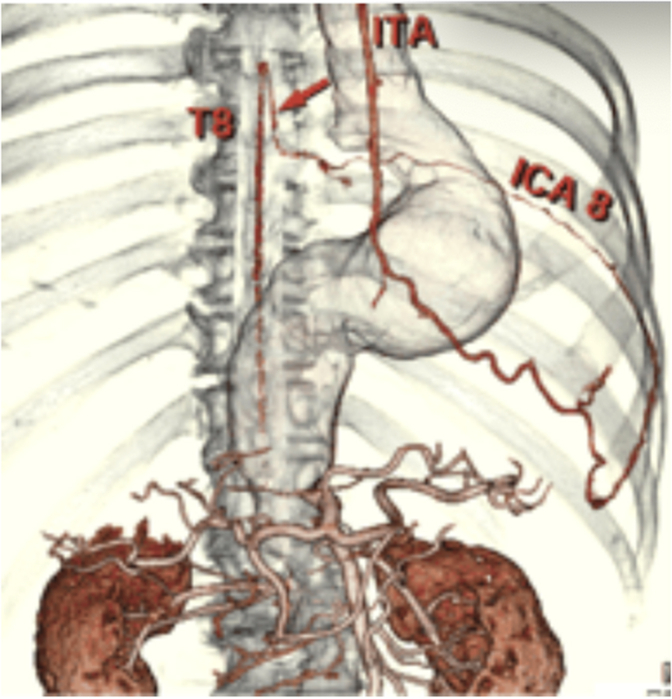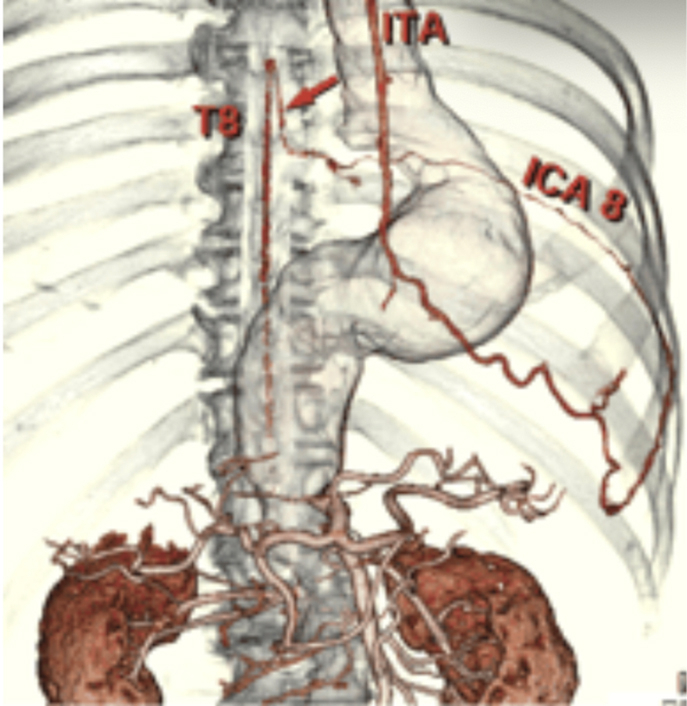
CT demonstrating variant collateral circulation of the LIMA and artery of Adamkiewicz.
Central Message.
We propose a potential benefit in preoperative screening for abdominal aortic occlusions prior to undergoing LIMA-LAD grafting to prevent a potentially devastating complication.
Paraplegia is a well-documented complication after surgery involving the thoracic aorta, such as aortic dissection or aneurysm repair, affecting up to 22% of patients after type II thoracoabdominal aneurysm repair using a conventical open method.1 However, there is sparse literature on the mechanism and prevention of the much rarer postoperative paraplegia after nonaortic cardiac surgery, such as during coronary artery bypass grafting (CABG).2 Leriche syndrome is one known cause of acute paraplegia, attributable to decreased perfusion through the common iliac arteries. A second mechanism is through spinal ischemia after surgery.1,3 The goal of this report is to expand the discussion to determine changes in medical management of patients undergoing CABG to avoid this debilitating complication. Written informed consent was obtained from patient. Institutional review board approval was not required.
Case Presentation
A 61-year-old man with a 40 pack-year smoking history was admitted to the emergency department for shortness of breath. Medical history included hypertension, chronic obstructive pulmonary disease, and coronary artery disease treated with balloon angioplasty. In the emergency department, the patient was treated with antihypertensive medications, antiplatelet medications, and bronchodilators.
Laboratory studies showed elevated brain natriuretic peptide (2432 pg/mL), troponin (27 ng/mL), and D-dimer (0.72 μ/mL). Echocardiography and left heart catheterization through the right radial artery showed multiple-vessel disease and severe aortic insufficiency with an ejection fraction of 30%. Aortic valve replacement and CABG were recommended.
The patient was placed on cardiopulmonary bypass with the average mean arterial pressure in the 60 to 80 mm Hg range. Anterograde and retrograde cardioplegia was administered and the aortic valve was replaced with a 21-mm Carpentier-Edwards Perimount Magna Ease bioprothesis. The surgery also included a saphenous vein graft to the distal right coronary artery and left internal mammary to the left anterior descending artery. Aortic crossclamp time was 143 minutes. Blood pressure on weaning from bypass was 70 to 90 mm Hg systolic on 3 inotropes, including epinephrine, norepinephrine, and milrinone. Attempts to place a percutaneous femoral intra-aortic balloon pump were unsuccessful. With time, the patient improved hemodynamically, with systolic blood pressure into the 120 mm Hg range. The patient was then transferred to the cardiac intensive care unit.
On postoperative day 1, the patient was unable to move both lower extremities. Lower extremities were warm to touch with no signs of ischemia. Blood pressure was similar to preoperative values of approximately 140/60 mm Hg. Computed tomography of the abdomen (Figure 1) and arterial duplex scans showed thrombosis from the ostia of the renal arteries extending down to the common iliac arteries. Arterial studies demonstrated dorsalis pedis and posterior tibial pulses on Doppler bilaterally as well as low ankle-brachial indexes of 0.38 (left) and 0.42 (right). Computed tomography with and without contrast of the spine was taken and demonstrated no emboli or abnormal enhancement. In addition, serum lactate was 0.6 mmol/L. By postoperative day 10, there was little change in lower extremity status. Magnetic resonance imaging demonstrated a thoracic infarct via gray matter hyperintensity starting at the T9-T10 level and extending to the upper lumbar spine. The patient was discharged a week later with a plan to pursue subacute rehabilitation placement with a neurologic focus.
Figure 1.
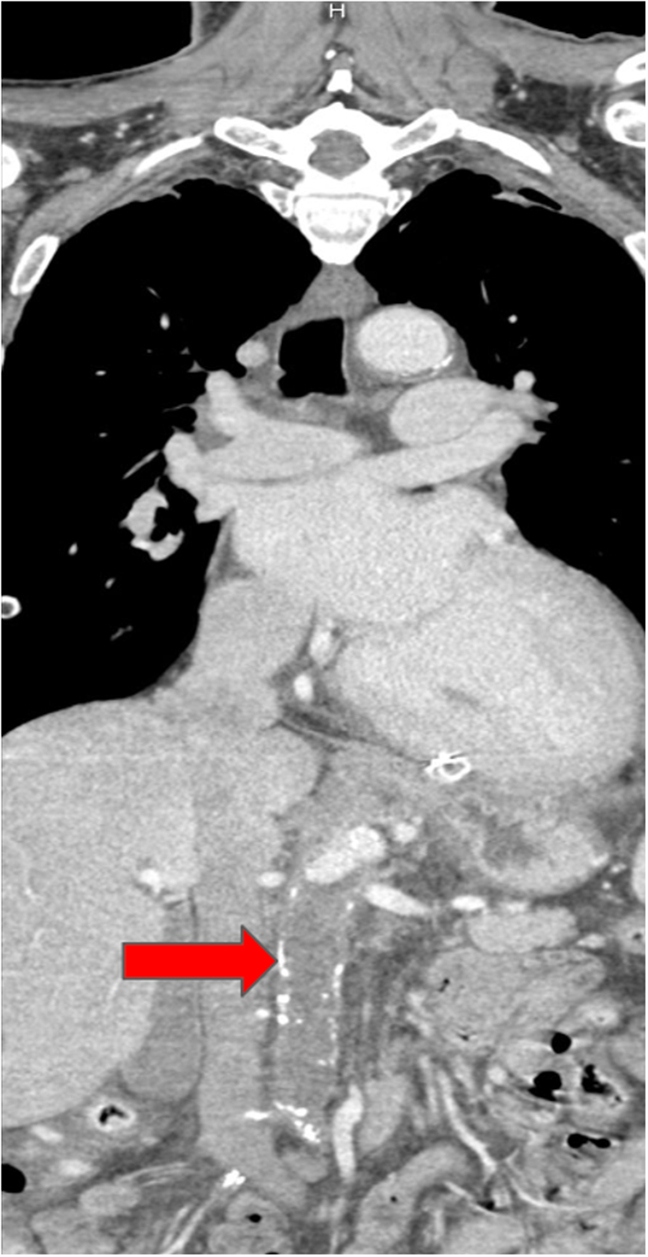
Computed tomography angiography on postoperative day 1 highlighting thrombosis in the abdominal aorta extending to the into the common iliac arteries. The red arrow points to the near-total occlusion of the abdominal aorta on CT angiography.
Discussion
Spinal cord ischemia (SCI) has been a problematic event after operations directly involving the aorta, such as during an aneurysm or dissection repair.1 SCI after nonaortic cardiac surgery (ie, CABG) has also been documented. This phenomenon is rare but still devastating in its effects. Currently, there are fewer than 50 reported cases of paraplegia after nonaortic surgery. When cases involving hemorrhage, dissection, severe hemodynamic instability, and paraplegia following intra-aortic balloon pump placement and use are excluded, there are very few cases. Risk factors for paraplegia after CABG may include hypertension, hypotension, diabetes, hypercholesterolemia, and peripheral vascular disease.2 To the knowledge of the authors, this is the first report of paraplegia after nonaortic cardiac surgery in a patient with unrecognized Leriche syndrome.
One hypothesized cause of SCI during a CABG is altered collateral circulation of spinal cord via the artery of Adamkiewicz.4 The artery of Adamkiewicz is typically on the left side and arises between the T7 and L2 levels in most individuals. The artery of Adamkiewicz typically stems from the 9th, 10th, 11th, or 12th left posterior intercostal artery, which arises from the thoracic aorta. The artery of Adamkiewicz then aids in the blood supply to the anterior lower two-thirds of the spinal cord through the anterior spinal artery.5,6
One possible significant collateral pathway to the artery of Adamkiewicz, which has been previously documented, is the internal mammary artery. Figure 2 demonstrates a proposed collateral pathway from the left internal mammary artery (LIMA) through the eighth intercostal artery to the artery of Adamkiewicz, which has been recorded as a clinically insignificant normal variant.6,7
Figure 2.
Sixteen-slice computed tomography demonstrating variant collateral circulation of the left internal mammary artery and artery of Adamkiewicz.7 The red arrow shows the collateral that between the Artery of Adamkiewicz connecting through the eighth intercostal artery to the internal mammary artery.
In our case, we hypothesize that a collateral was a significant contributor of spinal perfusion as a result of increased flow through the LIMA due to the downstream thrombus in the distal abdominal aorta.7 As the result of chronically decreased flow through the distal aortic occlusion, the internal mammary artery and its branches were likely engorged. After the dissection of the internal mammary artery during the CABG, we hypothesize that this led to the downstream loss of adequate flow to the watershed area between the internal mammary artery and the artery of Adamkiewicz, thus to the anterior spinal artery, causing a localized spinal infarct.
The perfusion pressures during the operation were adequate; however, there was a brief period of hypotension on weaning the patient from cardiopulmonary bypass, which may have been another contributor to SCI. Aortic emboli are an unlikely source of infarction in this case, as the result of imaging findings and thrombosed infrarenal aorta. The presence of Dopplerable pulses postoperatively and presence of sufficient collateral blood flow through the iliac arteries makes acute Leriche syndrome and limb hypoperfusion as the primary cause of paraplegia unlikely.
As CABG with LIMA is commonplace, it may be beneficial to screen for Leriche syndrome before surgery to avoid a significant surgical complication. If stenosis of the lower aorta is found, an alternative arterial conduit for the CABG may be preferred in order to preserve collateral flow.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Bicknell C.D., Riga C.V., Wolfe J.H. Prevention of paraplegia during thoracoabdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2009;37(6):654–660. doi: 10.1016/j.ejvs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Wanat-Hawthorne A., Akorede R., Floyd T. Perioperative spinal cord ischemia after cardiac surgery not involving the aorta: a review of the literature. J Cardiothorac Vasc Anesth. 2022;36(3):776–784. doi: 10.1053/j.jvca.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Mahendrakar S.M., Sandhu H.S., Khan A.H., Loya Y.S. Leriche syndrome: acute onset painful paraplegia of vascular origin with catastrophic consequences. J Clin Diagn Res. 2017;11(5):OD22–OD23. doi: 10.7860/JCDR/2017/26369.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirhosseini S.M., Meghdadi S., Moghaddam A.S. Anterior spinal artery syndrome following coronary artery bypass grafting: a case report. Braz J Cardiovasc Surg. 2017;32(2):136–137. doi: 10.21470/1678-9741-2016-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindeire S., Hauser J.M. StatPearls [Internet] StatPearls Publishing; 2024. Anatomy, back, artery of Adamkiewicz; pp. 2–3. [PubMed] [Google Scholar]
- 6.Taterra D., Skinningsrud B., Pękala P.A., et al. Artery of Adamkiewicz: a meta-analysis of anatomical characteristics. Neuroradiology. 2019;61(8):869–880. doi: 10.1007/s00234-019-02207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka K., Niinuma H., Kawazoe K., Ehara S. Three-dimensional demonstration of the collateral circulation to the artery of Adamkiewicz via internal thoracic artery with 16-row multi-slice CT. Eur J Cardiothorac Surg. 2005;28(3):492. doi: 10.1016/j.ejcts.2005.04.043. [DOI] [PubMed] [Google Scholar]