Abstract
Background
Posterior glenoid bone loss in glenohumeral osteoarthritis poses significant challenges in shoulder arthroplasty. Anatomic total shoulder arthroplasty (TSA) with a humeral head autograft to address the glenoid bone deficiency is an option for these difficult cases. Variable results with this procedure are reported in the literature. This article describes the surgical technique of posterior glenoid bone grafting in TSA using a glenoid implant with hybrid fixation and a series of reported patient functional and radiographic outcomes.
Methods
A retrospective chart review of cases from 2015 to 2020 by a single surgeon revealed 10 patients who underwent primary TSA with hybrid glenoid component and posterior glenoid bone grafting. Preoperative and postoperative radiographs were assessed for glenoid inclination, glenoid version, acromiohumeral distance, humeral stem status, and glenoid implant status. Functional outcomes were evaluated by range of motion, strength, and patient-reported clinical outcomes (pain and function on a visual analog scale, Disabilities of the Arm, Shoulder, and Hand score, Single Assessment Numeric Evaluation, American Shoulder and Elbow Surgeons score). Complications and reoperations were also evaluated.
Results
Average follow-up was 31.4 months, active forward flexion and external rotation improved on average from 105° to 150° and 20° to 60°, respectively (P < .001) and average abduction improved from 100° to 140° (P < .002). At an average of 26.7 months, patients reported assessments (visual analog scale pain and function, Disabilities of the Arm, Shoulder, and Hand score, Single Assessment Numeric Evaluation, American Shoulder and Elbow Surgeons scores) reveal significant (P < .05) improvement in pain and functional outcomes. Radiographically, at 23.0 ± 20.1 months, all patients demonstrated a well-fixed humeral and glenoid component without evidence of lucent lines. The bone graft used in each patient was well unionized and no radiographic complications were reported. No patients underwent revision surgery, and there was one clinical complication reported, which consisted of a suspected rotator cuff injury at follow-up.
Conclusion
Hybrid fixation with structural glenoid bone grafting in TSA resulted in excellent outcomes with no evidence of graft or component failure on follow-up radiographs and significantly reduced pain, improved functional scores, and improved active range of motion.
Keywords: Total shoulder arthroplasty, Posterior glenoid bone loss, Posterior glenoid bone graft, Humeral head autograft, Hybrid fixation, Cemented fixation
Osteoarthritis of the glenohumeral is one of the most common etiologies of shoulder pain in patients over 60 years old.10 Primary glenohumeral osteoarthritis prevalence has been reported to be as high as 94% in women and 85% in men over the age of 80.10 Over the past few decades, rates of shoulder arthroplasty have rapidly increased. From 1993 to 2007, data from the National Inpatient Sample suggest an increase in primary total shoulder arthroplasty (TSA) of 369%.3 By 2025, the growth rate of TSA and reverse shoulder arthroplasty (RSA) is estimated to remain steady at 349% which corresponds to 439,206 procedures, 66,086 of those being TSA.4
When preoperatively planning for shoulder arthroplasty, glenoid bone loss is an important factor that can have significant implications related to operative technique.1 The classification system described by Walch et al can be a useful tool in classifying the various types of glenoid bone loss. Substantial glenoid bone loss poses significant challenges in terms of functional and radiographic outcomes following arthroplasty. A posterior glenoid defect (Walch B1-3 glenoid type) of as little as 5o increases posterior humeral head translation, which is associated with subluxation and subsequent asymmetric glenoid loading leading to accelerated wear.13,16 Surgical attempts to correct glenoid version may result in joint line medialization, decreased glenoid vault volume, decreased glenohumeral surface contact area, and increased contact pressures.13,16 Due to the challenges posed secondary to posterior glenoid bone loss, B type glenoids are associated with worse functional outcomes.9
Current options to address glenoid bone loss include TSA with a retroverted implant, TSA with version correction using eccentric reaming, bone grafting, or an augmented glenoid component. Other surgical options for severe bone loss include reverse TSA. Multiple studies suggest eccentric reaming can only correct the degree of retroversion up to 15°, beyond which bone graft or augmented glenoid implants are necessary to address posterior deficits. 2,6,14 The technique for structural bone grafting first described by Neer and Morrison in 1988 serves as a guide and has been cited by other studies that report the use of bone graft in TSA.8.12 Previous studies which have reported surgical techniques of bone grafting for posterior glenoid deficits in TSA differ based on graft fixation technique, use of cement, glenoid components, and additional procedural steps such as subscapularis lengthening and posterior capsulorrhaphy. Options for glenoid components range from metal-backed to all-polyethylene and pegged vs. keeled.5,7,8,11, 12, 13,15,17,18 The use of cement in the implantation of the glenoid component is also controversial as cement can interdigitate with the interface between the native glenoid and bone graft and subsequently increase the risk of nonunion, graft failure, and component loosening.11, 12, 13,15,17 One option to address this concern is the use of hybrid fixation, which utilizes structural bone grafting and a partially cemented, pegged, bony-ingrowth glenoid component with smaller peripheral pegs to limit cement use and promote bone unionization. The purpose of this article is to describe the surgical technique using hybrid fixation with structural glenoid bone graft taken from the humeral head in TSA and to report a series of patients who underwent this procedure and their functional and radiographic outcomes.
Methods
Patient population
This retrospective non-randomized study was reviewed and approved by the University at Buffalo Institutional Review Board. Electronic medical records were queried to find 10 patients who underwent primary TSA with a humeral head allograft from January 2016 through September 2018. All participants underwent the procedure at a single site and the operation was performed by a single surgeon. Inclusion criteria were as follows: (1) age between 18 and 100 years old; (2) underwent surgery in the specified time frame; and (3) had available clinical, patient-reported, and radiographic follow-up.
Indications
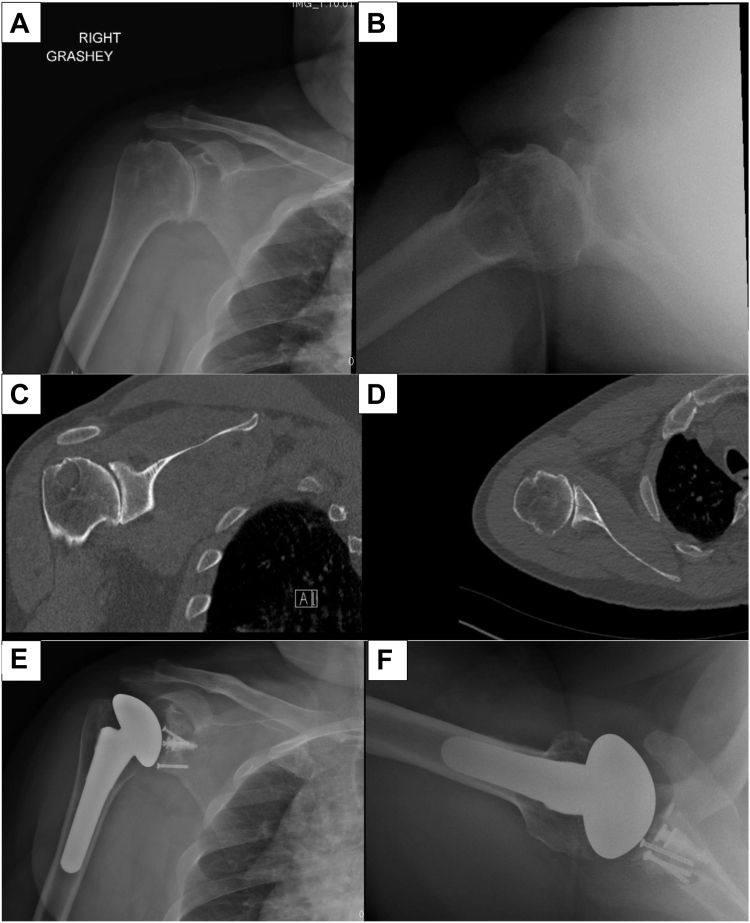
Preoperative planning was performed using plain radiographs and computed tomography imaging in all cases. The extent of posterior glenoid bone loss is evaluated and 3D planning is performed to assess ability to perform anatomic TSA. Our indications for bone grafting with an anatomic hybrid glenoid implant are as follows: (1) glenoid version cannot be corrected to 5 degrees or less of retroversion with high-side reaming; (2) high-side reaming results in medialization of the joint line by more than 5 mm; (3) the patient is physiologically young and active with good bone quality (typically <60); (4) patient has an intact rotator cuff; and (5) there is no evidence of glenoid dysplasia based on the Walch classification (no type C glenoid morphology). Patients who meet these criteria are offered the option of an anatomic total shoulder with a glenoid bone graft. Alternative options are discussed with the patient including anatomic total shoulder with an augmented glenoid implant or reverse TSA. A representative case is depicted in Figure 1.
Figure 1.
Preoperative Grashey (A) and axillary (B) radiographs and coronal (C) and axial (D) CT scan images of a patient with a B2 type glenoid with severe posterior glenoid bone loss, glenoid retroversion, and posterior humeral head subluxation who underwent anatomic total shoulder arthroplasty with humeral head autografting for posterior glenoid defect. Postoperative Grashey (E) and axillary (F) radiographs of the same patient at final follow-up demonstrating components in good position with no evidence of lucency or component loosening. The graft appears to be unionized, and the version has been corrected to be close to neutral.
Surgical technique
The patient was taken to the operating room and placed in a beach chair position. A deltopectoral approach was used. The skin is incised using an 8-12 cm incision extending from just lateral to the coracoid process distally to the deltoid insertion. Dissection is carried down through skin and soft tissue, and the cephalic vein is identified and protected. The deltopectoral interval is identified and entered. The deltoid is mobilized and the subdeltoid and subacromial space are developed by blunt dissection. The rotator cuff musculature is evaluated at this time, it was found to be intact in all patients included in the study, thus allowing implantation of an anatomic TSA. Reverse TSA is performed if the rotator cuff was found to be deficient.
A subscapularis tenotomy is performed 5 mm medial to the lesser tuberosity and the rotator interval is split at the level of the glenoid. The long head of the biceps tendon is identified and tenodesed to the pectoralis muscle with a #2 Ethibond suture (Johnson & Johnson MedTech, New Brunswick, NJ, USA), the biceps is then transected at the articular margin of the humerus with electrocautery. The inferior capsule is released starting anteriorly progressing around the humerus posteriorly with care to mobilize around any inferior osteophytes. The capsule is released just past the central aspect of the medial calcar leaving the posterior capsule attached to the humerus. The posterior capsule is typically loose in these patients and limiting release helps prevent posterior subluxation after implant placement. If glenoid exposure is limited, additional posterior capsule can be released at that time to improve exposure as needed. The humeral head is then able to be dislocated anteriorly.
Humeral preparation is begun with the use of humeral reamers up to appropriate size. This is followed by a humeral head osteotomy performed at 30 degrees of retroversion. The humeral head is harvested and preserved for bone grafting later in the case. The humeral canal is then broached to appropriate size allowing good purchase of the humeral stem. Remainder of osteophytes are then débrided from the neck of the humerus.
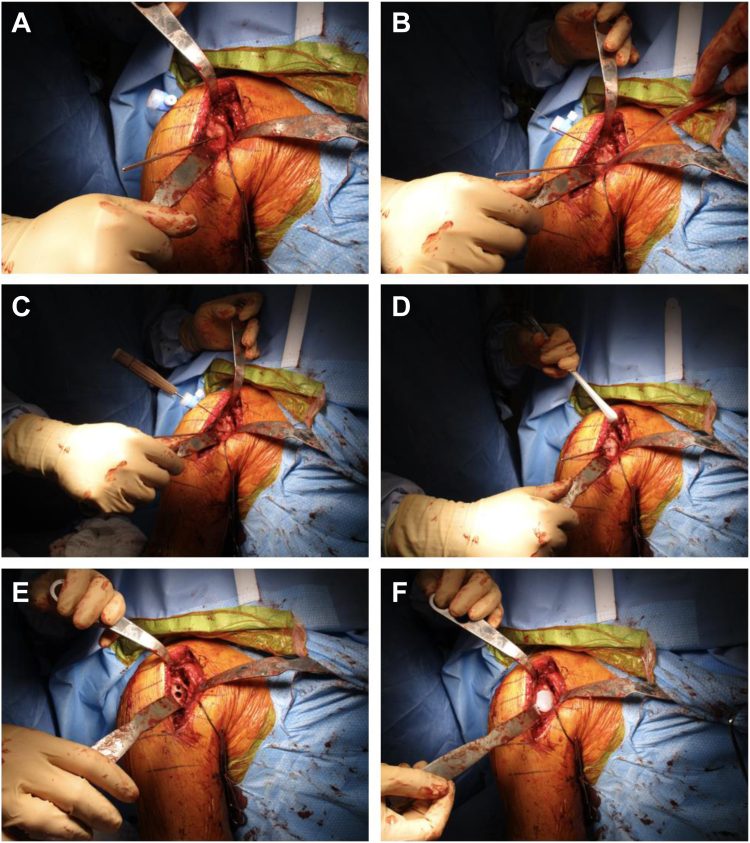
Attention is then turned to the glenoid. The subscapularis is carefully mobilized from the surrounding capsule around the glenoid. A complete release around the subscapularis is performed with care taken to protect the axillary nerve. The anterior capsule and anterior labral tissue are excised. The posterior labrum is excised and débrided, and the inferior capsule is released to the 6 o'clock position on the glenoid. Patient-specific guides are helpful for correcting version and were used in most cases in this series. The guide is positioned over the anterior rim of the glenoid with correct positioning confirmed with the patient-specific model provided. The guidepin for the glenoid reamer is placed through the hole in the patient's specific guide at the planned version and inclination (Fig. 2, A). The glenoid is then reamed to allow for contact of at least 50% of the glenoid implant surface with native bone. The size of the remaining defect is then measured.
Figure 2.
Images of a patient undergoing anatomic total shoulder arthroplasty (TSA) with humeral head autografting for a posterior glenoid defect. This figure shows glenoid preparation, graft placement and fixation, and glenoid component fixation. (A) Central guide pin is placed through the patient’s specific guide and the glenoid surface is subsequently reamed. (B) Humeral head autograft is positioned over the posterior glenoid defect and provisionally fixed with two 1.0 mm pins, one transverse and one through a transdeltoid portal. (C) A cannulated screwdriver and 3.0 mm cannulated screw is placed over the pin in the transdeltoid portal and used to contact the superior aspect of the autograft and securely fix it to the glenoid. Usually, a second screw is used to fix the inferior aspect of the autograft (image not shown). (D) Provisional pins are removed, and the native glenoid and autograft are reamed to create a concave surface. (E) Using the central guide pin, one central hole and three peripheral holes are drilled. (F) The glenoid polyethylene component is impacted into place and secured with tobramycin mixed cement.
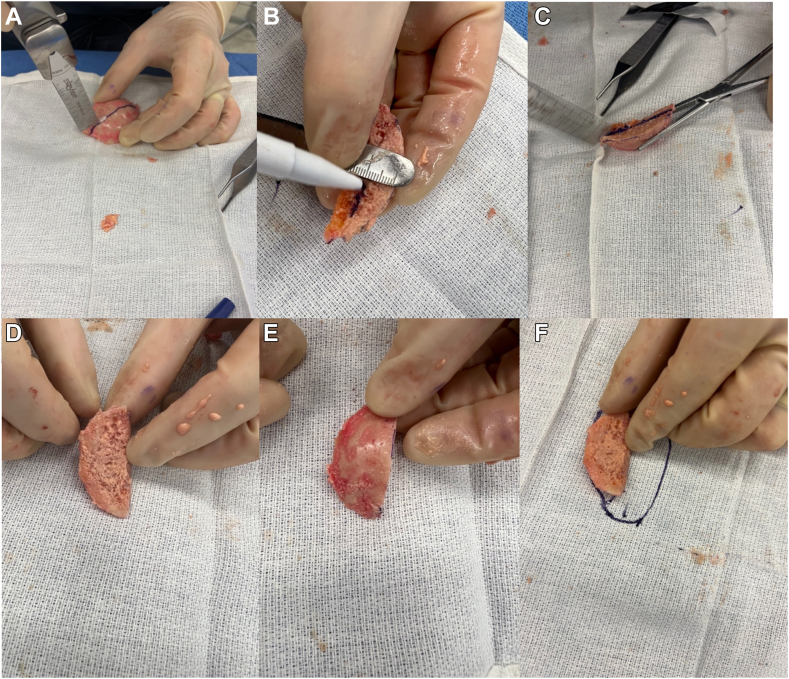
Attention is then drawn to the glenoid bone graft creation from the humeral head autograft. The anterior half of the humeral head is utilized as this matches with the posterior defect of the glenoid. The humeral head is trimmed in a wedge-shaped fashion to match the posterior defect of the glenoid. The graft is made slightly larger than the measured bony deficiency that was planned preoperatively using the patient-specific planning software and then further trimmed to perfectly match the bony defect (Fig. 3). The graft is then positioned over the posterior glenoid defect to ensure appropriate sizing. The articular surface of the humeral head is lightly abraded with a bur to improve graft healing, while maintaining the subchondral plate for structural fixation. A transdeltoid portal is created posteriorly in the appropriate trajectory for graft fixation. The graft is then provisionally fixed with 2 pins from a 3.0 mm cannulated screw set (Fig. 2, B). The screw placement for the graft does need to be directed to miss the central post. The graft is typically larger than the glenoid implant so there is space to avoid the central post. The first screw is placed in the superior part of the graft and the second at the inferior part of the graft. It can be helpful to place the drill guide for the central post and peripheral pegs over the central pin at this time to provide a visual aid while determining screw trajectory to avoid the post and pegs. Confirmation of position and alignment is performed and fixation of the graft is performed with 3.0 cannulated screws placed to avoid the porous central post and 4 peripheral poly pegs of the glenoid implant (Fig. 2, C). The screws are buried through the exposed cancellous bone of the humeral head until they engage the subchondral plate. Number and location of screw fixation is patient dependent, but two screws are typically sufficient to provide stability of the graft. After adequate fixation is obtained, the provisional pins are removed.
Figure 3.
Images of graft preparation with the resected humeral head. (A) The anterior to posterior dimensions of the glenoid defect are measured based on preoperative templating and intraoperative measurement and cut with a saw. (B) The depth of the glenoid defect is measured again based on preoperative templating and intraoperative measurements. (C) The graft is cut to the appropriate depth. (D) The completed graft demonstrating the exterior surface which will be reamed and the baseplate implanted. (E) The completed graft demonstrating the surface that will abut the native glenoid, prior to being abraded with the bur. (F) A representative drawing of how the graft will fit on the glenoid (left–posterior, right–anterior).
The glenoid surface reamer is used again to prepare the bone graft so that is flush with the native bone of the anterior glenoid face (Fig. 2, D). The central hole for the porous metal post is drilled, followed by the 3 peripheral holes (Fig. 2, E). Antibiotic-impregnated bone cement is mixed and pressurized into the superior and anterior holes of in the native glenoid and is loosely injected into the posterior hole in the bone graft to prevent extravasation of the cement at the bone graft host interface. The glenoid baseplate is then impacted into place and cement was allowed to fully cure (Fig. 2, F). Attention is then turned back to the humerus. Humeral trials are placed onto the broach and the shoulder is reduced. After appropriate-sized trials were identified resulting in excellent balance and no instability of the shoulder, the trials are removed. Final implants are placed based on the alignment during the humeral trailing. Range of motion (ROM) and stability is tested again. After appropriate ROM stability is confirmed, the wound is copiously irrigated and hemostasis obtained with electrocautery. The subscap is mobilized and repaired at the level of the tuberosity with #2 FiberWire (Arthrex, Naples, FL, USA) suture passed through the bone of the lesser tuberosity using a Mason-Allen suture configuration resulting in a suture bridge repair. Final radiographs were obtained in the operating room with flat plate radiographs and assessed to confirm the appropriate position and alignment of components as well as no evidence of fracture. The wound is irrigated again and closed from deep to superficial in standard fashion. A shoulder immobilizer was placed.
Postoperative plan
The patient was made non-weight-bearing on the extremity in a shoulder immobilizer. One day after the procedure, patients begin a therapy program with pendulum exercises, passive ROM exercises of the shoulder, and active elbow, wrist, and hand exercises. The patient is given an immobilizer to wear during the first 6 weeks after surgery; the immobilizer can be removed for hygiene activities and light below-shoulder-level activity as pain allows. Formal physical therapy for active assisted and passive ROM is initiated after 2 weeks. After 6 weeks, the immobilizer is discontinued, and patients are allowed to use the arm for light daily activity. Full-weight-bearing activity is permitted at 3 months after surgery.
Clinical outcomes
Forward flexion, abduction, external rotation ROM, incidence of postoperative complications, and deltoid, subscapularis, biceps, and triceps strength at final follow-up. Muscle strength was tested using resistance against the examiner’s hand on a five-point scale.
Patient-reported outcomes
Visual analog scale (VAS) pain (range 0-10), VAS function (0-10), Single Assessment Numeric Evaluation (SANE), American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, and Disabilities of the Arm, Shoulder, and Hand Score (QuickDash).
Radiological outcomes
Preoperative radiographs and computed tomography to assess glenoid morphology using the Walch and Sirveaux classifications (Fig. 1, A–D). Postoperative radiographs to assess glenoid implant and humeral stem status, humeral bone loss, humeral, glenoid, global, and acromiohumeral offset, humeral tuberosity-head height, acromiohumeral distance, glenoid version and inclination, bone graft unionization, and incidence of any radiological complications at final follow-up (Fig. 1, E and F).
Statistical analysis
Preoperative and postoperative clinical and radiographic data were compared. A repeated measures design was used, and continuous variables were compared using parametric t-tests. Mean scores from patient-reported outcome measures before surgery and at follow-up were compared. A P value of .05 was considered significant. Statistical analyses were performed using Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Results
From January 2016 to September 2018, a total of 10 primary TSA surgeries with a humeral head allograft were performed. All patients had postoperative data available and were included in the study. Groupwise demographics are presented for the patients included in Table I.
Table I.
Groupwise demographics (n = 10).
| Characteristic | Value |
|---|---|
| Age (y) (mean ± SD) | 63.4 ± 4.2 |
| Sex (%) | |
| Male | 100% |
| Female | 0% |
| BMI (in kgm−2) (mean ± SD) | 29.2 ± 2.2 |
| Tobacco use (n) | |
| Active smokers | 0 |
| Past smokers | 1 |
| No history | 9 |
| Alcohol use (n) | |
| No use | 5 |
| Rare/Social | 5 |
| Regular | 0 |
| Medical history (%) | |
| Cardiovascular disease | 60% |
| Gastrointestinal | 20% |
| Endocrine | 10% |
| Musculoskeletal | 40% |
SD, standard deviation; BMI, body mass index.
Preoperative and surgical characteristics are presented in Table II. The mean glenoid retroversion was 25.2 ± 10.6 degrees. Two of the patients had previous surgery on the operative shoulder, which included arthroscopy and débridement in one patient and multiple open and arthroscopic surgeries in the other patient. There was one intraoperative complication, which occurred during structural graft fixation. The distal end of a guide pin broke inside one of the cannulated screws used to fix the graft into the glenoid. The decision was made to leave the pin inside the screw/bone since it did not protrude through the scapula and did not obstruct the glenoid implant.
Table II.
Preoperative and surgical characteristics.
| Characteristic | Value |
|---|---|
| Operative side (n) | |
| Right | 8 |
| Left | 2 |
| Previous surgery on operative shoulder (n) | |
| No | 8 |
| Yes∗ | 2 |
| Preoperative diagnosis (n) | |
| Primary osteoarthritis + glenoid bone loss | 9 |
| Primary osteoarthritis + glenoid bone loss + biceps tendonitis | 1 |
| Walch classification (n) | |
| A2 | 1 |
| B1 | 1 |
| B2 | 6 |
| B3 | 2 |
| Sirveaux classification (n) | |
| E1 | 9 |
| E3 | 1 |
| Glenoid defect location (n) | |
| Posterior | 10 |
| Glenoid retroversion (mean ± SD) | −25.2 ± 10.6 |
| Glenoid defect depth (mean ± SD) | 7.8 ± 2.9 |
| Structural graft source (n) | |
| Humeral head | 10 |
| Structural graft fixation method (n) | |
| 3mm cannulated screws | 8 |
| 3.5mm cortical screws | 1 |
| Unspecified | 1 |
| Glenoid implant size (n) | |
| Medium | 7 |
| Large | 3 |
| Humeral off-set (n) | |
| Versa dial A | 2 |
| Versa dial C | 2 |
| Versa dial D | 1 |
| Versa dial E | 5 |
| Intraoperative complications (n)† | 1 |
SD, standard deviation.
Two patients had previous surgeries on their operative shoulder. One patient received an arthroscopic debridement for shoulder osteoarthritis, impingement syndrome, and acromioclavicular joint osteoarthritis. The other patient received four surgeries consisting of two open shoulder reconstructions and two arthroscopic debridement procedures to address injuries related to chronic anterior shoulder dislocations.
During structural graft fixation, the end of a guide pin broke inside a cannulated screw that was fixed into bone. The pin was ultimately left inside since the glenoid implant was not obstructed.
Clinical outcomes are presented in Table III. At an average follow-up of 31.4 ± 24.0 months, active forward flexion, abduction, and external rotation were significantly improved (P < .01). Patient-reported measures revealed improvements at 26.7 ± 24.0 months, with significantly improved scores (P < .05) in VAS pain and function, QuickDASH, SANE, and ASES scores. There was one postoperative clinical complication, which consisted of one patient with a suspected rotator cuff injury after a traumatic fall.
Table III.
Clinical outcomes.
| Assessment | Patient-reported scores (mean ± SD) |
P value | |
|---|---|---|---|
| Preoperative | Postoperative∗ | ||
| Shoulder pain (VAS range 0-10) | 5.3 ± 2.4 | 2.1 ± 2.9∗ | <.001 |
| Shoulder function (VAS range 0-10) | 3.8 ± 2.0 | 7.1 ± 3.5∗ | .012 |
| QuickDASH | 50.87 ± 14.79 | 14.8 ± 14.7∗ | <.001 |
| SANE | 33.20 ± 17.45 | 60.9 ± 34.2∗ | .021 |
| ASES shoulder (surgery side) | 41.86 ± 18.09 | 80.8 ± 25.3∗ | <.001 |
| Active ROM | |||
| Forward flexion | 105 ± 30 | 150 ± 30† | <.001 |
| Abduction | 100 ± 30 | 140 ± 40† | .002 |
| External rotation @ 0° | 20 ± 10 | 60 ± 20† | <.001 |
| Muscle strength | |||
| Deltoid | 5 ± 0.0 | 5 ± 0.3† | >.999 |
| Subscapularis | 5 ± 0.3 | 4 ± 1.1† | .046 |
| Biceps | 5 ± 0.0 | 5 ± 0.0† | >.999 |
| Triceps | 5 ± 0.0 | 5 ± 0.0† | >.999 |
SD, standard deviation; VAS, visual analog scale; QuickDASH, disabilities of the arm, shoulder, and hand score; SANE, single assessment numeric evaluation; ASES, American Shoulder and Elbow Surgeons; ROM, range of motion.
Bold P values were determined to be statistically significant if P < .05.
Time since surgery (mean ± SD) was 26.7 ± 24.0 months.
Time since surgery (mean ± SD) was 31.4 ± 24.0 months.
Postoperative radiographic outcomes are presented in Table IV. At 23.0 ± 20.1 months follow-up, all humeral stems and glenoid implants remained well fixed without lucent lines. There was no evidence of bone loss or scapular notching in each case. The humeral head autograft used in each TSA procedure showed adequate unionization in all cases. Acromiohumeral distance was significantly greater compared to preoperative measurements (P < .05). Glenoid version was significantly improved (P < .001), and glenoid inclination was unchanged from preoperative measurements. No postoperative radiographic complications were reported.
Table IV.
Follow-up radiological findings∗.
| Description | Value |
|---|---|
| Humeral stem status (n) | |
| Well fixed, no lucent lines | 10 |
| Well fixed, lucent lines | 0 |
| Loose | 0 |
| Humeral stem position (n) | |
| Neutral | 10 |
| Valgus | 0 |
| Varus | 0 |
| Humeral bone loss (n) | |
| None | 0 |
| Glenoid implant status (n) | |
| Well fixed, no lucent line | 10 |
| Well fixed, lucent line | 0 |
| Loose | 0 |
| Scapular notching (n) | 0 |
| Bone graft unionized (n) | |
| Yes | 10 |
| No | 0 |
| Preoperative | Postoperative | P value | |
|---|---|---|---|
| Acromiohumeral distance (mm) | 8.8 ± 2.0 | 10.9 ± 2.6 | .044 |
| Glenoid inclination (degrees) | 4.00° ± 4.9° | 5.1° ± 3.1° | .298 |
| Glenoid version (degrees) | −25.2° ± 10.7° | −7.4° ± 0.9° | <.001 |
Bold P values were determined to be statistically significant if P < .05.
Follow-up time (mean ± SD) was 23.0 ± 20.1 months.
Discussion
TSA in patients with glenoid bone loss can be a technically challenging procedure, with multiple reported methods of addressing the area of bone loss reported. Some of these methods include TSA with high side ream, TSA with structural bone grafting, RSA, or RSA with augmented baseplates. Multiple studies have suggested that eccentric reaming of the glenoid has poorer outcomes when the degree of retroversion being corrected is greater than 15 degrees. Over-reaming results in excessive medialization of the joint as well as decreased glenoid contact area and increased contact pressure. For patients with more substantial glenoid bone loss requiring greater correction, TSA with bone grafting or RSA with augmented baseplates are recommended.
Multiple techniques for TSA with structural bone grafting report the use of a fully cemented baseplate. While Neer and Morrison were the first to describe this technique in 1988, studies by Steinmann & Cofield, Hill & Norris, Walch et al, Sabesan et al, Kilka et al, and Nicholson et al utilized cement to fix the metal-backed or all-polyethylene glenoid baseplate. 5,7,8,11, 12, 13,15,17,18 While these studies demonstrate good functional and radiographic outcomes, the use of cement in the area of graft placement may result in incomplete incorporation of the graft into the native glenoid due to leakage of the cement into the interface between the structural graft and native bone. This may portend an increased risk of graft failure in the long term.
In this report, we describe a hybrid technique to perform structural bone grafting in the setting of substantial glenoid bone loss in patients undergoing TSA. We feel that the use of a partially cemented glenoid baseplate with minimal cement surrounding the peripheral pegs in the area of the bone graft may result in a decreased risk of cement leakage into the interface between the graft and native glenoid and therefore decreased risk for nonunion of the graft site. Our experience with this method of graft and baseplate fixation demonstrates that this is a safe and reproducible procedure that adequately addresses glenoid defects in primary TSA with substantial glenoid bone loss.
Prior studies report varying degrees of improvement in ROM postoperatively. Nicholson et al and Kilka et al reported improvement in forward flexion and active elevation of approximately 60° and external rotation of 60° compared to preoperative levels.11,13 Other studies that have investigated bone grafting in TSA showed similar or lesser degrees of improvement in forward flexion and external rotation. Improvements in forward flexion reported by Walch et al, Hill & Norris, and Sabesan et al range from 19° to 44.9°.8,15,18 The same studies as well as Steinmann & Cofield report average improvements in external rotation to range from 15.9° to 30.3°.8,15,17,18
Postoperatively, all patients included in our study experienced improvements in clinical outcomes at 1-year follow-up. Active ROM significantly improved from 105 to 150 degrees of forward flexion (average increase of 45°), 100 to 140 degrees of abduction (average increase of 40°), and 20 to 60 degrees of external rotation (average increase of 40°) at the final follow-up (P < .01). Overall, these results compare similarly to the reported outcomes of recent literature.
Patient-reported outcome scores also improved from preoperative values in this study. VAS pain, VAS function, QuickDASH, SANE, and ASES scores were each observed to have a significant improvement at follow-up (P < .05). These data are similar to prior studies that demonstrated significant improvements in constant, VAS pain, and ASES scores postoperatively.7,13,18
Despite reporting improvements in clinical and patient-reported outcomes, prior studies have reported variable radiographic outcomes. Kilka et al reported high rates of radiolucent lines (RLL) in up to 80% of their patients with 40% of implants deemed at risk of clinical failure, while other studies report various rates of incomplete RLL ranging from 7 to 50%.5,7,8,11, 12, 13,15,17,18 Interestingly, the only study to report no RLL had only 2 of 77 TSA procedures utilize cement.7
All the patients included in this study demonstrated excellent radiographic outcomes observed at follow-up. No radiographic complications were recorded, and each humeral stem and glenoid implant was well fixed without lucent lines at the final follow-up. Compared to preoperative measurements, acromiohumeral distance and glenoid version were significantly improved and optimized for implant mobility and decreased risk of impingement. Furthermore, each autograft showed signs of unionization at follow-up.
One clinical complication was reported at follow-up, which consisted of one patient with decreased ROM and strength due to a suspected rotator cuff injury. This patient-reported adequate function of daily living and did not pursue revision surgery options. There were no patients in this study who required revision surgery, which is similar to revision rates reported by Nicholson et al and Neer & Hill. This contrasts with older studies which report revision rates to be as high as 23.8%.5,7,8,11, 12, 13,15,17,18
This case series demonstrates significant improvements in active shoulder ROM, patient reported, and radiographic outcomes with the use of TSA and structural bone grafting with hybrid fixation in the setting of significant glenoid bone loss. Despite successful results, there are limitations to this study, including a small patient population, lack of a control group, and short-term follow-up. Additionally, the power of this study is low due to the small patient cohort. Thus, future studies are required to obtain more data from larger cohorts with longer-term follow-up to confirm the benefit and reproducibility of TSA with structural bone grafting with hybrid fixation.
Conclusion
The use of TSA with structural bone graft and hybrid fixation technique described in this paper to address significant glenoid bone loss in primary shoulder arthroplasty resulted in improved clinical and radiographic outcomes at short-term follow-up with only one complication reported that was related to trauma sustained during the postoperative period. Hybrid fixation of the glenoid component may decrease the risk of cement leaking into the interface between the native glenoid and bone graft, thus reducing the risk for nonunion and component failure. We believe this is a reliable method to treat substantial glenoid bone loss that results in improved patient outcomes following surgery.
Disclaimers:
Funding: No funding was disclosed by the authors.
Conflicts of interest: Dr. Duquin received royalties and consultant payments from Zimmer Biomet, which is related to the subject of this work. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
This study was approved by the University at Buffalo Institutional Review Board, approval number STUDY00006997.
References
- 1.Bryce C.D., Davison A.C., Okita N., Lewis G.S., Sharkey N.A., Armstrong A.D. A biomechanical study of posterior glenoid bone loss and humeral head translation. J Shoulder Elbow Surg. 2010;19:994–1002. doi: 10.1016/j.jse.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Clavert P., Millett P.J., Warner J.J. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16:843–848. doi: 10.1016/j.jse.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Day J.S.P., Lau E.M.S., Ong K.L.P., Williams G.R., Ramsey M.L., Kurtz S.M.P. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19:1115–1120. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Farley K.X., Wilson J.M., Daly C.A., Gottschalk M.B., Wagner E.R. The incidence of shoulder arthroplasty: rise and future projections compared to hip and knee arthroplasty. JSES Open Access. 2019;3:244. doi: 10.1016/j.jses.2019.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Gazielly D.F., Scarlat M.M., Verborgt O. Long-term survival of the glenoid components in total shoulder replacement for arthritis. Int Orthop. 2015;39:285–289. doi: 10.1007/s00264-014-2637-y. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie R., Lyons R., Lazarus M. Eccentric reaming in total shoulder arthroplasty: a cadaveric study. Orthopedics. 2009;32:21. doi: 10.3928/01477447-20090101-07. [DOI] [PubMed] [Google Scholar]
- 7.Habermeyer P., Magosch P., Lichtenberg S. Recentering the humeral head for glenoid deficiency in total shoulder arthroplasty. Clin Orthop Relat Res. 2007;457:124–132. doi: 10.1097/BLO.0b013e31802ff03c. [DOI] [PubMed] [Google Scholar]
- 8.Hill J.M., Norris T.R. Long-term results of total shoulder arthroplasty following bone grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. [PubMed] [Google Scholar]
- 9.Iannotti J.P., Norris T.R. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85:251–258. doi: 10.2106/00004623-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Khazzam M., Gee A.O., Pearl M. Management of glenohumeral joint osteoarthritis. J Am Acad Orthop Surg. 2020;28:781–789. doi: 10.5435/jaaos-d-20-00404. [DOI] [PubMed] [Google Scholar]
- 11.Klika B.J., Wooten C.W., Sperling J.W., Steinmann S.P., Schleck C.D., Harmsen W.S., et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1066–1072. doi: 10.1016/j.jse.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Neer C.S., Morrison D.S. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70:1154–1162. [PubMed] [Google Scholar]
- 13.Nicholson G.P., Cvetanovich G.L., Rao A.J., O'Donnell P. Posterior glenoid bone grafting in total shoulder arthroplasty for osteoarthritis with severe posterior glenoid wear. J Shoulder Elbow Surg. 2017;26:1844–1853. doi: 10.1016/j.jse.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Nowak D.D., Bahu M.J., Gardner T.R., Dyrszka M.D., Levine W.N., Bigliani L.U., et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18:680–688. doi: 10.1016/j.jse.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Sabesan V., Callanan M., Ho J., Iannotti J.P. Clinical and radiographic outcomes of total shoulder arthroplasty with bone graft for osteoarthritis with severe glenoid bone loss. J Bone Joint Surg Am. 2013;95:1290–1296. doi: 10.2106/JBJS.L.00097. [DOI] [PubMed] [Google Scholar]
- 16.Sears B.W., Johnston P.S., Ramsey M.L., Williams G.R. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20:604–613. doi: 10.5435/jaaos-20-09-604. [DOI] [PubMed] [Google Scholar]
- 17.Steinmann S.P., Cofield R.H. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9:361–367. doi: 10.1067/mse.2000.106921. [DOI] [PubMed] [Google Scholar]
- 18.Walch G., Moraga C., Young A., Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21:1526–1533. doi: 10.1016/j.jse.2011.11.030. [DOI] [PubMed] [Google Scholar]