Abstract
Background:
The global outbreak of coronavirus disease 2019 (COVID-19) presents numerous obstacles for healthcare professionals. The present study aimed to evaluate and compare the role of serum biomarkers like- C-reactive protein (CRP), interleukin-6 (IL-6), and D-dimers in the severity of COVID-19 infection.
Methodology:
A cross-sectional, observational retrospective pilot study was conducted in Udaipur, Rajasthan, wherein data was collected from 250 subjects, out of which, data of 100 subjects were included as per the inclusion criteria. The data was recorded retrospectively among the health professionals via Google Forms in Udaipur, Rajasthan.
Results:
There were 1 (1%), 3 (3%), 31 (31%) and 65 (65%) participants with minor elevation (0.3-1.0), moderate elevation (1-10), marked elevation (10-50) and severe elevation (>50) of CRP respectively. The difference between the groups was statistically highly significant with a significantly higher number of study participants with a severe elevation of CRP levels (χ2 = 107.84, P < 0.001). The results showed that there was a significant difference between the groups with IL6 in 0-7 range while 96 (96%) study participants had >7 IL6, and the difference was statistically highly significant (2 = 84.640, P 0.001).
Conclusion:
In conclusion, the existing body of research indicates a discernible correlation between COVID-19 infection and the fluctuation of biomarker levels. This supplement has the potential to be utilised in clinical practice as a means of informing treatment decisions and determining the necessity of admission to the intensive care unit (ICU).
Keywords: ABO blood grouping, biomarker, covid-19, CRP, D-dimer, IL-6
INTRODUCTION
The global COVID-19 pandemic creates many challenges for healthcare practitioners. Rapid diagnosis and hospitalization, risk classification, good use of intensive care resources, appropriate therapies, close patient monitoring, and prompt discharge are essential to saving lives. Clinicians must use clinical assessment, but biomarkers can provide additional, objective data that can dramatically impact healthcare. Instead of a localised 11 respiratory infection, COVID-19 is a systemic disease caused by complex interactions between the immunological, inflammatory, and coagulative pathways.[1]
The SARS-CoV-2 virus was formally identified as the cause of COVID-19. Although a large percentage of people have recovered from COVID-19, over 45% may experience protracted COVID symptoms for four months after the initial SARS-CoV-2 infection.[2,3] A study[4] suggests that elderly people may have a lower risk of long-term COVID. Females are more likely than males to have persistent COVID-19 symptoms.[5] Long-term COVID causes neurological, neuropsychiatric, cardiac, and gastrointestinal symptoms.[4] Several studies have connected protracted COVID to tiredness (29–58%), headache (10–44%), and anxiety or depression (22–28%).[6,7,8,9] Many long-term COVID patients with pulmonary symptoms report dyspnea or respiratory discomfort (21–24%) and anosmia or ageusia (12–15%).[8,9] Female patients with protracted COVID syndrome are more likely than male patients to develop neurological, neuropsychiatric, cardiac, gastrointestinal, and rheumatological issues.[10] Moreover, long-term COVID pulmonary or neurological problems may affect work performance and domestic responsibilities.[7,11]
COVID-19 has a wide range of clinical severity. Research shows that 30-60% of COVID-19 patients had no symptoms or minimal symptoms. About 5% of those with symptoms are extremely unwell.[12] Multiorgan failure and respiratory compromise characterise severe COVID-19. Advanced age, male gender, and chronic health conditions as such diabetes, cardiovascular disease, immunosuppression, and obesity are risk factors for severe illness. Clinical symptoms very quickly, and severe cases can cause hypoxia, organ failure, and death. Still, no reliable indications exist for illness severity and progression.[13] Biomarkers are quantifiable attributes used to identify disease presence, monitor clinical progression, interpret responses to interventions, predict treatment response, identify high-risk populations, and identify susceptibility or risk factors.[14,15,16,17,18] Biomarkers are becoming a key diagnostic tool for SARS-CoV-2 treatment. Immunological indicators can measure the immune response and predict COVID-19 prognosis.[19] Biomarkers for severe organ failure have been found recently. Serum ferritin, CRP, d-dimer, and procalcitonin are biomarkers. These studies also underline the use of biomarkers in identifying patients at risk of poor outcomes. There is little study on immunological markers and COVID-19 prognosis and severity in India. Thus, this study examined how IL-6, CRP, and D-dimer levels affect severe COVID-19 infection in hospitalised patients. SARS-CoV-2, the cause of severe acute respiratory syndrome, has many symptoms, including multiorgan dysfunction. COVID-19 can alter biomarkers, which can be used to identify, predict, and assess illness progression and consequences.[20] Hence, this study investigated the impact of blood biomarkers such as CRP, IL-6, and D-dimers on the severity of COVID-19 infection.
METHODOLOGY
In the present retrospective study, wherein data was collected from over 250 subjects, out of which, data of 100 subjects were included as per the inclusion criteria. The data was collected from private laboratories, and it included the Age, Gender, IL-6, CRP and D-dimer values of hospitalised. The data was recorded retrospectively among the health professionals via Google Forms in Udaipur, Rajasthan.
The age group taken into consideration was between 20 and 50 years. The patients with comorbidities or any other systemic illness were excluded from the study. The data was tabulated and sent for statistical correlation. Statistical analysis was performed using IBM, SPSS version 26. Standard descriptive and analytics statistics will be used to analyze the data. The Chi-square test was used to test for significant differences and P-value ≤0.05 will be considered significant.
RESULTS
Table 1 and Figure 1 show the distribution of study participants according to gender. There were 65 (65%) males and 35 (35%) females in the study.
Table 1.
Distribution of study participants according to demographic details
| Demographic Parameter | Frequency | Percentage | Mean Age with Std. deviation |
|---|---|---|---|
| Males | 65 | 65% | 53.03±13.11 |
| Females | 35 | 35% | 56.68±12.03 |
| Total | 100 | 100% |
Figure 1.
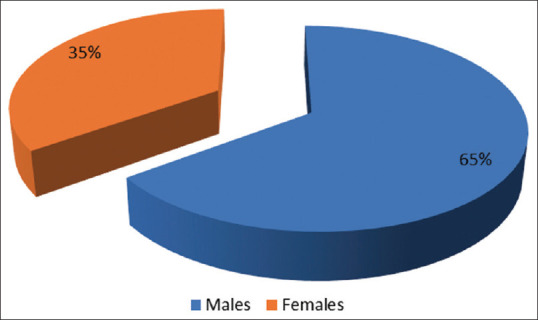
Distribution of study participants according to the Gender
Table 2 and Figure 2 show the distribution of study participants according to CRP levels. There were 1 (1%), 3 (3%), 31 (31%) and 65 (65%) participants with minor elevation (0.3-1.0), moderate elevation (1-10), marked elevation (10-50) and severe elevation (>50) of CRP respectively. The difference between the groups was statistically highly significant with a significantly higher number of study participants with a severe elevation of CRP levels (χ2 = 107.84, P < 0.001).
Table 2.
Distribution of study participants according to CRP levels
| CRP levels | n | % |
|---|---|---|
| 0-0.3 (Normal) | 0 | 0% |
| 0.3-1.0 (Minor elevation) | 1 | 1% |
| 1.0-10 (Moderate elevation) | 3 | 3% |
| 10-50 (Marked elevation) | 31 | 31% |
| >50 (Severe elevation) | 65 | 65% |
| Total | 100 | 100% |
| χ2, P | χ2=107.84, P<0.001** | |
**Highly Significant
Figure 2.
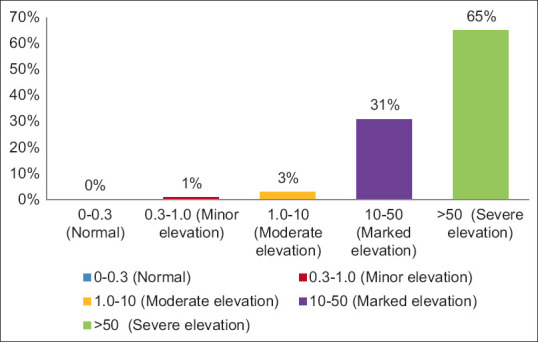
Distribution of study participants according to CRP levels
Table 3 and Figure 3 show the distribution of study participants according to IL6 levels. There were 4 (4%) study participants with IL6 in 0-7 range while 96 (96%) study participants had >7 IL6 levels and the difference between the groups was statistically highly significant (χ2 = 84.640, P < 0.001).
Table 3.
Distribution of study participants according to IL6 levels
| IL6 | n | % |
|---|---|---|
| 0-7 | 4 | 4% |
| >7 | 96 | 96% |
| Total | 100 | 100% |
| χ2, P | χ2=84.640, P<0.001** | |
**Highly Significant
Figure 3.
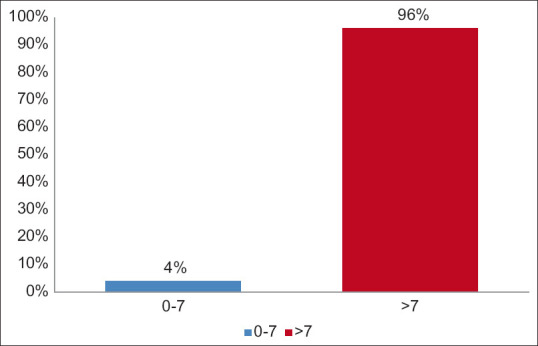
Distribution of study participants according to IL6 levels
Table 4 and Figure 4 show the distribution of study participants according to D-dimer levels. All the study participants [100 (100%)] had positive D-dimer values.
Table 4.
Distribution of study participants according to D-dimer levels
| D-dimer | n | % |
|---|---|---|
| 0-0.5 (Normal) | 0 | 0% |
| >0.5 (Positive) | 100 | 100% |
| Total | 100 | 100% |
| χ2, P | a-Cannot be computed | |
Figure 4.
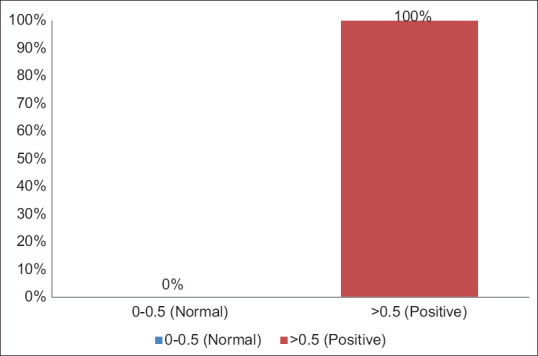
Distribution of study participants according to D-dimer levels
DISCUSSION
The emergence of Coronavirus disease 2019 (COVID-19) may be traced back to the West District of the Southern China Seafood Wholesale Market in late December 2019. This infectious disease has garnered global attention and has been officially classified as a pandemic by the World Health Organization (WHO). COVID-19 predominantly induces infection in the lower respiratory tract, manifesting as symptoms such as cough, fever, dyspnea, and lethargy.[21] However, it is noteworthy that this viral illness can also give rise to consequences affecting the cardiovascular and immunological systems, including single or multi-organ failure and disseminated intravascular coagulation (DIC).[21,22]
The utilization of biomarkers in the evaluation of patients diagnosed with COVID-19 infection can provide valuable assistance to doctors in initiating appropriate therapeutic interventions and facilitating diligent surveillance. The potential utility of biomarkers in enhancing prognosis and outcomes is tempered by the substantial interpatient heterogeneity observed, which may introduce confounding factors into research findings.[23,24] The majority of research have employed many biomarkers longitudinally and have observed their utility in evaluating the prognosis and adjusting the therapeutic approach for individuals affected by COVID-19. The current investigation aimed to evaluate the contribution of a composite of three blood biomarkers, specifically C-reactive protein (CRP), interleukin-6 (IL-6), and D-dimers. The threshold values for all three biomarkers were determined based on the criteria established by Nehring et al.,[25] Said et al.[26] and Bounds et al.[27]. The patients were classified based on the predetermined threshold values of each biomarker.
The CRP levels were compared in the study participants, and it was noted that there were 1 (1%), 3 (3%), 31 (31%) and 65 (65%) participants with minor elevation (0.3-1.0), moderate elevation (1-10), marked elevation (10-50) and severe elevation (>50) of CRP respectively. The difference between the groups was statistically highly significant with a significantly higher number of study participants with a severe elevation of CRP levels (χ2 = 107.84, P < 0.001). Most of our participants who were hospitalised showed a severe elevation in CRP and this shows that CRP levels may have a significant role in the progression of COVID-19 infection. The retrospective single-center study conducted in Wuhan; China has brought attention to the utilization of CRP in the context of COVID-19. The study revealed that the severe cohort exhibited notably elevated levels of CRP compared to the non-severe cohort (57.9 mg/L vs 33.2 mg/L, P < 0.001).[28] In a subsequent retrospective cohort analysis, it was observed that individuals with C-reactive protein (CRP) levels over 41.8 mg/L exhibited an elevated probability of developing severe COVID-19 illness.[14] Both investigations indicate that levels of C-reactive protein (CRP) serve as a robust biomarker for assessing the presence and severity of COVID-19 infection.[24] Moreover, a study based on unpublished results posits that C-reactive protein (CRP) exhibits early alterations in blood plasma, serving as a potential biomarker for physiological problems. If corroborated, CRP could emerge as the most efficacious biomarker for prognosticating the advancement of COVID-19 infection. In contrast, another study also demonstrated instances of infection that exhibited alterations in serum amyloid A (SAA) levels, rather than eliciting notable changes in C-reactive protein (CRP) levels. Consequently, additional assessment is necessary in these cases.[24]
All COVID-19 patients in our study had D-dimer values >0.5, which is significant. Thus, group comparisons were impossible. Since most of our patients are hospitalised, severe COVID-19 infection raises D-dimer levels. After thrombotic episodes, D-dimer levels rise, indicating fibrinolysis.[29] Researchers examined D-dimer levels in critical COVID-19 pneumonia patients and their risk of VTE, disease severity, and fatality.[30] D-dimer levels were linked to poor prognosis and higher mortality in this illness.[31] COVID-19 patients with Systemic Inflammatory Response Syndrome (SIRS) had elevated D-Dimer levels due to the coagulation cascade.[32] Zou et al.[33] found a connection between high D-dimer levels and sickness severity in 129 Shanghai Public Health Clinical Centre COVID-19 patients. The data showed that mild and severe sickness increased D-dimer levels by 10 times the (upper limit of normal) ULN, respectively. Clearly, Mucha et al.[34] set the threshold value for high-risk patients as 6 times the upper limit, or 3000 ng/mL FEU. Artifoni[35] found that a D-dimer level of ≥1.0 μg/mL had a 44% positive predictive value, while a level of ≥3.0 μg/mL had a 67% positive predictive value in 65 out of 71 COVID-19 patients with VTE. The relationship has been found in deep vein thrombosis and pulmonary embolism. Demelo-Rodríguez et al.[29] found a correlation between D-dimer levels and DVT risk in 156 COVID-19 patients who were not admitted to the ICU. In DVT patients, D-dimer levels were 4527 ng/mL, while in those without DVT, they were 2050. In a separate cross-sectional investigation, COVID-19 patients with elevated D-dimer levels upon admission had a higher risk of proximal deep vein thrombosis (DVT).[23] In addition, D-dimer, urea, respiration rate, blood pressure, age 65 or older, and the CURB-65 score independently increased the risk of distal DVT in COVID-19 patients. Another study[36] found that 88.5% of patients with a D-dimer level above 1.0 μg/mL experienced deep vein thrombosis (DVT), but only 15.9% of COVID-19 patients with d-dimer had DVT.
In the present study, there were 4 (4%) study participants with IL6 in 0-7 range while 96 (96%) study participants had >7 IL6 levels and the difference between the groups was statistically highly significant (χ2 = 84.640, P < 0.001). Usually, the IL-6 levels are considered to be normal up to 7 pg/ml of blood. It is noted by Guirao et al.,[37] mild covid-19 infection patients had a mean IL-6 value of 7.66 whereas the IL-6 values were elevated to more than 320 in severely infected cases. This is in accordance with our study as we found the levels elevated above the normal IL-6 levels in our study subjects. In a study conducted by Mucha,[34] the phenomenon known as cytokine storm was elucidated as a central feature of the pathophysiology of COVID-19. This condition is distinguished by elevated concentrations of inflammatory markers, such as IL-1 and IL-6, which induce thrombosis by stimulating platelets, endothelial cells, monocytes, and the tissue factor VIIa pathway. Moreover, these substances impede the process of fibrinolysis and the functioning of endogenous anticoagulants, such as protein C and S.
Interleukin-6 (IL-6) is a versatile cytokine involved in the transmission of cellular signals and the regulation of immune cells. This factor exhibits a potent proinflammatory effect and possesses various biological roles, making it a significant contributor to the pathogenesis of inflammation, tumorigenesis, and hematological disorders.[34,35] Interleukin-6 (IL-6) serves as the principal initiator of cytokine storms.[36] According to the findings of Yang et al., it was shown that the levels of IL-6 in peripheral blood can serve as an independent predictor for the advancement of COVID-19. This aligns with the outcomes of the present investigation, emphasizing the significance of IL-6 in the context of this disease. Consequently, the role of IL-6 in COVID-19 warrants further consideration.[14] Ranucci et al.[37] established a correlation between the levels of interleukin-6 (IL-6) in patients with COVID-19 and the occurrence of acute respiratory distress syndrome (ARDS) necessitating mechanical ventilation. The findings indicated a direct correlation between the levels of IL-6 and fibrinogen, so establishing a connection between inflammation and alterations in procoagulant activity. A parallel analysis was conducted, which demonstrated a correlation between elevated levels of IL-6 and an increased risk of mortality in individuals diagnosed with COVID-19.[38]
Research findings have indicated that there is a significant increase in the levels of IL-6, which is the predominant cytokine generated by activated macrophages, in severe presentations of COVID-19.[39] Nevertheless, due to the predominantly observational nature of existing studies, it remains challenging to infer if the observed increase is of sufficient magnitude to induce the clinical signs observed in severe cases. A meta-analysis was conducted to examine six studies, which revealed that the average IL-6 concentrations were 2.9 times higher in patients with complex COVID-19 compared to those with non-complicated disease. The sample size for this analysis was 1302, and the confidence interval was reported as 1.17 to 7.19 with a 95% level of confidence.[40] The analysis encompasses many outcomes of the research, such as admission to the Intensive Care Unit (ICU), the development of acute respiratory distress syndrome (ARDS), and mortality.[41,42] Given the observed correlation between the proportional increase of IL-6 and the severity of the condition, this study has the potential to make significant advancements in the field. While clinicians can utilise this method to detect severity at an earlier stage and initiate oxygen therapy promptly, the diverse results pose challenges in determining the specific IL-6 level associated with each unfavourable event.[24]
CONCLUSION
In summary, the existing body of research indicates a discernible correlation between the severity of COVID-19 infection and the fluctuation of biomarker levels.[43,44] This supplement has the potential to be utilised in clinical practice as a means of informing treatment decisions and determining the necessity of admission to the intensive care unit (ICU). By implementing this approach, it has the potential to enhance the prognosis and mitigate the rates of mortality. The pathological occurrences are evident through abnormal laboratory indicators, such as CRP, D-dimers, and IL-6. Nevertheless, given the limited knowledge regarding the pathogenesis of this infectious disease, it is imperative to advocate for additional global research endeavors to enhance comprehension of the observed alterations highlighted in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Samprathi M, Jayashree M. Biomarkers in COVID-19: An up-to-date review. Front Pediatrics. 2021;8:607647. doi: 10.3389/fped.2020.607647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vodnar DC, Mitrea L, Teleky BE, Szabo K, Călinoiu LF, Nemeş SA, et al. Coronavirus disease (COVID-19) caused by (SARS-CoV-2) infections: A real challenge for human gut microbiota. Front Cell Infect Microbiol. 2020;10:575559. doi: 10.3389/fcimb.2020.575559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long Covid among hospitalized and non-hospitalized populations: A systematic review and meta-analysis. EClinicalMedicine. 2023;55:101762. doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28:1706–14. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis. 2022;226:1593–607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. Health outcomes in people 2 years after surviving hospitalization with COVID-19: A longitudinal cohort study. Lancet Respir Med. 2022;10:863–76. doi: 10.1016/S2213-2600(22)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, et al. A systematic review and meta-analysis of long COVID symptoms. Syst Rev. 2023;12:88. doi: 10.1186/s13643-023-02250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester SV, Rusu R, Chan B, Bellows M, O’Keefe C, Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: A review. Curr Med Res Opin. 2022;38:1391–9. doi: 10.1080/03007995.2022.2081454. [DOI] [PubMed] [Google Scholar]
- 11.Lai YJ, Liu SH, Manachevakul S, Lee TA, Kuo CT, Bello D. Biomarkers in long COVID-19: A systematic review. Front Med (Lausanne) 2023;10:1085988. doi: 10.3389/fmed.2023.1085988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. N Engl J Med. 2020;383:2451–60. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 13.Li X, To KKW. Biomarkers for severe COVID-19. EBioMedicine. 2021;68:103405. doi: 10.1016/j.ebiom.2021.103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243:213–21. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–99. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keddie S, Ziff O, Chou MKL, Taylor RL, Heslegrave A, Garr E, et al. Laboratory biomarkers associated with COVID-19 severity and management. Clin Immunol. 2020;221:108614. doi: 10.1016/j.clim.2020.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolopoulos ID, Aznaouridis SI, Tzani MA. Extracting possibly representative COVID-19 biomarkers from X-ray images with deep learning approach and image data related to pulmonary diseases. J Med Biol Eng. 2020;40:462–9. doi: 10.1007/s40846-020-00529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider M. The role of biomarkers in hospitalized COVID-19 patients with systemic manifestations. Biomark Insights. 2022;17:11772719221108909. doi: 10.1177/11772719221108909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battaglini D, Robba C, Ball L, Silva PL, Cruz FF, Pelosi P, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: A narrative review. Br J Anaesth. 2021;127:353–64. doi: 10.1016/j.bja.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L, et al. COVID-19 related coagulopathy: A distinct entity? J Clin Med. 2020;9:1651. doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eljilany I, Elzouki AN. D-Dimer, fibrinogen, and IL-6 in COVID-19 patients with suspected venous thromboembolism: A narrative review. Vasc Health Risk Manag. 2020;16:455–62. doi: 10.2147/VHRM.S280962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 –A systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nehring SM, Goyal A, Patel BC. StatPearls. StatPearls Publishing; Treasure Island (FL): 2023. C Reactive Protein. [PubMed] [Google Scholar]
- 26.Said EA, Al-Reesi I, Al-Shizawi N, Jaju S, Al-Balushi MS, Koh CY, et al. Defining IL-6 levels in healthy individuals: A meta-analysis. Journal of medical virology. 2021;93:3915–24. doi: 10.1002/jmv.26654. [DOI] [PubMed] [Google Scholar]
- 27.Bounds EJ, Kok SJ. StatPearls. StatPearls Publishing; Treasure Island (FL): 2023. D Dimer. [PubMed] [Google Scholar]
- 28.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macías M, Toledo-Samaniego N, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–6. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porfidia A, Pola R. Venous thromboembolism in COVID-19 patients. J Thromb Haemost. 2020;18:1516–7. doi: 10.1111/jth.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296:E189–91. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Y, Guo H, Zhang Y, Zhang Z, Liu Y, Wang J, et al. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci Trends. 2020;14:285–9. doi: 10.5582/bst.2020.03086. [DOI] [PubMed] [Google Scholar]
- 34.Mucha SR, Dugar S, McCrae K, Joseph D, Bartholomew J, Sacha GL, et al. Coagulopathy in COVID-19: Manifestations and management. Cleve Clin J Med. 2020;87:461–8. doi: 10.3949/ccjm.87a.ccc024. [DOI] [PubMed] [Google Scholar]
- 35.Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: Incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–6. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: Prevalence, risk factors, and outcome. Circulation. 2020;142:114–28. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 37.Guirao JJ, Cabrera CM, Jiménez N, Rincón L, Urra JM. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Molecular immunology. 2020;128:64–8. doi: 10.1016/j.molimm.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Lai HS, Lin WH, Lai SL, Lin HY, Hsu WM, Chou CH, et al. Interleukin-6 mediates angiotensinogen gene expression during liver regeneration. PLoS One. 2013;8:e67868. doi: 10.1371/journal.pone.0067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang P, Ding Y, Xu Z, Pu R, Li P, Yan J, et al. Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China. Epidemiology. 2020 doi:10.1101/2020.02.28.20028068. [Google Scholar]
- 41.Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–51. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]


