Abstract
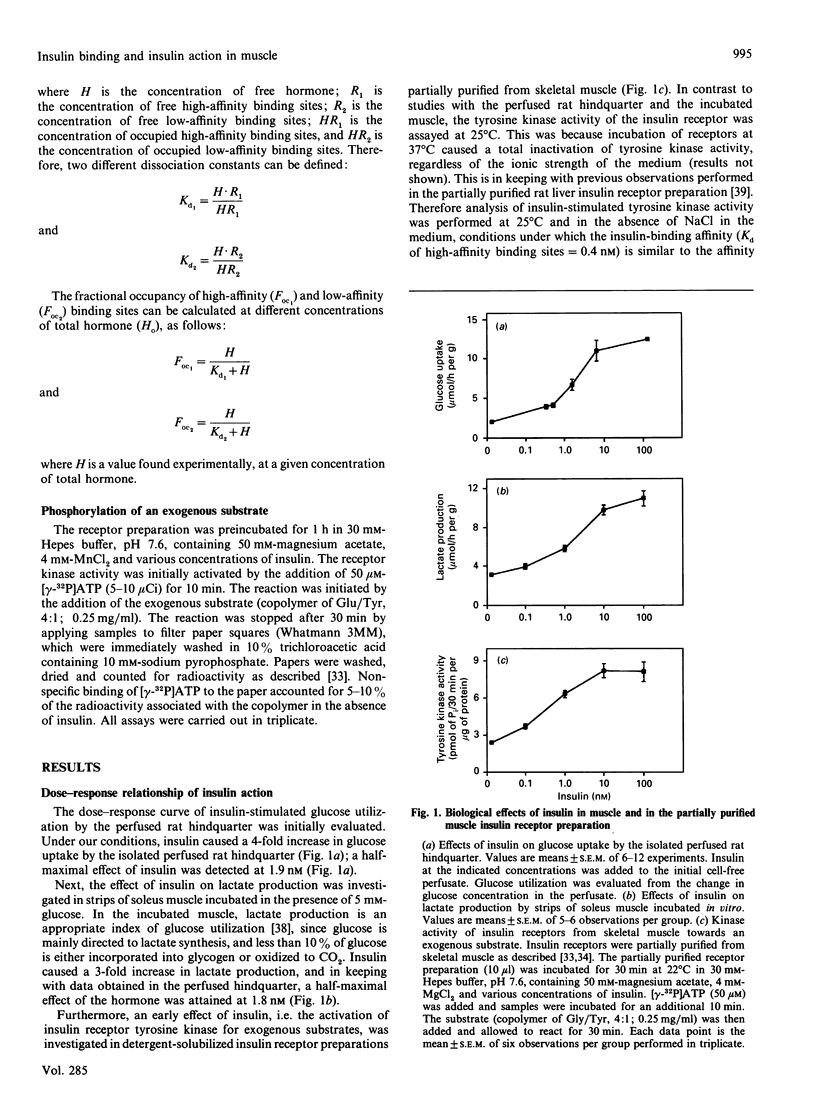
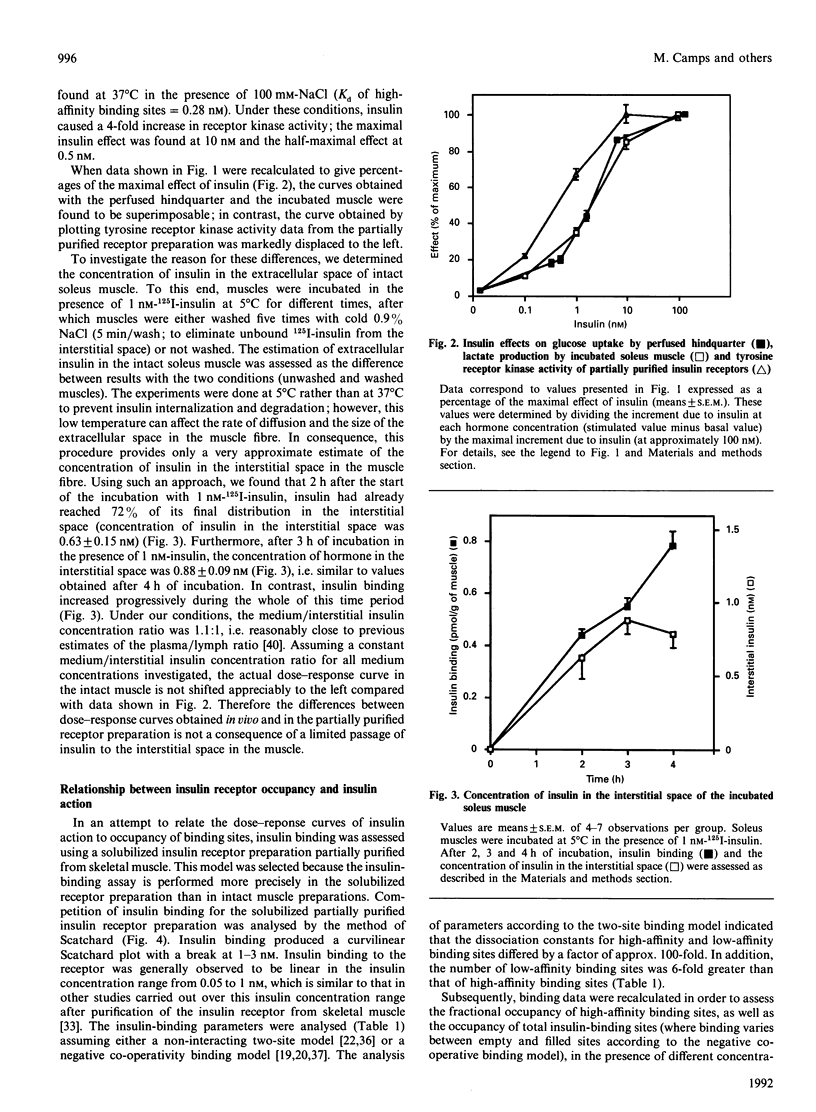
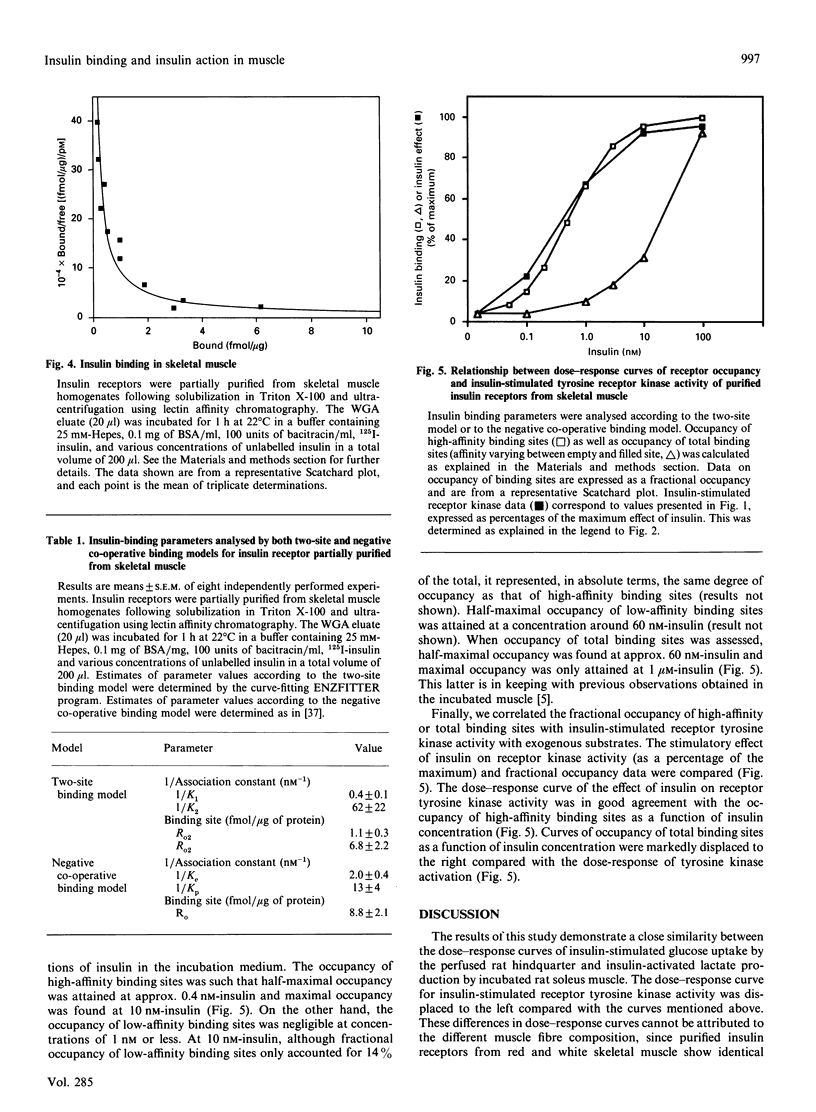
In this study, the relationship between the concentration of extracellular insulin, insulin binding and insulin action was evaluated in skeletal muscle. Initially we investigated the dose-response relationship of insulin action using three different experimental models that are responsive to insulin, i.e. the isolated perfused rat hindquarter, incubated strips of soleus muscle, and insulin receptors partially affinity-purified from skeletal muscle. We selected as insulin-sensitive parameters glucose uptake in the perfused hindquarter, lactate production in the incubated muscle preparation, and tyrosine receptor kinase activity in the purified receptor preparation. Our results showed that the dose-response curves obtained in the perfused hindquarter and in the incubated muscle were superimposable. In contrast, the dose-response curve for insulin-stimulated receptor tyrosine kinase activity in partially purified receptors was displaced to the left compared with the curves obtained in the perfused hindquarter and in the incubated muscle. The differences between the dose-response curve for receptor tyrosine kinase and those for glucose uptake and lactate production were not explained by a substantial insulin concentration gradient between medium and interstitial space. Thus the medium/interstitial insulin concentration ratio, when assayed in the incubated intact muscle at 5 degrees C, was close to 1. We also compared the dose-response curve of insulin-stimulated receptor tyrosine kinase with the pattern of insulin-binding-site occupancy. The curve of insulin-stimulated receptor kinase activity fitted closely with the occupancy of high-affinity binding sites. In summary, assuming that the estimation of the medium/interstitial insulin concentration ratio obtained at 5 degrees C reflects the actual ratio under more physiological conditions, our results suggest that maximal insulin action is obtained in skeletal muscle at insulin concentrations which do allow full occupancy of high-affinity binding sites. Therefore our data provide evidence for a lack of spare high-affinity insulin receptors in skeletal muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenis G., Livingston J. N. Alterations in the tyrosine kinase activity of the insulin receptor produced by in vitro hyperinsulinemia. J Biol Chem. 1986 Jan 5;261(1):147–153. [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Kemmer F. W., Becker K., Herberg L., Schwenen M., Gjinavci A., Berchtold P. Effect of physical training on glucose tolerance and on glucose metabolism of skeletal muscle in anaesthetized normal rats. Diabetologia. 1979 Mar;16(3):179–184. doi: 10.1007/BF01219795. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryer-Ash M. Rat insulin-receptor kinase activity correlates with in vivo insulin action. Diabetes. 1989 Jan;38(1):108–116. doi: 10.2337/diab.38.1.108. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Lemmon S. K., Treutelaar M. K., Buse M. G. Insulin resistance of denervated rat muscle: a model for impaired receptor-function coupling. Am J Physiol. 1984 Nov;247(5 Pt 1):E657–E666. doi: 10.1152/ajpendo.1984.247.5.E657. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Prentki M., Zaninetti D., Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J. 1980 Feb 15;186(2):525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Binding of insulin and other hormones to non-receptor materials: saturability, specificity and apparent "negative cooperativity". Biochem Biophys Res Commun. 1975 Jan 6;62(1):31–41. doi: 10.1016/s0006-291x(75)80401-3. [DOI] [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Espinal J., Dohm G. L., Newsholme E. A. Sensitivity to insulin of glycolysis and glycogen synthesis of isolated soleus-muscle strips from sedentary, exercised and exercise-trained rats. Biochem J. 1983 May 15;212(2):453–458. doi: 10.1042/bj2120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Suter S. L., Henry R. R., Reichart D., Olefsky J. M. In vivo stimulation of the insulin receptor kinase in human skeletal muscle. Correlation with insulin-stimulated glucose disposal during euglycemic clamp studies. J Clin Invest. 1991 Jun;87(6):2222–2229. doi: 10.1172/JCI115257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Green A., Newsholme E. A. Sensitivity of glucose uptake and lipolysis of white adipocytes of the rat to insulin and effects of some metabolites. Biochem J. 1979 May 15;180(2):365–370. doi: 10.1042/bj1800365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C., Tepperman H. M., Holohan P., Tepperman J. Insulin binding and insulin response of adipocytes from rats adapted to fat feeding. J Lipid Res. 1976 Nov;17(6):588–599. [PubMed] [Google Scholar]
- James D. E., Jenkins A. B., Kraegen E. W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985 May;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- James D. E., Kraegen E. W., Chisholm D. J. Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest. 1985 Aug;76(2):657–666. doi: 10.1172/JCI112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Zorzano A., Böni-Schnetzler M., Nemenoff R. A., Powers A., Pilch P. F., Ruderman N. B. Intrinsic differences of insulin receptor kinase activity in red and white muscle. J Biol Chem. 1986 Nov 15;261(32):14939–14944. [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Role of insulin receptors in insulin-resistant states. Metabolism. 1980 May;29(5):455–466. doi: 10.1016/0026-0495(80)90171-7. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Klein H. H., Matthaei S., Drenkhan M., Ries W., Scriba P. C. The relationship between insulin binding, insulin activation of insulin-receptor tyrosine kinase, and insulin stimulation of glucose uptake in isolated rat adipocytes. Effects of isoprenaline. Biochem J. 1991 Mar 15;274(Pt 3):787–792. doi: 10.1042/bj2740787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp R. H., Herrera E., Freinkel N. Carbohydrate metabolism in pregnancy. 8. Metabolism of adipose tissue isolated from fed and fasted pregnant rats during late gestation. J Clin Invest. 1970 Jul;49(7):1438–1446. doi: 10.1172/JCI106361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Kraegen E. W., James D. E., Jenkins A. B., Chisholm D. J. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985 Mar;248(3 Pt 1):E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Insulin binding to solubilized material from fat cell membranes: evidence for two binding species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2593–2597. doi: 10.1073/pnas.75.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Effect of fasting and streptozotocin diabetes on insulin binding and action in the isolated mouse soleus muscle. J Clin Invest. 1979 Nov;64(5):1505–1515. doi: 10.1172/JCI109609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Leturque A., Ferre P., Burnol A. F., Kande J., Maulard P., Girard J. Glucose utilization rates and insulin sensitivity in vivo in tissues of virgin and pregnant rats. Diabetes. 1986 Feb;35(2):172–177. doi: 10.2337/diab.35.2.172. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Shafer J. A. Evidence that insulin receptor from human placenta has a high affinity for only one molecule of insulin. J Biol Chem. 1984 Jul 10;259(13):8589–8596. [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Hormone-receptor interactions are noncooperative: application to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4340–4344. doi: 10.1073/pnas.77.7.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Kemmer F. W., Goodman M. N., Berger M. Oxygen consumption in perfused skeletal muscle. Effect of perfusion with aged, fresh and aged-rejuvenated erythrocytes on oxygen consumption, tissue metabolites and inhibition of glucose utilization by acetoacetate. Biochem J. 1980 Jul 15;190(1):57–64. doi: 10.1042/bj1900057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushakoff R. J., Kalkhoff R. K. Effects of pregnancy and sex steroid administration on skeletal muscle metabolism in the rat. Diabetes. 1981 Jul;30(7):545–550. doi: 10.2337/diab.30.7.545. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Lawrence J. C., Jr Insulin action in denervated rat hemidiaphragms. Decreased hormonal stimulation of glycogen synthesis involves both glycogen synthase and glucose transport. J Biol Chem. 1984 Feb 25;259(4):2201–2207. [PubMed] [Google Scholar]
- Smith R. L., Lawrence J. C., Jr Insulin action in denervated skeletal muscle. Evidence that the reduced stimulation of glycogen synthesis does not involve decreased insulin binding. J Biol Chem. 1985 Jan 10;260(1):273–278. [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Pessin J. E. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987 May 25;262(15):6939–6942. [PubMed] [Google Scholar]
- Whitson R. H., Grimditch G. K., Sternlicht E., Kaplan S. A., Barnard R. J., Itakura K. Characterization of rat skeletal muscle sarcolemmal insulin receptors and a sarcolemmal insulin binding inhibitor. J Biol Chem. 1988 Apr 5;263(10):4789–4794. [PubMed] [Google Scholar]
- Yang Y. J., Hope I. D., Ader M., Bergman R. N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989 Nov;84(5):1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Whittaker J., Roth J. Insulin stimulated phosphorylation of its own receptor. Activation of a tyrosine-specific protein kinase that is tightly associated with the receptor. J Biol Chem. 1983 Mar 25;258(6):3431–3434. [PubMed] [Google Scholar]
- Zorzano A., Balon T. W., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol. 1985 May;248(5 Pt 1):E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]
- Zorzano A., James D. E., Ruderman N. B., Pilch P. F. Insulin-like growth factor I binding and receptor kinase in red and white muscle. FEBS Lett. 1988 Jul 18;234(2):257–262. doi: 10.1016/0014-5793(88)80093-0. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


