anne-Laure Martin, Laurence Vanlemmens, Idris Guessous, Ines Vaz-Luis, Gwenn Menvielle
Administrative support: Anne-Laure Martin, Sibille Everhard, Idris Guessous
Provision of study materials or patients: Sibille Everhard, Christelle Jouannaud, Philippe Rouanet, Laurence Vanlemmens, Asma Dhaini-Merimeche, Paul Cottu, Christelle Levy
Collection and assembly of data: José Luis Sandoval, Anne-Laure Martin, Sibille Everhard, Christelle Jouannaud, Marion Fournier, Philippe Rouanet, Asma Dhaini-Merimeche, Paul Cottu, Christelle Levy, Ines Vaz-Luis
Data analysis and interpretation: José Luis Sandoval, Maria Alice Franzoi, Antonio di Meglio, Fabrice André, Baptiste Sauterey, Silvia Stringhini, Idris Guessous, Ines Vaz-Luis, Gwenn Menvielle
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Magnitude and Temporal Variations of Socioeconomic Inequalities in the Quality of Life After Early Breast Cancer: Results From the Multicentric French CANTO Cohort
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
José Luis Sandoval
Travel, Accommodations, Expenses: Pfizer
Maria Alice Franzoi
Speakers' Bureau: Novartis (Inst)
Research Funding: Resilience Care (Inst)
Antonio di Meglio
Expert Testimony: Kephren, Techspert.io
Arlindo R. Ferreira
Employment: Resilience Care
Honoraria: Roche, Novartis, Gilead Sciences, Merck Sharp & Dohme, Seagen
Travel, Accommodations, Expenses: Daiichi Sankyo Europe GmbH, Lilly
Alessandro Viansone
Honoraria: Seagen
Consulting or Advisory Role: Seagen
Speakers' Bureau: AstraZeneca/Daiichi Sankyo
Research Funding: Pfizer
Expert Testimony: Seagen
Travel, Accommodations, Expenses: Eisai Europe
Fabrice André
Consulting or Advisory Role: Guardant Health (Inst), AstraZeneca (Inst), Lilly, Daiichi Sankyo (Inst), Roche (Inst), Lilly (Inst), Pfizer (Inst), Owkin (Inst), Novartis (Inst), N-Power Medicine (Inst), Servier (Inst), Gilead Sciences (Inst), Boston Pharmaceuticals (Inst)
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Lilly (Inst), Roche (Inst), Daiichi (Inst), Owkin (Inst), Guardant Health (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Christelle Jouannaud
Honoraria: Pfizer, Daiichi Sankyo/Astra Zeneca, Gilead Sciences
Consulting or Advisory Role: Daiichi Sankyo/Astra Zeneca
Travel, Accommodations, Expenses: MSD Oncology, Viatris
Paul Cottu
Honoraria: Pfizer, Novartis (Inst), Roche, NanoString Technologies (Inst), Lilly, Daiichi Sankyo, AstraZeneca, Gilead Sciences
Consulting or Advisory Role: Pfizer, Lilly
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Lilly
Christelle Levy
Consulting or Advisory Role: Seagen, Gilead Sciences, Lilly
Travel, Accommodations, Expenses: Gilead Sciences, Pfizer
Ines Vaz-Luis
Honoraria: AstraZeneca (Inst), Novartis (Inst), Sandoz
Research Funding: Resilience Care (Inst)
Travel, Accommodations, Expenses: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Park JH, Anderson WF, Gail MH: Improvements in US breast cancer survival and proportion explained by tumor size and estrogen-receptor status. J Clin Oncol 33:2870-2876, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleyer A, Welch HG: Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 367:1998-2005, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Giordano SH, Buzdar AU, Smith TL, et al. : Is breast cancer survival improving? Trends in survival for patients with recurrent breast cancer diagnosed from 1974 through 2000. Cancer 100:44-52, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F, Kyriakides S, Ohno S, et al. : Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1674, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Moran MS, Abraham J, et al. : Breast cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20:691-722, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Weigelt B, Geyer FC, Reis-Filho JS: Histological types of breast cancer: How special are they? Mol Oncol 4:192-208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, et al. : Molecular portraits of human breast tumours. Nature 406:747-752, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Howlader N, Altekruse SF, Li CI, et al. : US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106:dju055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbeck N, Penault-Llorca F, Cortes J, et al. : Breast cancer. Nat Rev Dis Primers 5:66, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Shapiro CL, Recht A: Side effects of adjuvant treatment of breast cancer. N Engl J Med 344:1997-2008, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Franzoi MA, Agostinetto E, Perachino M, et al. : Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol 22:e303-e313, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Marmot M: Social determinants of health inequalities. Lancet 365:1099-1104, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Marmot M, Allen J, Bell R, et al. : WHO European review of social determinants of health and the health divide. Lancet 380:1011-1029, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Marmot M, Friel S, Bell R, et al. : Closing the gap in a generation: Health equity through action on the social determinants of health. Lancet 372:1661-1669, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Quinn MJ, Cooper N, Rachet B, et al. : Survival from cancer of the breast in women in England and Wales up to 2001. Br J Cancer 99:S53-S55, 2008. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orsini M, Tretarre B, Daures JP, et al. : Individual socioeconomic status and breast cancer diagnostic stages: A French case-control study. Eur J Public Health 26:445-450, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Lyratzopoulos G, Barbiere JM, Rachet B, et al. : Changes over time in socioeconomic inequalities in breast and rectal cancer survival in England and wales during a 32-year period (1973-2004): The potential role of health care. Ann Oncol 22:1661-1666, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Lyratzopoulos G, Abel GA, Brown CH, et al. : Socio-demographic inequalities in stage of cancer diagnosis: Evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann Oncol 24:843-850, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graells-Sans A, Serral G, Puigpinos-Riera R, Grupo Cohort DAMA : Social inequalities in quality of life in a cohort of women diagnosed with breast cancer in Barcelona (DAMA Cohort). Cancer Epidemiol 54:38-47, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Molero J, Dierssen-Sotos T, Gomez-Acebo I, et al. : Quality of life in a cohort of 1078 women diagnosed with breast cancer in Spain: 7-year follow-up results in the MCC-Spain study. Int J Environ Res Public Health 17:8411, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn SH, Park BW, Noh DY, et al. : Health-related quality of life in disease-free survivors of breast cancer with the general population. Ann Oncol 18:173-182, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Vaz-Luis I, Cottu P, Mesleard C, et al. : Unicancer: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO). ESMO Open 4:e000562, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loibl S, Andre F, Bachelot T, et al. : Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 35:159-182, 2024 [DOI] [PubMed] [Google Scholar]
- 24.Fayers P, Bottomley A: Quality of life research within the EORTC—The EORTC QLQ-C30. Eur J Cancer 38:125-133, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Scott NW, Fayers P, Aaronson NK, et al. : EORTC QLQ-C30 Reference Values Manual. 2008 [Google Scholar]
- 26.Sprangers MA, Groenvold M, Arraras JI, et al. : The European Organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol 14:2756-2768, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Fayers P, Aaronson NK, Bjordal K, et al. : EORTC QLQ–C30 Scoring Manual: European Organisation for Research and Treatment of Cancer. 1995 [Google Scholar]
- 28.OECD : What are Equivalence Scales? 2009. https://www.oecd.org/els/soc/OECD-Note-EquivalenceScales.pdf [Google Scholar]
- 29.Le Gall G, Noal S, Heutte N, et al. : Impact of adjuvant trastuzumab treatment on fatigue, emotional status and quality of personal and work life of patients with localised breast cancer: Results of the 'HER-ception' study. Support Care Cancer 31:38, 2022 [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, Negassa A, Edwardes MD, et al. : Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol 157:364-375, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Mackenbach JP, Kunst AE: Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Soc Sci Med 44:757-771, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Donaldson GW: Ridit scores for analysis and interpretation of ordinal pain data. Eur J Pain 2:221-227, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Bross ID: How to use ridit analysis. Biometrics 14:18-38, 1958 [Google Scholar]
- 34.Ferreira AR, Di Meglio A, Pistilli B, et al. : Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann Oncol 30:1784-1795, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Schneider P, Love-Koh J, McNamara S, et al. : Socioeconomic inequalities in HRQoL in England: An age-sex stratified analysis. Health Qual Life Outcomes 20:121, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musoro JZ, Coens C, Sprangers MAG, et al. : Minimally important differences for interpreting EORTC QLQ-C30 change scores over time: A synthesis across 21 clinical trials involving nine different cancer types. Eur J Cancer 188:171-182, 2023 [DOI] [PubMed] [Google Scholar]
- 37.Musoro JZ, Coens C, Fiteni F, et al. : Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr 3:pkz037, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivier T, Smith CEP, Haslam A, et al. : Quality of life in the adjuvant setting: A meta-analysis of us Food and Drug Administration approved anti-cancer drugs from 2018 to 2022. J Cancer Policy 37:100426, 2023 [DOI] [PubMed] [Google Scholar]
- 39.Chevreul K, Berg Brigham K, Durand-Zaleski I, et al. : France: Health system review. Health Syst Transit 17:1, 2015 [PubMed] [Google Scholar]
- 40.Mackenbach JP: The persistence of health inequalities in modern welfare states: The explanation of a paradox. Soc Sci Med 75:761-769, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Franken M, Koolman X: Health system goals: A discrete choice experiment to obtain societal valuations. Health Policy 112:28-34, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Marmot M: The health gap: Doctors and the social determinants of health. Scand J Public Health 45:686-693, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Dumas A, Vaz Luis I, Bovagnet T, et al. : Impact of breast cancer treatment on employment: Results of a multicenter prospective cohort study (CANTO). J Clin Oncol 38:734-743, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray CJ: Quantifying the burden of disease: The technical basis for disability-adjusted life years. Bull World Health Organ 72:429-445, 1994 [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein MC, Torrance G, McGuire A: QALYs: The basics. Value Health 12:S5-S9, 2009. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 46.Mackenbach JP, Meerding WJ, Kunst AE: Economic costs of health inequalities in the European Union. J Epidemiol Community Health 65:412-419, 2011 [DOI] [PubMed] [Google Scholar]
- 47.INSEE : Immigrés et descendants d'immigrés. 2023 [Google Scholar]


