Abstract
Oncolytic adenoviruses have emerged as a promising therapeutic approach for cancer therapy. However, systemic delivery of the viruses to metastatic tumors remains a major challenge. Mesenchymal stem cells (MSCs) possess tumor tropism property and can be used as cellular vehicles for delivering oncolytic adenoviruses to tumor sites. Since telomerase activity is found in ~90% of human carcinomas, but undetected in normal adult cells, the human telomerase reverse transcriptase gene (TERT) promoter can be exploited for regulating the replication of oncolytic adenoviruses. Here, we evaluated the antitumor effects of syngeneic murine MSCs loaded with the luciferase-expressing, telomerase-dependent oncolytic adenovirus Ad.GS2 (MSC-Ad.GS2) and Ad.GS2 alone on metastatic MBT-2 bladder tumors. MSCs supported a low degree of Ad.GS2 replication, which could be augmented by coculture with MBT-2 cells or tumor-conditioned medium (TCM), suggesting that viral replication is increased when MSC-Ad.GS2 migrates to tumor sites. MBT-2 cells and TCM enhanced viral replication in Ad.GS2-infected MSCs. SDF-1 is a stem cell homing factor. Our results suggest that the SDF-1/STAT3/TERT signaling axis in MSCs in response to the tumor microenvironment may contribute to the enhanced replication of Ad.GS2 carried by MSCs. Notably, we demonstrate the potent therapeutic efficacy of systemically delivered MSC-Ad.GS2 in pleural disseminated tumor and experimental metastasis models using intrapleural and tail vein injection of MBT-2 cells, respectively. Treatment with MSC-Ad.GS2 significantly reduced tumor growth and prolonged the survival of mice bearing metastatic bladder tumors. Since telomerase is expressed in a broad spectrum of cancers, this therapeutic strategy may be broadly applicable.
Keywords: oncolytic adenovirus, mesenchymal stem cell, telomerase, TERT, tumor microenvironment, metastasis, bladder cancer, SDF-1
Graphical Abstract
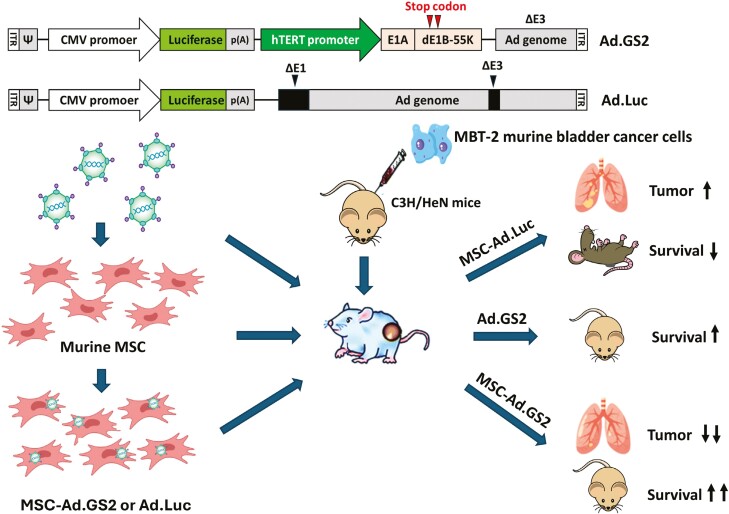
Graphical Abstract.
Significance Statement.
Given the tumor-tropic property of mesenchymal stem cells (MSCs) and the conditionally replicative property of oncolytic adenoviruses, we investigated the therapeutic efficacy of systemic delivery of murine MSCs loaded with the telomerase-dependent oncolytic adenovirus Ad.GS2 in pleural disseminated tumor and experimental metastasis models using syngeneic MBT-2 bladder tumors. MSCs exposed to MBT-2 cells or tumor-conditioned medium (TCM) increased SDF-1 secretion and STAT3 activation to upregulate TERT promoter activity. Treatment with MSC-Ad.GS2 significantly reduced tumor growth and prolonged the survival of mice bearing metastatic bladder tumors.
Introduction
Oncolytic adenoviruses, such as E1B-55 kD-deleted adenoviruses, which selectively replicate in and lyse tumor cells without affecting normal cells, have emerged as a promising therapeutic approach for cancer therapy.1 To further improve the specificity of viral replication in tumor cells while sparing normal cells, tumor-specific promoters can be exploited to control oncolytic viral replication. We have previously constructed several E1B-55 kD-deleted adenoviruses in which E1A gene expression, and therefore viral replication, is under the control of various tumor-specific promoters or responsive elements. These oncolytic adenoviruses exhibit more potent antitumor efficacy than the one with the E1A gene driven by its own promoter in murine tumor models.2-6 Telomerase activity is found in ~90% of human carcinomas and ~99% of urothelial carcinomas of the bladder (USB), but undetected in normal adult cells.7 Mutations of the human telomerase reverse transcriptase gene (TERT) cause telomerase reactivation in 60%-80% of USB.8 The human TERT (hTERT) promoter has been exploited in cancer gene therapy.9,10 Because the phenotype of rheumatoid synovium is similar to that of an aggressive tumor, such as expression of telomerase and mutant p53, we have demonstrated the therapeutic effect of an hTERT promoter-driven oncolytic adenovirus, designated Ad.GS1, in rats with collagen-induced arthritis that resembles human rheumatoid arthritis.11
Mesenchymal stem cells (MSCs) are nonhematopoietic adult stem cells with multilineage potential.12 They can be isolated from bone marrow and other tissues and have the ability to differentiate into osteoblasts, adipocytes, chondrocytes, and other lineages.13 The circulating MSCs can migrate to and repair wounded tissues in response to tissue stress or damage.14 The tumor microenvironment resembles injured tissues and is a preferred site for MSC homing.15,16 The tumor tropism of MSCs encourages investigators to explore the use of MSCs as a tumor-targeting platform.17,18 MSCs have been exploited to deliver various therapeutic interventions, such as cytokines, apoptosis-inducing agents, cytotoxic drugs, and oncolytic viruses, to tumor sites for cancer therapy.17,18 MSCs have unique characteristics of tumor tropism, self-renewing/proliferative capability, and low immunogenicity, making them the most attractive vehicle for systemic delivery of oncolytic viruses for cancer therapy.19,20 Moreover, MSC-based delivery of oncolytic adenoviruses has been evaluated in clinical trials for treating several malignancies, including metastatic tumors.19,21,22
In the present study, by taking advantages of the tumor-homing capability of MSCs and the high telomerase activity of bladder cancer cells, we tested whether syngeneic murine MSCs loaded with the luciferase-expressing, telomerase-dependent oncolytic adenovirus Ad.GS2 (designated MSC-Ad.GS2) was more efficacious than Ad.GS2 alone in treating metastatic tumors in the syngeneic MBT-2 murine bladder tumor model. Our results indicate that MSCs carrying telomerase-dependent oncolytic adenoviruses have therapeutic potential for treating metastatic tumors. Since telomerase is expressed in a broad spectrum of cancer cells but not in normal adult cells, this therapeutic strategy may be broadly applicable.
Materials and methods
MSCs, cell lines, and mice
Primary MSCs were obtained by flushing the bone marrow from femurs and tibias of 6- to 8-week-old female C3H/HeN mice.23 They were cultured in MesenCult MSC Basal Medium (Mouse; STEMCELL Technologies, Vancouver, Canada) supplemented with Mesenchymal Stem Cell Stimulatory Supplements (Mouse; STEMCELL Technologies) in a humidified incubator at 37°C with 5% CO2. After 72 h, nonadherent hematopoietic cells were washed out, and adherent MSCs were further cultured and fed every 3 days by replacing the old medium with fresh medium. When MSCs were grown to confluence, their phenotypes were analyzed with cell differentiation and surface markers. Murine MBT-2 bladder cancer cells derived from a carcinogen-induced bladder tumor in C3H mice, NMuMG normal murine mammary gland epithelial cells, and human 293 embryonic kidney cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM l-glutamine, and 50 μg/mL gentamicin. NMuMG cells were additionally supplemented with 10 μg/mL insulin. Female C3H/HeN mice at 6-8 weeks of age were purchased from the National Cheng Kung University (NCKU) Laboratory Animal Center or BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). All animal work was conducted in animal biosafety level 2 facilities at NCKU. The experimental protocols adhered to the rules of the Animal Protection Act of Taiwan and were approved by the Animal Care and Use Committee of NCKU (IACUC number: 106170).
Analysis of the TERT promoter activity
MSCs grown in 24-well plates were cotransfected with 1 μg of pGL3-hTERT,9 a luciferase reporter plasmid driven by the hTERT promoter, and 0.5 μg of pTRELacZ, a β-galactosidase reporter vector derived from pTREd2EGFP (Clontech, Palo Alto, CA), with the Neon Transfection System (Invitrogen, Carlsbad, CA). Subsequently, MSCs were incubated with 100 ng/mL recombinant stroma-derived factor-1 (SDF-1; Peprotech, Rocky Hill, NJ), tumor-conditioned medium (TCM) collected from MBT-2 cells cultured for 3 days, TCM in the presence of 3.5 μg/mL anti-CXCL12/SDF-1 antibody (ARG56613, Arigo Biolaboratories, Hsinchu, Taiwan), MSC medium containing 2% FBS, or MBT-2 cells at 24 h post-transfection. Cell lysates were harvested at 48 h post-transfection, and their relative luciferase activity was measured by a dual-light luciferase and β-galactosidase reporter gene assay system (Tropix, Bedford, MA) and divided by the β-galactosidase activity to normalize transfection efficiency.24
Production of recombinant adenoviruses
Ad5WS1, an E1B-55 kD-deleted, human adenovirus type 5 (Ad5)-based oncolytic adenovirus driven by adenoviral E1 promoter, has been described.24,25 Ad.GS2 is an E1B-55 kD-deleted adenovirus, in which the E1A gene is driven by the hTERT promoter. Additionally, Ad.GS2 carries firefly luciferase gene under the control of the cytomegalovirus (CMV) promoter, allowing viral replication and localization to be monitored. Construction of Ad.GS2 has been previously described.24,25 In addition to the two replication-competent adenoviruses, an E1-deleted replication-defective adenovirus expressing luciferase, designated Ad.Luc, was also used as the negative control virus.26 All recombinant adenoviruses were propagated in 293 cells and quantified by the plaque assay.2
Chemotaxis assay, immunoblot analysis, and ELISA
In vitro migration of MSCs toward TCM was assessed using a 48-well chemotaxis chamber (Neuro Probe, Cabin John, MD).3 MBT-2 and NMuMG cells, as well as MSCs were cultured in the MSC medium containing 2% FBS and plated separately (5 × 104 cells/well) in the upper chambers (6.5-mm diameter, 0.4-μm pore size; Corning Costar, Cambridge, MA), whereas MSCs (1 × 105 cells/well) were plated in the lower chambers. After 3 days, the supernatant (0.4 mL) in lower chambers was collected for detecting SDF-1 secretion by immunoblotting with polyclonal anti-SDF-1 antibody (1:5000, ARG56613, Arigo) and by ELISA (R&D, Minneapolis, MN). Total cell lysates in lower chambers were harvested for detecting STAT3 and phospho-STAT3 by immunoblotting. Immunoblot analysis was performed using standard methods.27 The primary antibodies include monoclonal anti-STAT3 antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-phospho-STAT3 antibody (1:2000, GeneTex, Irvine, CA), and monoclonal anti-β-actin peroxidase antibody (1:10000, Sigma-Aldrich, St. Louis, MO). Horseradish peroxidase (HRP)-conjugated secondary antibody (1:4000, Jackson, West Grove, PA) was used where appropriate.
Analysis of replication-dependent luciferase expression in MSCs infected with Ad.GS2
MSCs were infected with Ad.GS2 or transduced with Ad.Luc at various multiplicities of infection (MOI). Luciferase activities were determined after 48 h. Whether MBT-2 cells, TCM, and SDF-1 could promote Ad.GS2 replication was tested next by assessing replication-dependent luciferase expression in MSCs. MSCs (3 × 105) were infected with Ad.GS2 at an MOI of 250 on day 0. Subsequently, MSC-Ad.GS2 was cocultured with MBT-2 cells (1 × 105), treated with TCM with or without anti-SDF-1 antibody (3.5 μg/mL), or incubated with various concentrations of recombinant SDF-1 on day 1. After 48 h, their luciferase activities were measured and normalized with the total protein concentration of cell lysates.
Detection of adenoviral capsid proteins in MSCs infected with Ad.GS2
Expression of viral hexon proteins indicative of productive viral replication in Ad.GS2-infected MSCs was detected by immunoblotting and immunohistochemistry. Cell lysates harvested from Ad.GS2-infected MSCs cocultured with MBT-2 cells or treated with TCM on days 3 and 5 were subjected to immunoblotting with monoclonal anti-adenovirus hexon protein antibody (1:3000, Abcam, Cambridge, UK) and monoclonal anti-β-actin peroxidase antibody (1:10000, Sigma-Aldrich). Furthermore, the cells were fixed with 4% formaldehyde and permeabilized by 0.1% Triton X-100. Then, virus-infected cells were detected by polyclonal anti-Ad5 antibody (1:200, Abcam). After sequential incubation with HRP-conjugated secondary antibody at room temperature and AEC as the chromogen substrate, the cells were subsequently stained with hematoxylin. Capsid-expressing cells were quantified using the ImageJ software (U.S. National Institute of Health).
Assay of cytolytic effects of MSC-Ad.GS2 on MBT-2 cells
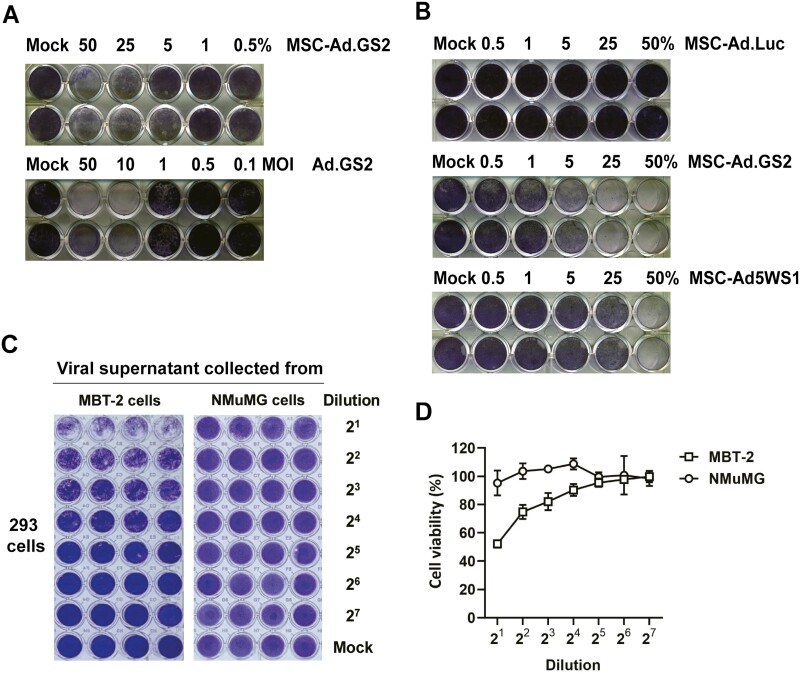
MSCs were infected with Ad.GS2, Ad5WS1, or Ad.Luc at an MOI of 100. After 24 h, MSCs loaded with different adenoviral vectors were mixed with MBT-2 cells in different ratios. MBT-2 cells were also directly infected with Ad.GS2 at different MOI. After 7 days, MBT-2 cells were examined for cytopathic effect (CPE) by crystal violet staining.
Assessment of Ad.GS2 replication in MBT-2 and MSCs by detecting infectious viruses
MBT-2 cells (2 × 105) were infected with Ad.GS2 at an MOI of 200 on day 0. Normal NMuMG cells, which are non-permissive for oncolytic adenoviral replication,2 were also tested in parallel as the negative control. Culture supernatants and freeze-thaw cell lysates from Ad.GS2-infected cells were collected and pooled (1 mL) to serve as crude viral supernatants on day 5. Then, 150 μL of serially diluted viral supernatants (600 μL virus supernatant plus 600 μL culture medium containing 2% FBS as 2 × dilution) were used to infect 3 × 104 293 cells (a human embryonic kidney cell line which complements adenoviral E1A and E1B functions) cultured in 96-well plates for detecting infectious viruses. After 6 days, CPE was observed by crystal violet staining. The wells were scanned, and crystal violet staining was quantified to determine cell survival using the ImageJ software. Values are shown as the percentages of cell survival, with the levels in the mock-infected cells arbitrarily set to 100. For assessing Ad.GS2 replication in murine MSCs, 2 × 105 MSCs were infected with Ad.GS2 at an MOI of 250 on day 0 and then treated with or without 0.4 mL TCM or incubated with 1 × 105 MBT-2 cells on day 1. The TCM (10 mL) was collected from 4 × 106 MBT-2 cells that had been cultured for 3 days. Culture supernatants and freeze-thaw cell lysates were collected and pooled (400 μL) on day 3. Then, 50 μL viral supernatants were used to infect 293 cells (3 × 104). After 6 days, cells were photographed to observe virus-induced CPE. Direct infection of Ad.GS2 to 293 cells served as the positive control for detecting virus-induced CPE.
In vivo bioluminescence imaging
To facilitate monitoring of tumor-targeting capability of MSC-Ad.GS2 and Ad.GS2 by bioluminescence imaging, we established disseminated peritoneal tumors by intraperitoneal inoculation of MBT-2 cells (1.2 × 106) into C3H/HeN mice on day 0. On day 11, the tumor-bearing mice were injected intraperitoneally with either 2 × 106 MSC-Ad.GS2 or 5 × 108 plaque-forming units (PFU) of Ad.GS2. On days 12 and 14, mice were anesthetized with 5% isoflurane (IsoSol; Medeva Pharmaceuticals, Rochester, NY), followed by intraperitoneal injection with d-luciferin (125 mg/kg; Synchem, Felsberg, Germany). The luminescence intensity in the intraperitoneal tumors was visualized with the Xenogen IVIS spectrum noninvasive quantitative molecular imaging system (Xenogen, Alameda, CA). Luminescent activities in photons per second for selected and defined areas were quantified. The value for the luminescence measured in each group on day 12 was arbitrarily set to 1. The luminescence value determined on day 14 was expressed relative to this baseline.
Animal studies
For monitoring pleural disseminated tumors, 1 × 105 MBT-2 cells in 100 μL PBS were intrapleurally inoculated into the thoracic cavity of C3H/HeN mice on day 0. On day 4, groups of the mice were injected with 1 × 105 MSC-Ad.GS2 or MSC-Ad.Luc, or saline via the tail vein. All mice were sacrificed, and their lungs and tumors around the thoracic cavity were excised and weighed on day 10. Tissue sections of the mouse lungs were prepared, formalin-fixed, and paraffin-embedded for hematoxylin and eosin (H&E) staining.2 The numbers and sizes of pleural disseminated tumor nodules were assessed in H&E-stained sections. In another set of the experiment for survival analysis, mice were injected with 1 × 105 MBT-2 via the tail vein to develop experimental lung metastasis on day 0, followed by injection with 2 × 106 MSC-Ad.GS2 or MSC-Ad.Luc (MOI = 500), or direct injection with Ad.GS2 (1 × 109 PFU) or saline via the tail vein on day 7. All mice were monitored for survival until death.
Statistical analysis
Data are expressed as the mean ± SD. The Kaplan-Meier survival curve and the log-rank test were used to analyze mouse survival. Statistical differences were compared by Student’s t test between two groups and by one-way ANOVA with Tukey’s multiple comparisons test among three or more groups. Any P value less than .05 was considered statistically significant. Statistical tests were performed using GraphPad Prism (version 8.0, GraphPad software, San Diego, CA).
Results
MSCs exposed to MBT-2 cells or TCM increase SDF-1 secretion and STAT3 activation to upregulate TERT promoter activity
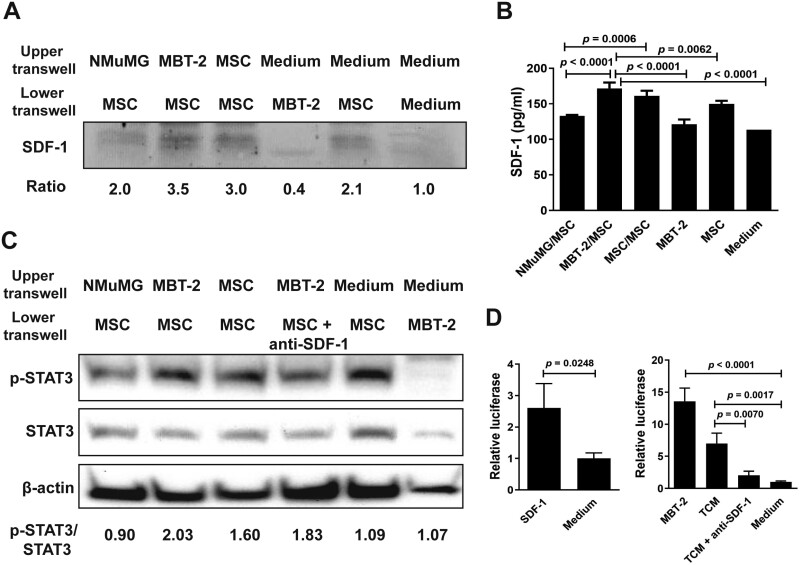
A previous study has identified a set of genes, including SDF-1, which are upregulated in rat MSCs cocultured with the TCM of human C85 colorectal cancer cells.28 Furthermore, knockdown of SDF-1 expression in MSCs inhibits their migration to the TCM. To mimic a clinical scenario, we tested whether SDF-1 expression was enhanced in murine MSCs exposed to syngeneic bladder tumor cells. First, we isolated bone marrow MSCs from C3H/HeN mice. Murine MSCs were induced with osteoinductive medium and adipogenic differentiation medium to differentiate into osteoblast and adipocyte lineages, respectively. The differentiation potential of MSCs to osteoblasts and adipocytes was validated by alkaline phosphatase activity and lipid vacuole formation detected by alkaline phosphatase and oil red-O staining, respectively (Supplementary Figure S1A). Furthermore, murine MSCs obtained from C3H/HeN mice expressed high levels of Sca-1 and CD44, which are surface markers for MSCs, as well as H-2DK antigen, as examined by flow cytometric analysis (Supplementary Figure S1B). These results confirm that the isolated cells from the bone marrow of C3H/HeN mice were indeed MSCs exhibiting progenitor stem cell properties. Next, MSCs were cocultured with MBT-2 or NMuMG cells, or MSCs. Figure 1A shows that MSCs cocultured with MBT-2 cells exhibited higher secretion of SDF-1 compared to those cocultured with NMuMG cells or MSCs. Quantitation of SDF-1 in the supernatant by ELISA also revealed increased SDF-1 production in MSCs cocultured with MBT-2 cells compared to those cocultured with NMuMG cells or MSCs (Figure 1B). Given that SDF-1 secretion activates Janus kinase 2 (JAK2)/STAT3 to promote MSC migration to the tumor microenvironment,29 we investigated the downstream signaling triggered by secreted SDF-1 in murine MSCs. As shown in Figure 1C, phosphorylation of STAT3 (phospho-STAT3) at tyrosine residue 705 indicative of STAT3 activation was increased in MSCs cocultured with MBT-2 cells. By contrast, levels of phospho-STAT3 remained similar in MSCs cocultured with NMuMG cells when compared with MSCs cultured alone. In addition, levels of phospho-STAT3 were decreased when anti-SDF-1 neutralizing antibody was present in the coculture of MSCs/MBT-2. These results suggest that SDF-1 may positively regulate STAT3 phosphorylation. Since phospho-STAT3 binds to the hTERT promoter region and upregulates hTERT expression in both human cancer and normal somatic cells,30 we investigated whether SDF-1-mediated STAT3 phosphorylation could activate the TERT promoter activity of MSCs. MSCs cocultured with MBT-2 cells and TCM had 13.5- and 7-fold upregulation of the promoter activity, respectively, as compared to MSCs cultured in the medium (Figure 1D, right panel). Notably, the addition of anti-SDF-1 antibody abrogated TCM-mediated increases in the TERT promoter activity of MSCs. By contrast, MSCs treated with recombinant SDF-1 protein (100 ng/mL) upregulated the TERT promoter activity (Figure 1D, left panel). Collectively, these results indicate that TERT promoter activity is significantly upregulated via the SDF-1 signaling when MSCs are exposed to MBT-2 cells or TCM.
Figure 1.
MSCs exposed to MBT-2 cells or TCM increase SDF-1 secretion, STAT3 activation, and TERT promoter activity. (A-C) Detection of SDF-1, STAT3, and phospho-STAT3 (p-STAT3) in MSCs exposed to MBT-2 cells. NMuMG cells, MBT-2 cells, MSCs, and culture medium were separately added to the upper chamber of the 48-well transwell, whereas MSCs, MBT-2 cells, MSCs plus anti-SDF-1 antibody, and culture medium were separately added to the lower chamber. After culture for 72 h, cells and culture medium in the lower chamber were collected. Levels of SDF-1, p-STAT3, and STAT3 in the cell lysate were detected by immunoblot analysis (A, C). Ratios shown below the blot are the fold increase in the ratio in each lane relative to the ratio obtained from the medium-only control lane analyzed by densitometry (A). SDF-1 content in the culture medium was quantified by ELISA (B). Values shown below the blots are ratios between the intensity of the bands corresponding to p-STAT3 and those corresponding to STAT3 analyzed by densitometry (C). Expression of β-actin served as the loading control. (D) Determination of the TERT promoter activity in MSCs exposed to MBT-2 cells, TCM, or SDF-1. MSCs that had been cotransfected with the luciferase reporter plasmid pGL3-hTERT and pTRELacZ were cocultured with MBT-2 cells, TCM, recombinant SDF-1 protein, TCM plus anti-SDF-1 antibody, or culture medium. Relative luciferase activity was measured by a dual-light luciferase and β-galactosidase reporter gene assay system (Tropix, Bedford, MA) and divided by the β-galactosidase activity to normalize transfection efficiency. (B, D) Values shown are means ± SD (n = 3).
Replication-dependent luciferase expression occurs in Ad.GS2-infected MSCs
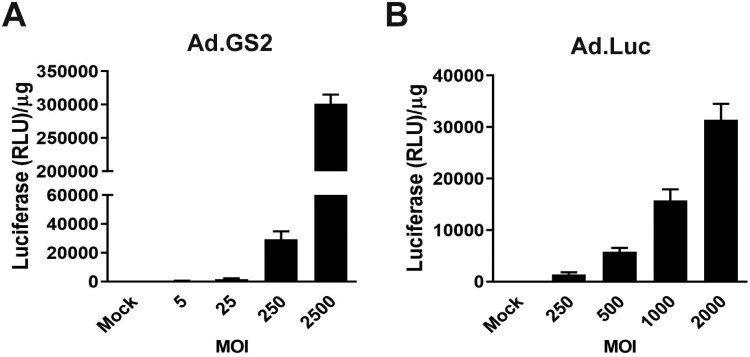
We next examined whether MSCs could be permissive for the replication of Ad.GS2, an hTERT promoter-driven oncolytic adenovirus whose luciferase gene is under the control of the CMV promoter. Additionally, Ad.Luc, an E1-deleted replication-defective adenoviral vector, was tested in parallel. To optimize the efficiency of adenovirus infection and transduction into MSCs, Ad.GS2 and Ad.Luc were infected and transduced into murine MSCs at various MOI, respectively, and luciferase signals were detected at 48 h post-treatment. Luciferase activities were increased in a dose-dependent manner in MSCs infected with replication-competent Ad.GS2 (Figure 2A) or transduced with replication-defective Ad.Luc (Figure 2B). Notably, Ad.GS2 produced much higher luciferase activities than Ad.Luc. Since the level of luciferase activity reflects the extent of viral replication, our results indicate that the expression of luciferase in Ad.GS2-infected MSCs is replication-dependent. As luciferase-based assay is very sensitive, low levels of Ad.GS2 replication in murine MSCs can be detected.
Figure 2.
Luciferase gene can be expressed in MSCs infected with Ad.GS2 or transduced with Ad.Luc. MSCs were infected with Ad.GS2 at MOI of 5-2500 (A) or transduced with Ad.Luc at MOI of 250-2000 (B). Luciferase activities were determined at 48 h post-treatment. The relative luciferase was normalized by the total protein concentration in cell lysates. Values shown are mean ± SD (n = 3).
MBT-2 cells and TCM enhance replication-dependent luciferase expression and adenoviral capsid production in Ad.GS2-infected MSCs
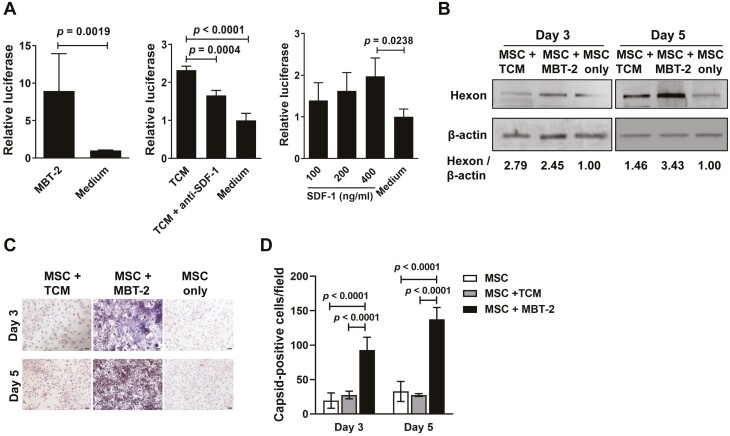
As TCM upregulated the TERT promoter activity of MSCs (Figure 1D), we examined whether MBT-2 cells or TCM could promote the replication of Ad.GS2, in which the E1A gene is under the control of the hTERT promoter. MSCs that had been infected with Ad.GS2 on day 0 were cocultured with MBT-2 cells or treated with TCM or SDF-1 on day 1, and their luciferase activities were measured 48 h later. As shown in Figure 3A, expression of luciferase was significantly higher in Ad.GS2-infected MSCs cocultured with MBT-2 cells (left panel) or incubated with TCM (middle panel) than in those incubated with culture medium. Furthermore, the addition of anti-SDF-1 antibody partially abrogated TCM-mediated enhancement of luciferase expression in MSCs (middle panel). Notably, luciferase activities of MSC-Ad.GS2 treated with recombinant SDF-1 protein were increased in a dose-dependent manner (right panel). These results collectively suggest that SDF-1 secreted by MSCs may transactivate TERT transcription and thus enhance the TERT promoter activity (Figure 1D), thereby increasing Ad.GS2 replication in MSCs (Figure 3A). We next assessed the productive replication of Ad.GS2 in murine MSCs by detecting adenoviral hexon protein, which is the major capsid protein of the virion. After infection with Ad.GS2 for 3 and 5 days, levels of hexon proteins in MSCs cocultured with MBT-2 cells or incubated with TCM increased by 1.5- to 3.4-fold as compared to those in MSCs cultured alone (Figure 3B). Furthermore, viral capsid proteins were detected in Ad.GS2-infected MSCs cocultured with MBT-2 cells by immunohistochemical staining, whereas only background expression was observed in MSCs incubated with TCM (Figure 3C). Moreover, coculture of Ad.GS2-infected MSCs with MBT-2 cells resulted in significant increases in capsid-positive cells, particularly in MBT-2 cells (Figure 3C). Quantification of capsid-expressing cells indicative of productive replication of Ad.GS2 revealed that MSCs cocultured with MBT-2 cells significantly enhanced Ad.GS2 replication compared with MSCs treated with TCM or culture medium after viral infection for 3 and 5 days, in particular at day 5 (Figure 3D). Taken together, these results indicate that Ad.GS2 can replicate in MSCs cocultured with MBT-2 cells. Our findings also suggest that the tumor microenvironment may promote the TERT promoter activity and increase Ad.GS2 replication in both murine MSCs and MBT-2 cells. We further verified the production of infectious Ad.GS2 particles by MSCs (Supplementary Figure S2). Culture supernatants and cell lysates collected from MSC-Ad.GS2 cocultured with MBT-2 cells or treated with TCM for 2 days induced a typical grape-like cluster CPE in 293 cells, whereas no CPE was observed in the samples collected from MSCs cultured alone. These results indicate that MSCs cocultured with MBT-2 cells or TCM result in the enhancement of Ad.GS2 replication.
Figure 3.
MBT-2 cells and TCM enhance replication-dependent luciferase expression and adenoviral capsid production in MSCs infected with Ad.GS2. (A, B) Detection of luciferase activity (A) and hexon protein (B) in MSCs infected with Ad.GS2 in the presence of MBT-2 cells, TCM, or SDF-1. MSCs (3 × 105) were infected with Ad.GS2 at an MOI of 250 on day 0, and cocultured with MBT-2 cells (1 × 105) or 0.4 mL TCM (10 mL conditioned medium collected from a 3-day culture of 4 × 106 MBT-2 cells) with or without anti-SDF-1 antibody (3.5 μg/mL), or incubated with 100, 200, or 400 ng/mL of SDF-1 on day 1. Luciferase activities of Ad.GS2-infected MSCs were measured on day 3 and normalized with the total protein concentration of cell lysates (A). Values shown are mean ± SD (n = 3). Hexon proteins produced in Ad.GS2-infected MSCs were detected on days 3 and 5 by immunoblot analysis (B). Expression of β-actin served as the loading control. Values shown below the blots are ratios between the intensity of the bands corresponding to hexon and those corresponding to β-actin analyzed by densitometry. (C) Detection of adenoviral capsid proteins in MSC-Ad.GS2 cocultured with MBT-2 cells or TCM by immunohistochemical staining with anti-Ad5 antibody. Representative images are shown (original magnification ×200, scale bar = 20 μm). (D) Capsid-positive cells were quantified by averaging the number of stained cells in 4 randomly selected fields. Values shown are the mean ± SD (n = 4).
MSCs enhance the cytolytic activity of Ad.GS2 against MBT-2 cells
Next, we compared the oncolytic effects of MSC-Ad.GS2 and Ad.GS2. Both MSC-Ad.GS2 and Ad.GS2 induced CPE on MBT-2 cells (Figure 4A). Furthermore, MSC-Ad.GS2 was more potent than MSC-Ad5WS1 in inducing cytolytic effects on MBT-2 cells (middle and lower panels, Figure 4B). As Ad.Luc is replication-defective, no CPE was observed induced by MSC-Ad.Luc (upper panel, Figure 4B). These results collectively indicate that MSC-Ad.GS2 is superior to Ad.GS2 alone in inducing cytolytic effects on MBT-2 cells. Furthermore, crude viral supernatants collected from the infected MBT-2 cells, but not NMuMG cells, induced CPE in 293 cells, as examined by crystal violet staining (Figure 4C). The staining was quantified to determine cell survival (Figure 4D). Taken together, we confirm that Ad.GS2 is capable of replicating and producing infectious viral particles in MBT-2 cancer cells, but not in normal NMuMG cells.
Figure 4.
MSC-Ad.GS2 exhibits potent cytolytic effects on MBT-2 cells. (A) MSC-Ad.GS2 is superior to Ad.GS2 in lysing MBT-2 cells. MSCs were infected with Ad.GS2 at an MOI of 100. After 24 h, Ad.GS2-infected MSCs (MSC-Ad.GS2) were detached and cocultured with MBT-2 cells in ratios ranging from 0.5% to 50%. Direct cytolytic effect of Ad.GS2 on MBT-2 cells was estimated in parallel by direct infection with Ad.GS2 at different MOI. After 7 days, MBT-2 cells were examined for CPE by crystal violet staining. (B) MSC-Ad.GS2 exerts higher cytolytic effects on MBT-2 cells than MSC-Ad5WS1. MSCs were infected with Ad.GS2, Ad5WS1, or Ad.Luc at an MOI of 100, followed by the same procedure as described in A. (C, D) Ad.GS2 replicates in MBT-2 cells, but not in normal NMuMG cells. MBT-2 and NMuMG cells (2 × 105) were infected with Ad.GS2 at an MOI of 200 on day 0. Culture supernatants and freeze-thaw cell lysates from Ad.GS2-infected cells were collected and pooled (1 mL) to serve as crude viral supernatants on day 5. Then, 150 μL of serially diluted viral supernatants (600 μL virus supernatant plus 600 μL culture medium as 2 × dilution) were used to infect 3 × 104 293 cells cultured in 96-well plates for detecting infectious viruses. After 6 days, CPE was detected by crystal violet staining (C). The wells were scanned, and crystal violet staining was quantified to determine cell survival using the ImageJ software (D). Values shown are the percentages of cell survival, with the levels in the mock-infected cells arbitrarily set to 100.
MSCs serve as vehicles for delivering Ad.GS2 to the tumor site
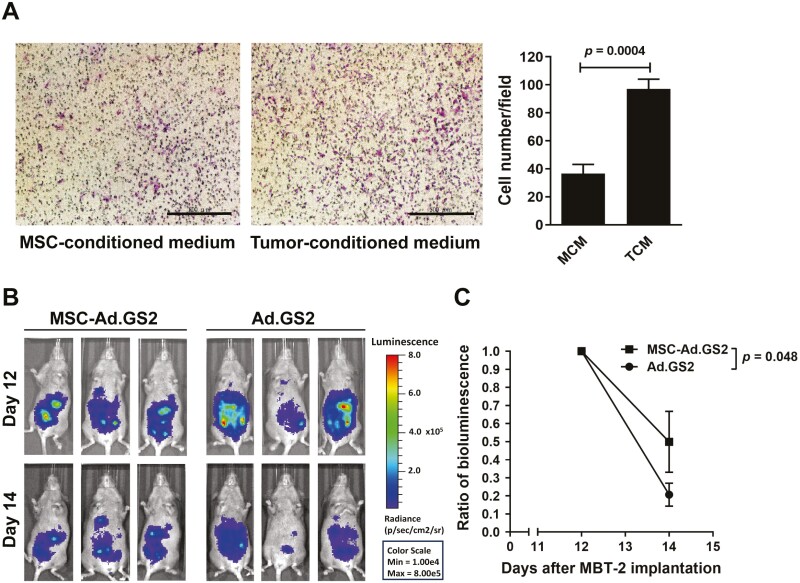
To investigate whether the tumor microenvironment attracted MSC migration, we used a modified Boyden chamber transwell for the migration assay. The TCM derived from MBT-2 cells, but not MSC-conditioned medium, significantly enhanced the migratory capability of MSCs (Figure 5A). To confirm tumor tropism of MSCs, bioluminescence imaging for noninvasive estimation of tumor targeting and adenoviral replication of MSC-Ad.GS2 and Ad.GS2 alone in vivo was established. C3H/HeN mice were inoculated intraperitoneally with MBT-2 cells on day 0 and treated with MSC-Ad.GS2 or Ad.GS2 via the same route on day 11. Mice injected with MSC-Ad.GS2 had higher luminescence signals in comparison with those injected with Ad.GS2 on day 12 (Figure 5B). Although luminescence signals decreased in both groups, mice injected with MSC-Ad.GS2 retained higher luminescence signals than those injected with Ad.GS2 on day 14. When the percentage change of bioluminescence signals was compared, the signals decreased by 80% in mice injected with Ad.GS2, whereas only 50% reduction was detected in those injected with MSC-Ad.GS2 on day 14 (Figure 5C). Collectively, our results confirm the property of tumor tropism of syngeneic MSCs and demonstrate the feasibility and advantage of MSCs for retaining Ad.GS2 with limited replication before migrating to the tumor site.
Figure 5.
MSC-Ad.GS2 shows tumor tropism in vitro and in vivo. (A) MSCs exhibit tropism toward tumor-conditioned medium (TCM) as assessed using a 48-well chemotaxis chamber. TCM or MSC-conditioned medium (MCM) was placed in the lower chamber, whereas MSCs were added to the upper chamber. Migratory cells on the lower surface of the membrane were fixed, stained with Giemsa solution, and photographed after culture for 6 h (magnification × 40; scale bar = 200 μm; left panel). Migratory cells were counted in 3 high-power fields using the ImageJ software (right panel). Values shown are mean ± SD (n = 3). (B) Biodistribution analysis of MSC-Ad.GS2 and Ad.GS2 in MBT-2 tumor-bearing mice by in vivo bioluminescence imaging. C3H/HeN mice were inoculated intraperitoneally with 1.2 × 106 MBT-2 cells on day 0 and injected via the same route with 2 × 106 MSC-Ad.GS2 or 5 × 108 PFU of Ad.GS2 on day 11. Bioluminescent signals and images of the mice were detected on days 12 and 14. (C) Quantification of bioluminescent signals. The bioluminescence associated with MSC-Ad.GS2 and Ad.GS2 was detected and colocalized in the abdominal tumor area after intraperitoneal injection with d-luciferin. The ratio of bioluminescence is shown as the bioluminescent signal on day 14 divided by that on day 12. Values shown are mean ± SD (n = 3).
Systemic therapy with MSC-Ad.GS2 reduces tumor growth and prolongs the survival of mice bearing bladder tumors with pulmonary metastasis
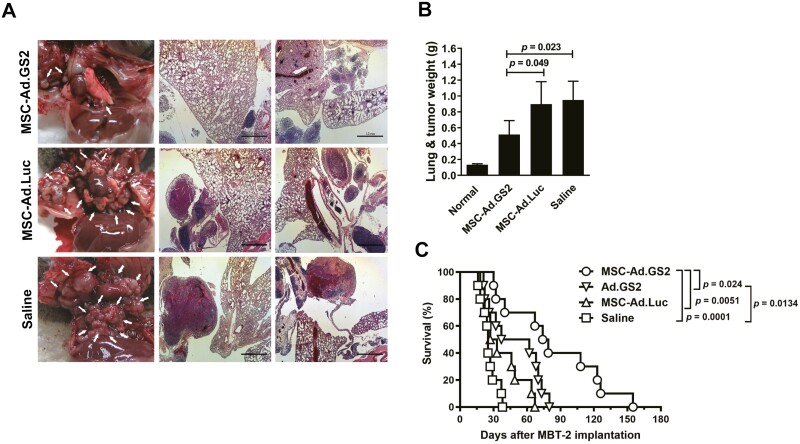
Therapeutic efficacy of MSC-Ad.GS2 delivered systemically was evaluated in pleural disseminated tumor and experimental metastasis models by direct intrapleural inoculation and via tail vein injection of MBT-2 cells, respectively, in syngeneic C3H/HeN mice. In the pleural disseminated tumor model, smaller and fewer lung tumor nodules were observed in mice administered with syngeneic MSC-Ad.GS2 compared to those treated with MSC-Ad.Luc or saline via tail vein injection (Figure 6A). Accordingly, lung and tumor weights were also lower in mice administered with MSC-Ad.GS2 (0.514 g) than those with MSC-Ad.Luc (0.898 g) or saline (0.95; Figure 6B). These results suggest that MSC-Ad.GS2 migrates to the tumor sites to induce active replication of Ad.GS2, resulting in effective oncolysis in the pleural disseminated bladder tumor model. To further evaluate the therapeutic efficacy on the survival in the experimental metastasis model, mice were inoculated with MBT-2 cells via the tail vein on day 0 and administered with MSC-Ad.GS2, Ad.GS2, MSC-Ad.Luc, or saline through the same route on day 7. Mice treated with MSC-Ad.GS2 or Ad.GS2 alone significantly survived longer than those treated with MSC-Ad.Luc or saline (Figure 6C). Notably, mice treated with MSC-Ad.GS2 had significant survival benefits compared with those treated with Ad.GS2 alone. Collectively, syngeneic MSC-Ad.GS2 is superior to Ad.GS2 in inducing potent oncolytic effects on distant metastatic tumors. Taken together, MSC-Ad.GS2 delivered systemically displays the tumor tropism property and induces remarkable therapeutic benefits in mice bearing metastatic tumors compared with Ad.GS2 injection alone.
Figure 6.
Systemic administration of MSC-Ad.GS2 reduces tumor growth and prolongs the survival in mice bearing bladder tumors with pulmonary metastasis. (A-C) MBT-2 cells (1 × 105) that had been intrapleurally inoculated into the thoracic cavity of C3H/HeN mice on day 0 were injected with 1 × 105 MSC-Ad.GS2 or MSC-Ad.Luc, or saline via the tail vein on day 4. Mice were sacrificed on day 10. Representative gross appearance (left panel) and H&E-stained histological images (scale bar = 1 mm; middle and right panels) of lung lobes from the treated mice (A). Note that metastases of tumor nodules were smaller after MSC-Ad.GS2 treatment compared to MSC-Ad.Luc or saline treatment. White arrows in the gross pathological images denote tumor nodules. At sacrifice, the mean total weights of lungs and tumor nodules in each treatment group of mice were calculated (B). Values shown are mean ± SD (n = 4 or 5). (C) Kaplan-Meier survival curves of tumor-bearing mice receiving different treatments. C3H/HeN mice were injected with 1 × 105 MBT-2 cells via the tail vein on day 0, followed by injection with 2 × 106 MSC loaded with Ad.GS2 or Ad.Luc (MOI = 500), or direct injection with Ad.GS2 (1 × 109 PFU) or saline via the same route on day 7. All mice were monitored for survival until death. The survival analysis was performed using the Kaplan-Meier survival curve and the long-rank test.
Discussion
This study demonstrates that intravenously injected syngeneic murine MSCs can deliver oncolytic adenoviruses controlled by the hTERT promoter against metastatic bladder cancers in immunocompetent mouse models. Our findings may be easily transferred to clinical application. In immunocompetent mouse models, our results show that syngeneic MSC-Ad.GS2 administered via the tail vein is a therapeutic tool for treating metastatic murine tumors. Clinical trials have been reported for human MSCs carrying oncolytic adenoviruses, showing that the treatment is a safe strategy for systemic administration of repeated doses.31 In the present study, the oncolytic adenovirus Ad.GS2 was derived from Ad5 whose E1B-55 kD gene was deleted, and E1A gene expression, and therefore viral replication, was under the control of the hTERT promoter. The E1B-55 kD-deleted adenovirus selectively replicates in tumor cells but not in normal cells.24,25 However, it replicates significantly less well than the wild-type Ad5.32 The E1A expression is the first adenoviral protein expressed after viral infection and regulates the temporal transcription pattern of adenoviral genes necessary for viral replication. In addition to the deletion of the E1B-55 kD gene, the employment of the hTERT promoter to control adenoviral E1A expression reinforces tumor-specific oncolysis of Ad.GS2. Moreover, Ad.GS2 carries the luciferase gene that enables to track viral replication and distribution. For further studies, a similar virus without carrying the luciferase gene that we generated before, namely Ad.GS1, can be applied.11
A major limitation in evaluating the antitumor efficacy of MSC-delivered oncolytic adenoviruses is the lack of immune- and replication-competent tumor models. To this end, we used the syngeneic murine MBT-2 bladder cells as an oncolytic human adenovirus-permissive tumor model in immunocompetent C3H/HeN mice to examine the anti-metastatic efficacy of syngeneic MSCs loaded with Ad.GS2. Since most murine cells express low levels of coxsackievirus and adenovirus receptor, it is generally thought that human Ad5 does not efficiently infect murine cells nor does it replicate well in these cells. For viral entry, we have reported that the susceptibility of murine MBT-2 bladder cancer cells and human J82 bladder cancer cells to replication-defective adenoviral vectors is similar, especially when cancer cells are infected with high viral doses.9 In addition, we have shown that human oncolytic adenoviruses infect and lyse MBT-2 cells, as well as produce viral capsid proteins, which are viral late proteins for capsid assembly indicative of viral replication, in MBT-2 cells and tumors.2,3 Notably, we further show that infectious viral particles were produced in MBT-2 cells, but not in normal NMuMG cells after infection with Ad.GS2 (Figure 4C, 4D). In this regard, some murine tumor cell lines, such as CMT-64 and KLN205 lung carcinoma cell lines, were reported to support the replication cycle of human adenoviruses with reduced efficacy.33-35 Diverse genetic alterations present in murine cancer cell lines might result in increased permissiveness to human adenovirus replication.
In the current study, we used syngeneic MSCs isolated from the bone marrow of C3H/HeN mice. In most studies on oncolytic viruses delivered by MSCs, human MSCs obtained from different sources have been applied.36,37 Xenogeneic or even allogeneic MSCs can elicit immune responses when transplanted into the parenchyma of wild-type mice,38 suggesting that the elicited immune responses may affect the therapeutic effect of MSCs, which should be considered. We also collected crude viral supernatants from Ad.GS2-infected MSCs to detect infectious virus particles in 293 cells. While Ad.GS2-infected MSCs did not produce infectious viruses, Ad.GS2-infected MSCs exposed to MBT-2 cells or TCM did, albeit at a low degree (Supplementary Figure S2). MSCs infected with Ad.GS2 expressed much higher levels of luciferase activity in a dose-dependent manner compared to those transduced with the replication-defective Ad.Luc (Figure 2), suggesting that Ad.GS2 can replicate in murine MSCs. Since luciferase-based assay is very sensitive, making the assessment more sensitive than CPE detection. Moreover, Ad.GS2-infected MSCs exposed to MBT-2 cells or TCM expressed higher levels of viral hexon proteins than Ad.GS2-infected MSCs cultured alone (Figure 3B). Collectively, these results indicate that murine MSCs support a low degree of Ad.GS2 replication, which can be augmented by coculture with MBT-2 cells or TCM. Previous literature has shown that oncolytic adenoviruses can slightly replicate in and lyse human MSCs, leading to viral release.39,40 Although it is ideal that MSCs can function as a biological factory for viral replication, excess viral replication may induce premature lysis of MSCs before they migrate to the tumor site. In the present study, we show that murine MSCs alone provide limited viral replication. However, when MSCs migrate to the tumor microenvironment, viral replication is increased, leading to viral release from MSCs and infection to neighboring tumor cells.
Secretion of chemokines/cytokines from the tumor microenvironment can recruit MSCs to tumor sites. Previous studies have reported that SDF-1 (also known as CXCL12) is an important chemokine secreted by human MSCs when exposed to the tumor microenvironment.28 SDF-1 binds to its cognate receptor CXCR4/7 existing on MSCs to activate downstream signaling via STAT3 and ERK/MAPK pathways in an autocrine manner.29 STAT3 can bind directly to the hTERT promoter region and thus upregulate hTERT expression in human cancer cells and normal somatic cells.30 We also show that MSCs secreted SDF-1 when interacting with MBT-2 cells or TCM. We propose that increased SDF-1 may, in turn, activate the STAT3 signaling to upregulate TERT promoter activity in MSCs. As the replication of Ad.GS2 is controlled by the hTERT promoter, Ad.GS2 replication in MSCs may be boosted, and more viral progeny may be released from MSCs to infect and kill surrounding cancer cells. Therefore, we propose that the SDF-1/STAT3/TERT signaling axis in MSCs in response to the tumor microenvironment may be involved in the enhanced antitumor efficacy of MSCs loaded with Ad.GS2 in the treatment of established bladder tumors with pulmonary metastasis. Treatment with MSC-Ad.GS2 significantly reduced tumor growth and prolonged the survival of mice bearing metastatic bladder tumors. Since we used immunocompetent C3H/HeN mice bearing syngeneic MBT-2 cells for evaluating the antitumor efficacy of syngeneic murine MSCs loaded with Ad.GS2, apart from direct viral oncolytic effects, antitumor immune responses may also contribute to the dramatic antitumor effects of MSC-Ad.GS2. Notably, MSCs loaded with the replication-defective Ad.Luc had no effects on tumor growth (Figure 6), suggesting that MSCs per se have no antitumor or pro-tumoral effects. We found that the side effects were not detected in the treated mice, including body-weight loss and death during the early stage of the treatment. Thus, systemic administration of syngeneic MSCs carrying Ad.GS2 provides a promising therapeutic tool for metastatic cancer and a safe strategy for patients with late-stage cancer. Since cancer metastasis is the major cause of cancer morbidity and mortality, systemic treatment with of MSCs loaded with telomerase-dependent oncolytic adenoviruses may be broadly applicable for treating metastatic cancer.
Supplementary material
Supplementary material is available at Stem Cells Translational Medicine online.
Acknowledgments
We thank the Laboratory Animal Center, College of Medicine, National Cheng Kung University, and Taiwan Animal Consortium for the technical support in IVIS analysis.
Contributor Information
Mei-Lin Yang, Department of Medical Research, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan; Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Che-Yuan Hu, Department of Urology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Ya-Che Lee, Department of Urology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan.
Chao-Ching Chang, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yi-Cheng Chen, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Pei-Ru Lee, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Bing-Hua Su, School of Respiratory Therapy, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Pi-Che Chen, Department of Urology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan.
Ai-Li Shiau, Department of Medical Research, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan; Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Gia-Shing Shieh, Department of Urology, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Urology, Tainan Hospital, Department of Health, Executive Yuan, Tainan, Taiwan.
Chao-Liang Wu, Department of Medical Research, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan; Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Pensee Wu, School of Medicine, Keele University, Staffordshire, United Kingdom; Department of Obstetrics and Gynaecology, University Hospital of North Midlands, Newcastle-under-Lyme, Staffordshire, United Kingdom.
Author contributions
Mei-Lin Yang: Collection and/or assembly of data, Data analysis and interpretation. Che-Yuan Hu, Ya-Che Lee: Provision of study material or patients, Data analysis and interpretation. Chao-Ching Chang, Yi-Cheng Chen, Pei-Ru Lee, Bing-Hua Su: Collection and/or assembly of data. Pi-Che Chen: Provision of study material or patients, Final approval of manuscript. Ai-Li Shiau: Conception and design, Administrative support, Manuscript writing, Final approval of manuscript. Gia-Shing Shieh: Conception and design, Financial support, Final approval of manuscript. Chao-Liang Wu: Conception and design, Financial support, Administrative support, Manuscript writing, Final approval of manuscript. Pensee Wu: Conception and design, Final approval of manuscript. Mei-Lin Yang, Che-Yuan Hu, and Ya-Che Lee contributed equally to this work.
Funding
This work was in part supported by research grants from Ditmanson Medical Foundation Chia-Yi Christian Hospital (NCKUCYC-P-11101) and National Science and Technology Council, Taiwan (108-2320-B-006-026 and 112-2314-B-006-045).
Conflicts of interest
The authors indicated no potential conflicts of interest.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373-376. 10.1126/science.274.5286.373 [DOI] [PubMed] [Google Scholar]
- 2. Wu CL, Shieh GS, Chang CC, et al. Tumor-selective replication of an oncolytic adenovirus carrying oct-3/4 response elements in murine metastatic bladder cancer models. Clin Cancer Res. 2008;14(4):1228-1238. 10.1158/1078-0432.CCR-07-1047 [DOI] [PubMed] [Google Scholar]
- 3. Chang CC, Shieh GS, Wu P, et al. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008;68(15):6281-6291. 10.1158/0008-5472.CAN-08-0094 [DOI] [PubMed] [Google Scholar]
- 4. Hsu KF, Wu CL, Huang SC, et al. Conditionally replicating E1B-deleted adenovirus driven by the squamous cell carcinoma antigen 2 promoter for uterine cervical cancer therapy. Cancer Gene Ther. 2008;15(8):526-534. 10.1038/cgt.2008.37 [DOI] [PubMed] [Google Scholar]
- 5. Lu CS, Hsieh JL, Lin CY, et al. Potent antitumor activity of Oct4 and hypoxia dual-regulated oncolytic adenovirus against bladder cancer. Gene Ther. 2015;22(4):305-315. 10.1038/gt.2014.122 [DOI] [PubMed] [Google Scholar]
- 6. Su BH, Shieh GS, Tseng YL, Shiau AL, Wu CL. Etoposide enhances antitumor efficacy of MDR1-driven oncolytic adenovirus through autoupregulation of the MDR1 promoter activity. Oncotarget. 2015;6(35):38308-38326. 10.18632/oncotarget.5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Günes C, Wezel F, Southgate J, Bolenz C. Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat Rev Urol. 2018;15(6):386-393. 10.1038/s41585-018-0001-5 [DOI] [PubMed] [Google Scholar]
- 8. Borah S, Xi L, Zaug AJ, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347(6225):1006-1010. 10.1126/science.1260200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shieh GS, Shiau AL, Yo YT, et al. Low-dose etoposide enhances telomerase-dependent adenovirus-mediated cytosine deaminase gene therapy through augmentation of adenoviral infection and transgene expression in a syngeneic bladder tumor model. Cancer Res. 2006;66(20):9957-9966. 10.1158/0008-5472.CAN-06-1138 [DOI] [PubMed] [Google Scholar]
- 10. Lanson NA Jr, Friedlander PL, Schwarzenberger P, Kolls JK, Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003;63(22):7936-7941. [PubMed] [Google Scholar]
- 11. Chen SY, Shiau AL, Shieh GS, et al. Amelioration of experimental arthritis by a telomerase-dependent conditionally replicating adenovirus that targets synovial fibroblasts. Arthritis Rheum. 2009;60(11):3290-3302. 10.1002/art.24940 [DOI] [PubMed] [Google Scholar]
- 12. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71-74. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- 13. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 14. Taheri M, Tehrani HA, Dehghani S, et al. Signaling crosstalk between mesenchymal stem cells and tumor cells: Implications for tumor suppression or progression. Cytokine Growth Factor Rev. 2024;76:30-47. 10.1016/j.cytogfr.2024.01.004 [DOI] [PubMed] [Google Scholar]
- 15. Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15(10):730-738. 10.1038/gt.2008.39 [DOI] [PubMed] [Google Scholar]
- 16. Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614-2623. 10.1002/stem.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med.. 2018;7(9):651-663. 10.1002/sctm.18-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 2020;8:43. 10.3389/fbioe.2020.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghasemi Darestani N, Gilmanova AI, Al-Gazally ME, et al. Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment. Cell Commun Signal. 2023;21(1):43. 10.1186/s12964-022-01012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghaleh HEG, Vakilzadeh G, Zahiri A, Farzanehpour M. Investigating the potential of oncolytic viruses for cancer treatment via MSC delivery. Cell Commun Signal. 2023;21(1):228. 10.1186/s12964-023-01232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takayama Y, Kusamori K, Nishikawa M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin Drug Deliv. 2021;18(11):1627-1642. 10.1080/17425247.2021.1960309 [DOI] [PubMed] [Google Scholar]
- 22. Yoon AR, Rivera-Cruz C, Gimble JM, Yun CO, Figueiredo ML. Immunotherapy by mesenchymal stromal cell delivery of oncolytic viruses for treating metastatic tumors. Mol Ther Oncolytics. 2022;25:78-97. 10.1016/j.omto.2022.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun S, Guo Z, Xiao X, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21(5):527-535. 10.1634/stemcells.21-5-527 [DOI] [PubMed] [Google Scholar]
- 24. Hsieh J-L, Wu C-L, Lee C-H, Shiau A-L. Hepatitis B virus X protein sensitizes hepatocellular carcinoma cells to cytolysis induced by E1B-deleted adenovirus through the disruption of p53 function. Clin Cancer Res. 2003;9(1):338-345. [PubMed] [Google Scholar]
- 25. Hsieh JL, Wu CL, Lai MD, et al. Gene therapy for bladder cancer using E1B-55 kD-deleted adenovirus in combination with adenoviral vector encoding plasminogen kringles 1-5. Br J Cancer. 2003;88(9):1492-1499. 10.1038/sj.bjc.6600908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bee YS, Tu L, Sheu SJ, et al. Gene delivery of calreticulin anti-angiogenic domain attenuates the development of choroidal neovascularization in rats. Hum Gene Ther. 2017;28(5):403-414. 10.1089/hum.2016.035 [DOI] [PubMed] [Google Scholar]
- 27. Su BH, Tseng YL, Shieh GS, et al. Prothymosin α overexpression contributes to the development of pulmonary emphysema. Nat Commun. 2013;4:1906. 10.1038/ncomms2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25(2):520-528. 10.1634/stemcells.2006-0257 [DOI] [PubMed] [Google Scholar]
- 29. Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27(4):857-865. 10.1002/stem.23 [DOI] [PubMed] [Google Scholar]
- 30. Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 2005;65(15):6516-6520. 10.1158/0008-5472.CAN-05-0924 [DOI] [PubMed] [Google Scholar]
- 31. Ruano D, López-Martín JA, Moreno L, et al. First-in-human, First-in-child trial of autologous MSCs carrying the oncolytic virus Icovir-5 in patients with advanced tumors. Mol Ther. 2020;28(4):1033-1042. 10.1016/j.ymthe.2020.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yew PR, Kao CC, Berk AJ. Dissection of functional domains in the adenovirus 2 early 1B 55K polypeptide by suppressor-linker insertional mutagenesis. Virology. 1990;179(2):795-805. 10.1016/0042-6822(90)90147-j [DOI] [PubMed] [Google Scholar]
- 33. Halldén G, Hill R, Wang Y, et al. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol Ther. 2003;8(3):412-424. 10.1016/s1525-0016(03)00199-0 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Hallden G, Hill R, et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21(11):1328-1335. 10.1038/nbt887 [DOI] [PubMed] [Google Scholar]
- 35. Woller N, Knocke S, Mundt B, et al. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J Clin Invest. 2011;121(7):2570-2582. 10.1172/JCI45585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moreno R, Fajardo CA, Farrera-Sal M, et al. Enhanced antitumor efficacy of oncolytic adenovirus-loaded menstrual blood-derived mesenchymal stem cells in combination with peripheral blood mononuclear cells. Mol Cancer Ther. 2019;18(1):127-138. 10.1158/1535-7163.MCT-18-0431 [DOI] [PubMed] [Google Scholar]
- 37. Shimizu Y, Gumin J, Gao F, et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J Neurosurg. 2022;136(3):757-767. 10.3171/2021.3.JNS203045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hwang JW, Lee NK, Yang JH, et al. A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int J Mol Sci . 2020;21(9):3052. 10.3390/ijms21093052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon AR, Hong J, Li Y, et al. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res. 2019;79(17):4503-4514. 10.1158/0008-5472.CAN-18-3900 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Liu C, Wang T, et al. Therapeutic effects of mesenchymal stem cells loaded with oncolytic adenovirus carrying decorin on a breast cancer lung metastatic mouse model. Mol Ther Oncolytics. 2022;24:486-496. 10.1016/j.omto.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.