Abstract
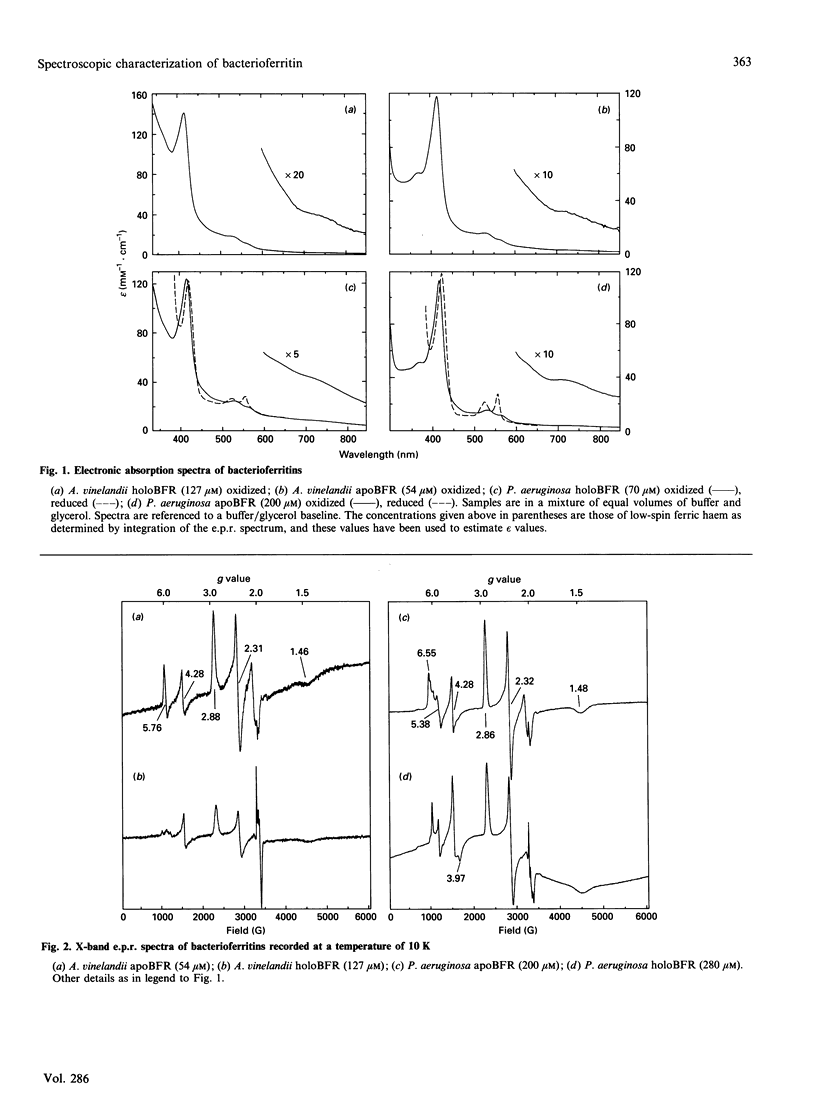
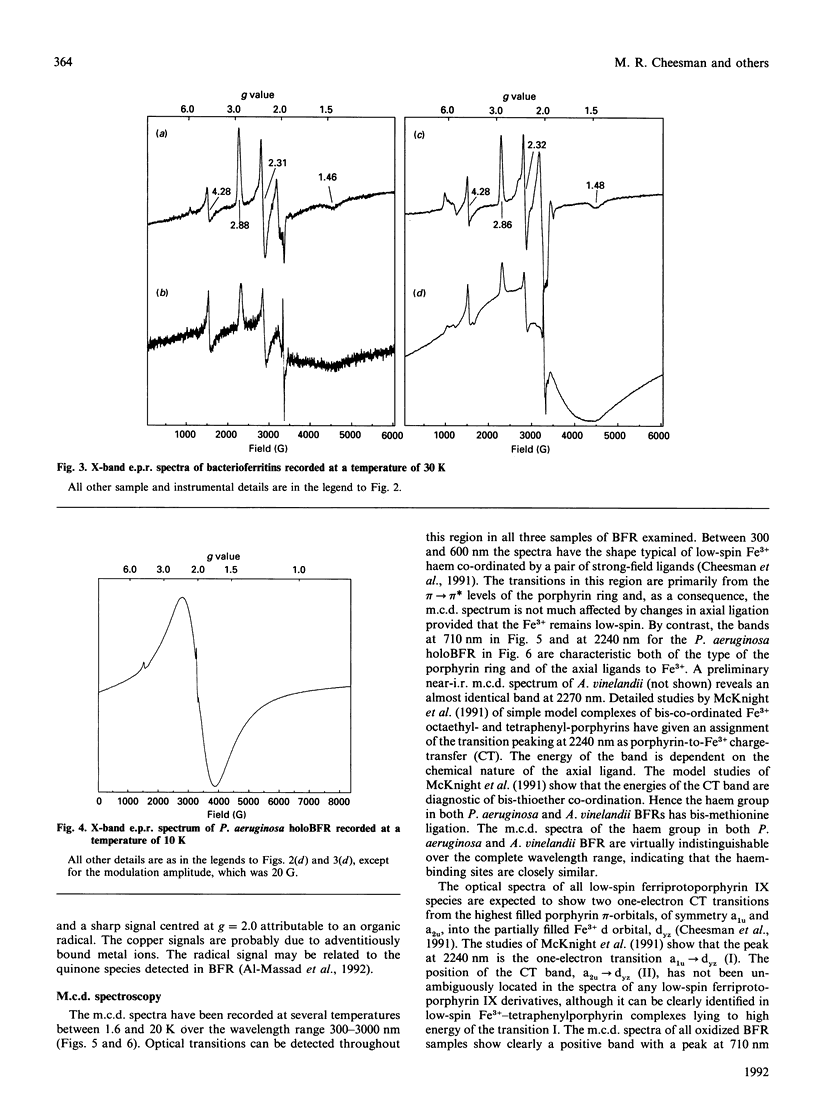
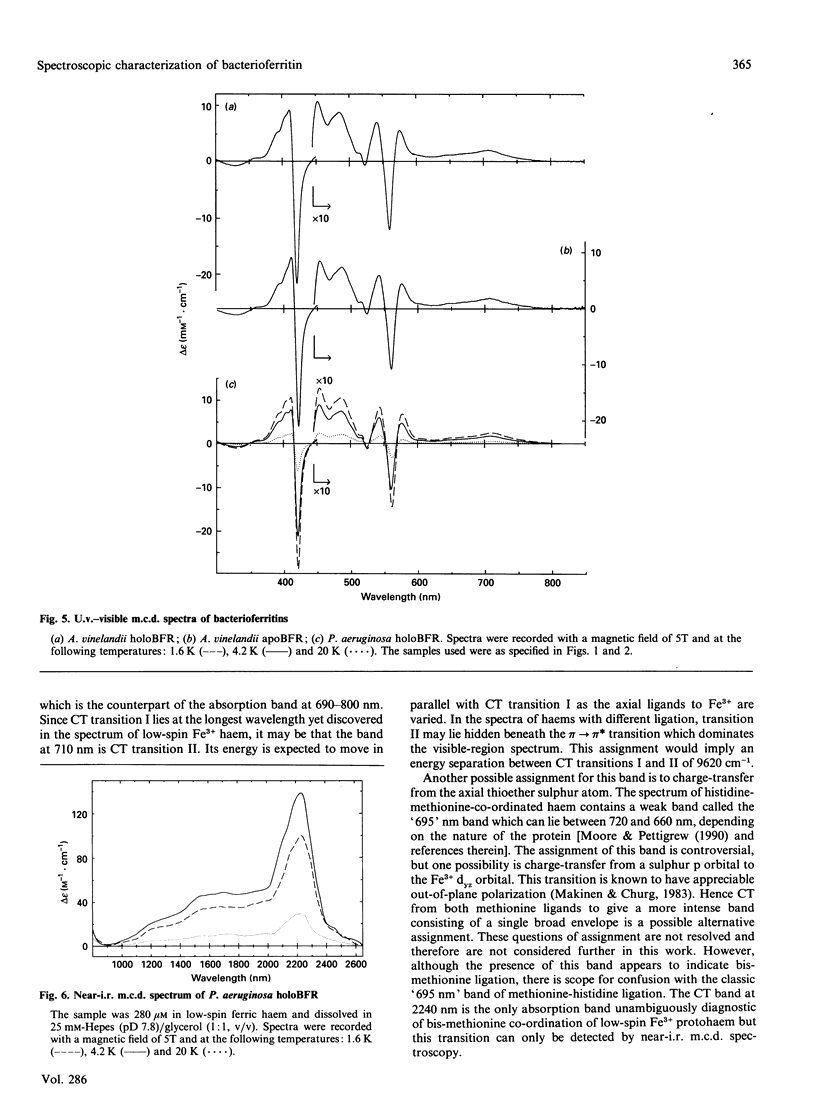
The e.p.r. and magnetic circular dichroism (m.c.d.) spectra of bacterioferritin (BFR) extracted from Pseudomonas aeruginosa and Azotobacter vinelandii have been studied over a wide temperature range down to liquid-helium temperature. The e.p.r. spectra show the presence of low-spin Fe3+ haem with g values of 2.86, 2.32, 1.48 (P. aeruginosa) and 2.88, 2.31, 1.46 (A. vinelandii), in both the presence and absence of the BFR core. Together with evidence from the porphyrin-to-Fe3+ charge-transfer band at 2240 and 2270 nm the axial haem ligands are identified as two methionines. The low-temperature m.c.d. spectra in the region 300-1000 nm of P. aeruginosa and A. vinelandii BFR are identical with one another and unaffected by removal of the iron core. Hence it can be concluded that the presence of the iron core has no detectable effect on the electronic states and on the stereochemistry of the haem group. This was unexpected, in view of the observations by Watt, Frankel, Papaefthymiou, Spartalian & Stiefel [(1986) Biochemistry 25, 4330-4336] that the redox potential of the haem group in A. vinelandii BFR shifts from -475 mV to -225 mV on removal of the core. The e.p.r. spectra of holoBFR show a broad symmetrical derivative-shaped band centred at g = 2.0 which decreases in bandwidth as the temperature is raised. This signal is assigned to the uncompensated electron spins of the iron core.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Smith J. M., Guest J. R., Harrison P. M. Amino acid sequence of the bacterioferritin (cytochrome b1) of Escherichia coli-K12. Biochem Biophys Res Commun. 1989 Jan 31;158(2):489–496. doi: 10.1016/s0006-291x(89)80075-0. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Smith J. M., Yewdall S. J., Guest J. R., Harrison P. M. Bacterioferritins and ferritins are distantly related in evolution. Conservation of ferroxidase-centre residues. FEBS Lett. 1991 Nov 18;293(1-2):164–168. doi: 10.1016/0014-5793(91)81177-a. [DOI] [PubMed] [Google Scholar]
- Boas J. F., Troup G. J. Electron spin resonance and Mössbauer effect studies of ferritin. Biochim Biophys Acta. 1971 Jan 19;229(1):68–74. doi: 10.1016/0005-2795(71)90319-9. [DOI] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R., Lough S. A hemoprotein from azotobacter containing non-heme iron: isolation and crystallization. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1274–1281. doi: 10.1016/0006-291x(73)91125-x. [DOI] [PubMed] [Google Scholar]
- Cheesman M. R., Thomson A. J., Greenwood C., Moore G. R., Kadir F. Bis-methionine axial ligation of haem in bacterioferritin from Pseudomonas aeruginosa. Nature. 1990 Aug 23;346(6286):771–773. doi: 10.1038/346771a0. [DOI] [PubMed] [Google Scholar]
- Crichton R. R. Proteins of iron storage and transport. Adv Protein Chem. 1990;40:281–363. doi: 10.1016/s0065-3233(08)60288-0. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Laulhère J. P., Labouré A. M., Van Wuytswinkel O., Gagnon J., Briat J. F. Purification, characterization and function of bacterioferritin from the cyanobacterium Synechocystis P.C.C. 6803. Biochem J. 1992 Feb 1;281(Pt 3):785–793. doi: 10.1042/bj2810785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch F. A., Moore G. R., Pettigrew G. W. Structural basis for the variation of pH-dependent redox potentials of Pseudomonas cytochromes c-551. Biochemistry. 1984 Apr 10;23(8):1831–1838. doi: 10.1021/bi00303a039. [DOI] [PubMed] [Google Scholar]
- Mann S., Bannister J. V., Williams R. J. Structure and composition of ferritin cores isolated from human spleen, limpet (Patella vulgata) hemolymph and bacterial (Pseudomonas aeruginosa) cells. J Mol Biol. 1986 Mar 20;188(2):225–232. doi: 10.1016/0022-2836(86)90307-4. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Mann S., Bannister J. V. Isolation and properties of the complex nonheme-iron-containing cytochrome b557 (bacterioferritin) from Pseudomonas aeruginosa. J Inorg Biochem. 1986 Oct-Nov;28(2-3):329–336. doi: 10.1016/0162-0134(86)80097-6. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- St Pierre T. G., Bell S. H., Dickson D. P., Mann S., Webb J., Moore G. R., Williams R. J. Mössbauer spectroscopic studies of the cores of human, limpet and bacterial ferritins. Biochim Biophys Acta. 1986 Mar 7;870(1):127–134. doi: 10.1016/0167-4838(86)90015-4. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Frankel R. B., Papaefthymiou G. C. Reduction of mammalian ferritin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3640–3643. doi: 10.1073/pnas.82.11.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Massad F. K., Kadir F. H., Moore G. R. Animal ferritin and bacterioferritin contain quinones. Biochem J. 1992 Apr 1;283(Pt 1):177–180. doi: 10.1042/bj2830177. [DOI] [PMC free article] [PubMed] [Google Scholar]


