Abstract
Background:
Cardiopulmonary assessment for lung resection is important for risk stratification, and the American College of Chest Physicians (ACCP) guidelines provide decision support. We ascertained thoracic surgeons’ cardiopulmonary assessment practices and determined whether they are guideline concordant.
Methods:
An anonymous survey was emailed to 846 thoracic surgeons who participate in the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD). We analyzed survey responses by practice type (general thoracic [GT] vs cardiothoracic [CT]) and years in practice (0–9, 10–19 and ≥20) using contingency tables. We compared adherence of survey responses to the guidelines.
Results:
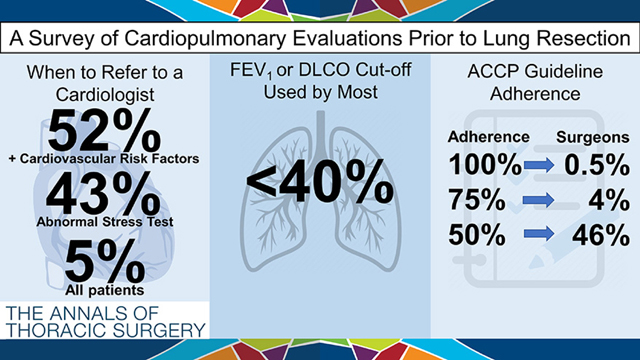
The response rate was 24.0% (n=203). Most surgeons, n=121 (59.6%), cited a predicted postoperative (ppo), FEV1 or DLCO threshold of 40% for further evaluation. Experienced surgeons (≥20 years) were more likely to have a threshold that varies by surgical approach (31.3% vs 23.5% 10–19 and 15.9% 0–9 years, p=0.007). Overall, 52.2% refer patients with cardiovascular risk factors to cardiology and 42.9% refer patients with abnormal stress testing. CT surgeons were more likely to refer all patients to cardiology than GT (17.6% vs 2.4%, p<0.001). Only one (0.5%) respondent was 100% adherent to ACCP guidelines, and 4.4% and 45.8% were 75% and 50% adherent, respectively.
Conclusions:
Among thoracic surgeons, there is variation in preoperative cardiopulmonary assessment practices, with differences by practice type and years in practice, and marked discordance with the ACCP guidelines. Further study of guideline adherence linked to postoperative morbidity and mortality is warranted to determine whether adherence impacts outcomes.
Keywords: Non-small cell lung cancer, preoperative cardiopulmonary testing, spirometry
Graphical Abstract
Patients with lung cancer often have comorbidities that necessitate a careful evaluation prior to lung resection. These comorbidities often stem from the high coincidence of long-term cigarette smoking, which is itself associated with significant risk of cardiovascular disease and chronic obstructive pulmonary disease (COPD) and therefore increased risk of perioperative morbidity and/or mortality [1,2]. Additionally, lung cancer is traditionally a disease of the elderly, with a median age at diagnosis of 70, who have higher rates of comorbidities [3]. The decision to undergo curative intent pulmonary resection for lung cancer is complex and must balance the benefit of resection with the risk of complications.
The preoperative cardiopulmonary evaluation is important for risk stratification. Pulmonary function testing (PFT) including preoperative and predicted postoperative (ppo) forced expiratory volume in one second (FEV1) and diffusion capacity for carbon monoxide (DLCO) as percentages of normal accurately predicts an increased risk of cardiopulmonary morbidity when these values are below certain thresholds [4–7]. Many international societies have developed preoperative evaluation guidelines [8–10]. One of the most commonly cited sources is the 2013 American College of Chest Physicians (ACCP) guidelines on physiological evaluation prior to lung resection [11]. Although the ACCP guidelines can be used as primary decision support for thoracic surgeons, it is unclear how closely these guidelines are followed. We surveyed thoracic surgeons to ascertain their cardiopulmonary assessment practices prior to elective lung resection. Additionally, we compared these practices to the ACCP guidelines to determine guideline adherence.
Material and Methods
We developed an anonymous, online survey utilizing a REDCap (Research Electronic Data Capture, Nashville, TN) application. The survey (Supplemental Material) queried thoracic surgeon’s practices regarding cardiopulmonary assessment prior to elective lung resection. The survey was emailed to 846 surgeons in the United States who participate in the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD). Survey questions were written to assess individual surgeons’ cardiopulmonary testing practices, but designed to allow evaluation of response adherence to specific components of the ACCP guidelines.
Demographic information obtained included sex, age, years in practice, practice type (general thoracic [GT] surgery vs cardiothoracic [CT] surgery with primarily cardiac cases), and practice setting (academic vs private/HMO). Surgeon’s years in practice were categorized: 0–9 years, 10–19 years, and ≥20 years. Non-respondent demographics were obtained via Internet search. Statistical Analyses
Statistical Analyses
Demographic data of respondents and non-respondents were compared using contingency tables. Response frequencies were analyzed, and subset analyses on each survey item were conducted based on sex, practice type, and years in practice using a χ2 test of proportions. Adherence to ACCP guidelines was determined for each participant utilizing a key of survey answers concordant with the ACCP guidelines [11]. Mean adherence rates were compared on subset analyses using independent sample Student’s t-tests or analysis of variance tests. A p-value <0.05 was statistically significant. SPSS Statistics Version 25 (IBM Crop., Armonk, NY) was used for analyses. This study was exempt from UC Davis Institutional Review Board review.
Results
Demographics
There were 203 (24.0%) respondents. The majority were male, 40–49 years old, practice at an academic center, and performed primarily general thoracic surgical cases (Table 1). Compared to non-respondents, a higher proportion of respondents practiced exclusively general thoracic surgery at an academic center, were female and were younger. .
Table 1:
Descriptive statistics of survey respondents versus non-respondents.
| Surgeon Characteristics | Respondents n=203 (24.0%) | Non-Respondents n=646 (76.0%) | p-value |
|---|---|---|---|
|
| |||
| Age | |||
| 30–39 years old | 13 (6.4%) | 22 (3.4%) | <0.001 |
| 40–49 years old | 89 (43.8%) | 181 (28.0%) | |
| 50–59 years old | 66 (32.5%) | 224 (34.7%) | |
| 60–69 years old | 31 (15.3%) | 147 (22.8%) | |
| ≥70 years old | 4 (2.0%) | 30 (4.6%) | |
| Missing | 42 (6.5%) | ||
| Gender | |||
| Female | 34 (17.0%) | 41 (6.4%) | <0.001 |
| Male | 168 (83.0%) | 600 (92.9%) | |
| Missing | 5 (0.7%) | ||
| Practice type | |||
| University/academic | 137 (67.5%) | 305 (47.2%) | <0.001 |
| Private/HMO | 65 (32.0%) | 336 (52.0%) | |
| Missing | 1 (0.5%) | 5 (0.8%) | |
| Practice Characterization | |||
| General Thoracic Surgery | 168 (82.8%) | 349 (54.0%) | <0.001 |
| Cardiothoracic Surgery with mostly Cardiac Surgery cases | 34 (16.7%) | 277 (42.9%) | |
| Missing | 1 (0.5%) | 20 (3.1%) | |
| Years in Practice | |||
| 0–9 years | 63 (31.0%) | 149 (23.1%) | 0.09 |
| 10–19 years | 68 (33.5%) | 233 (36.1%) | |
| ≥20 years | 67 (33.0%) | 243 (37.6%) | |
| Missing | 5 (2.5%) | 21 (3.2%) | |
Preoperative Pulmonary Assessment
When asked about assessment of pulmonary function prior to elective lung resection (excluding pneumonectomy) nearly all respondents always order preoperative spirometry (Table 2). The majority calculate the ppoFEV1 and obtain preoperative DLCO, although fewer calculate the ppoDLCO. There was variability regarding whether pulmonary function tests (PFTs) should be repeated after neoadjuvant therapy.
Table 2.
Survey responses regarding pulmonary assessment prior to elective lung resection.
| Preoperative Pulmonary Assessment | n (%) |
|---|---|
|
| |
| Order spirometry | |
| Always | 200 (98.5) |
| Usually | 3 (1.5) |
| Calculate ppoFEV 1 | |
| Always | 156 (77.2) |
| Usually | 16 (7.9) |
| Sometimes | 23 (11.4) |
| Rarely | 5 (2.5) |
| Never | 2 (1.0) |
| Order DLCO | |
| Always | 172 (84.7) |
| Usually | 19 (9.4) |
| Sometimes | 10 (4.9) |
| Rarely | 2 (1.0) |
| Calculate ppoDLCO | |
| Always | 117 (57.6) |
| Usually | 31 (15.3) |
| Sometimes | 31 (15.3) |
| Rarely | 13 (6.4) |
| Never | 11 (5.4) |
| Repeat PFTs after neoadjuvant therapy | |
| Always | 57 (28.1) |
| Usually | 30 (14.8) |
| Sometimes | 53 (26.1) |
| Rarely | 51 (25.1) |
| Never | 12 (5.9) |
| If ppoFEV1 or ppoDLCO is __ I further evaluate the patient | |
| < 60% | 20 (9.9) |
| <40% | 121 (59.6) |
| <30% | 15 (7.4) |
| My threshold varies by surgical approach | 47 (23.2) |
| My further evaluation consists of | |
| Cardiopulmonary exercise testing (CPET) - VO2 max testing | 116 (57.1) |
| Stair climbing test | 36 (17.7) |
| Shuttle walk test | 0 (0.0) |
| 6-minute walk test | 26 (12.8) |
| I do not order additional tests; If the FEV1 and/or DLCO are less than my threshold, I will not operate. | 18 (8.9) |
| Other | 7 (3.4) |
| I refer my patients with borderline pulmonary function for preoperative pulmonary rehabilitation | |
| Always | 13 (6.4) |
| Usually | 42 (20.7) |
| Sometimes | 69 (34.0) |
| Rarely | 56 (27.6) |
| Never | 23 (11.3) |
| I refer my patients with borderline pulmonary function for postoperative pulmonary rehabilitation | |
| Always | 43 (21.2) |
| Usually | 52 (25.6) |
| Sometimes | 69 (34.0) |
| Rarely | 27 (13.3) |
| Never | 12 (5.9) |
FEV1: forced expiratory volume in 1 second; DLCO: diffusion capacity of the lungs for carbon monoxide; ppoFEV1: predicted postoperative forced expiratory volume in 1 second; ppoDLCO: predicted postoperative diffusion capacity of the lungs for carbon monoxide; PFTs: pulmonary function tests
When asked what threshold value of FEV1 or DLCO was used to determine if a patient needs further pulmonary evaluation, the majority reported 40% (Table 2). Nearly a quarter of surgeons stated that their threshold varies based on surgical approach. When asked what further work-up is needed for those not meeting the aforementioned FEV1 or DLCO cut-off value, most use cardiopulmonary exercise testing (CPET) with maximal oxygen consumption (VO2 max). Other less commonly used modalities included the 6-minute walk test and stair climbing test. A shuttle walk test was not utilized by any surgeons. Few stated that if the FEV1 or DLCO was below the specified threshold, they would not offer surgery. There was variability regarding the use of pulmonary rehabilitation.
Preoperative Cardiac Assessment
Almost all surgeons inquire about a patient’s activity/functional status and most assess for cardiovascular risk factors (Table 3). Most surgeons never calculate a Thoracic Revised Cardiac Risk Index (ThRcRI), with very few always calculating it. Most surgeons refer their patient to a cardiologist only if they had an abnormal stress test or cardiovascular risk factors with very few referring all patients. Referral to a cardiologist was the primary method of assessment in those patients deemed to need additional cardiac evaluation, though many surgeons began a work-up with either a pharmacologic stress test, exercise stress test or echocardiogram.
Table 3.
Survey responses regarding cardiac assessment prior to elective lung resection
| Preoperative Cardiac Assessment | n (%) |
|---|---|
|
| |
| I inquire about activity/functional status | |
| Always | 196 (96.6) |
| Usually | 6 (3.0) |
| Never | 1 (0.4) |
| I calculate the Thoracic Revised Cardiac Risk Index (ThRcRI) | |
| Always | 15 (7.4) |
| Usually | 9 (4.4) |
| Sometimes | 20 (9.9) |
| Rarely | 32 (15.8) |
| Never | 127 (62.6) |
| I assess for cardiovascular risk factors | |
| Always | 185 (91.1) |
| Usually | 17 (8.4) |
| Never | 1 (0.5) |
| I refer my patients to a cardiologist to assess the risk of a perioperative major adverse cardiac event | |
| For all patients | 10 (4.9) |
| Only for patients with abnormal exercise or pharmacologic stress testing | 87 (42.9) |
| Only for patients with cardiovascular risk factors | 106 (52.2) |
| For patients who need additional cardiac evaluation, I begin the assessment with | |
| None, I refer directly to a Cardiologist | 77 (37.9) |
| Pharmacologic stress test | 53 (26.1) |
| Exercise stress test | 37 (18.2) |
| Echocardiogram | 33 (16.3) |
| Cardiopulmonary exercise testing (CPET) | 1 (0.5) |
| Other | 2 (1.0) |
Practice Differences by Sex
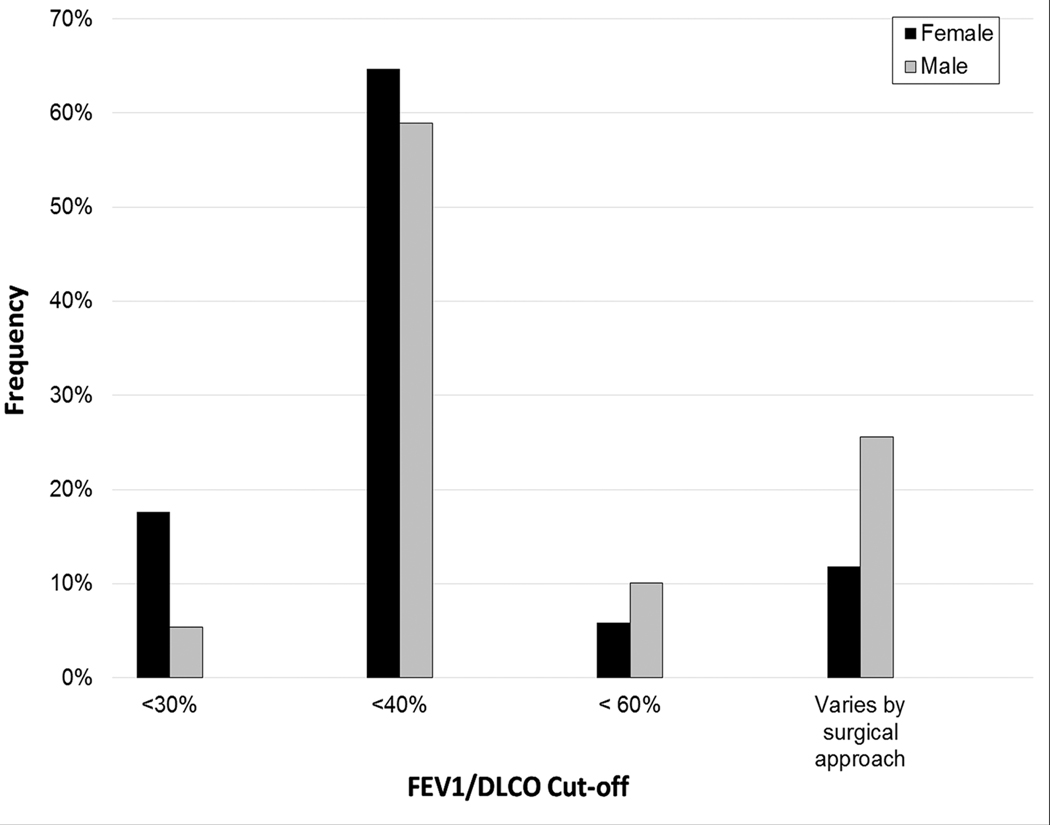
Male surgeons were more likely to use an FEV1/DLCO threshold mandating additional evaluation that varied based on surgical approach than female surgeons (25.6 vs 11.8%, p=0.032) (Figure 1a). No other survey questions differed by sex.
Figures 1.
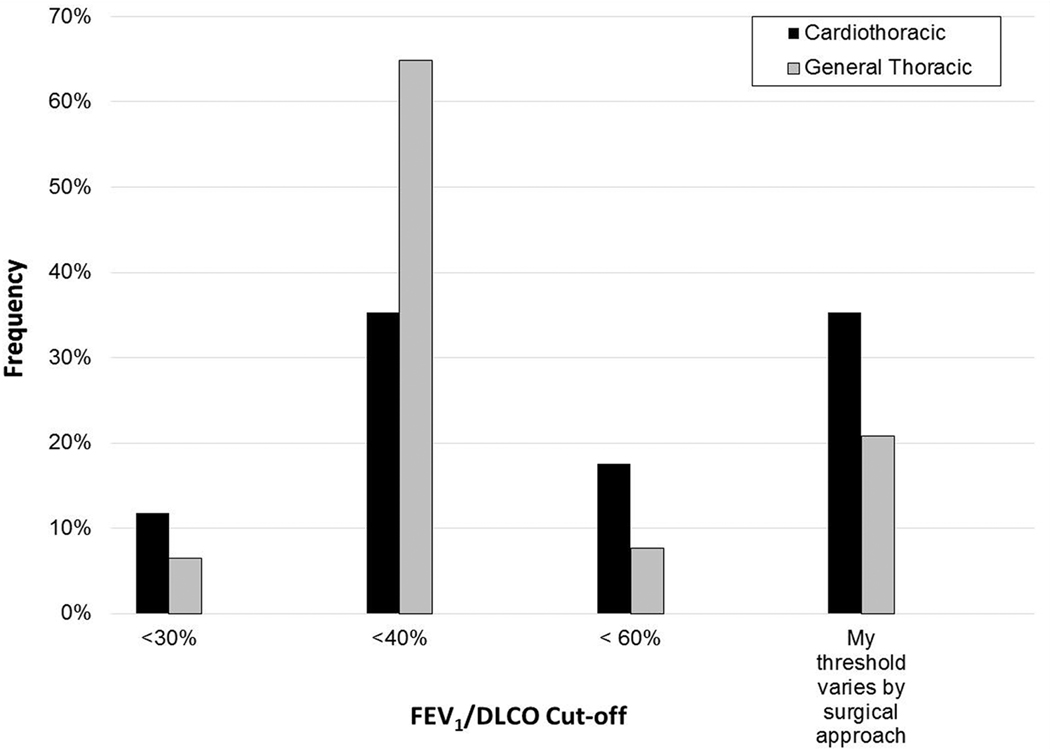
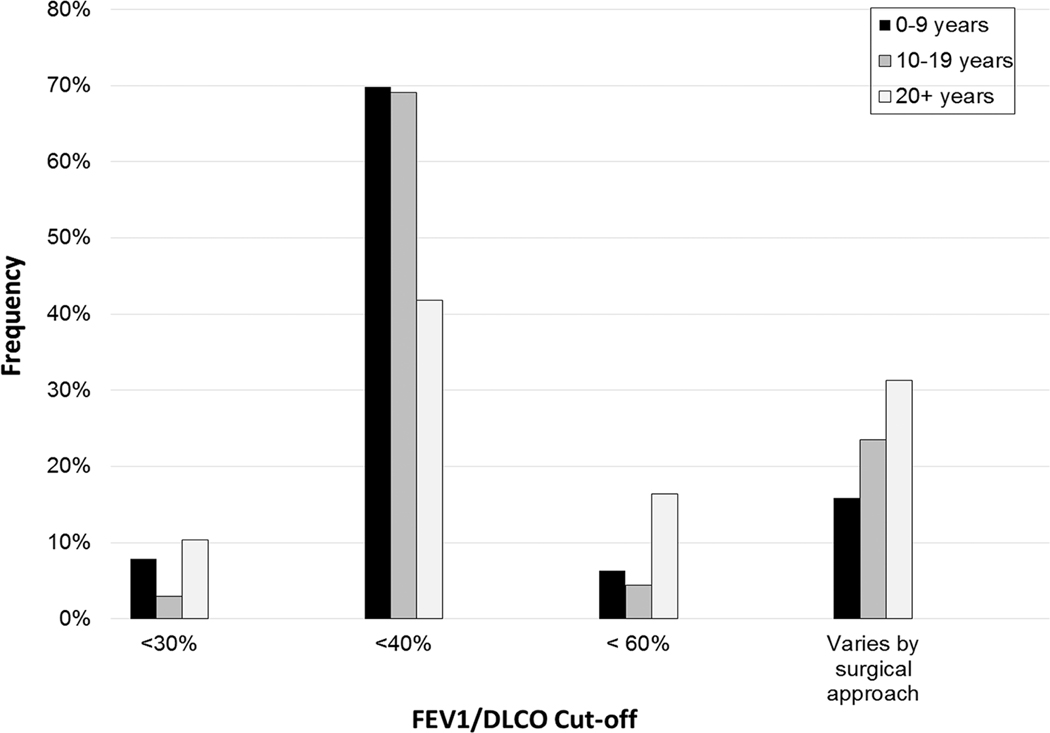
a-c. FEV1/DLCO thresholds for further evaluation (as percentage of normal values) stratified by sex (a), surgeon practice type (b), and years in practice (c).
Practice Differences by Practice Type
GT surgeons were more likely to use an FEV1/DLCO threshold of <40% than CT surgeons (64.9 vs 35.3%), while CT surgeons were more likely to use a threshold that varies by surgical approach (35.3% vs 20.8%, p=0.014) (Figure 1b). GT surgeons were more likely to always order DLCO testing (87.5% vs 70.6%, p=0.043). CT surgeons were more likely to always repeat PFTs after neoadjuvant therapy (35.3 vs 26.8%, p=0.012), refer all of their patients to a cardiologist for cardiac evaluation (17.6 vs 2.4%, p<0.001), and refer all patients with borderline pulmonary function for postoperative pulmonary rehabilitation (41.2 vs 17.3%, p=0.016).
Practice Differences by Years in Practice
Those in practice for ≥20 years were less likely to always order DLCO testing than those in practice 0–9 and 10–19 years (73.1 vs 88.2 vs 93.7%, p=0.05). Similarly, surgeons in practice ≥20 years were less likely to always calculate a ppoDLCO value than those in practice 0–9 and 10–19 years (44.8 vs 55.9 vs 74.6%, p=0.03). Those in practice 0–9 or 10–19 years were more likely to use an FEV1/DLCO threshold of <40% than those in practice ≥20 years (69.8 vs 69.1 vs 41.8%, p=0.007). Surgeons in practice ≥20 years were more likely to have a threshold that varies by surgical approach than surgeons in practice 0–9 or 10–19 years (31.3 vs 15.9 vs 23.5%, p=0.007) (Figure 1c).
Practice Differences by Practice Setting
Surgeons in private or HMO practice were more likely than academic surgeons to always repeat PTFs after neoadjuvant therapy (36.4 vs 24.1%, p=0.009), as well as refer their patients with borderline pulmonary function for postoperative pulmonary rehabilitation (38.2 vs 13.1%, p<0.001). The ThRCRI was more often always used by private or HMO practice surgeons than their academic counterparts (14.5 vs 3.6%, p<0.001).
Adherence to ACCP Guidelines
Survey responses were compared to a key of answers considered concordant with ACCP guidelines [11]. Only one respondent (0.5%) was 100% adherent to ACCP guidelines, while 4.4% were 75% adherent and 45.8% were 50% adherent. Mean guideline adherence was significantly higher for private practice than academic surgeons (58.9 vs 51.8%, p=0.002). There was no significant difference in mean guideline adherence by sex, years in practice, nor practice setting.
The survey items that most commonly had responses that were guideline adherent were ordering spirometry and assessing for cardiac risk factors/functional status/calculating the ThRCRI (Table 4). The survey items that least commonly had guideline adherent responses were FEV1/DLCO threshold and corresponding recommendation for further evaluation as defined by the ACCP guidelines. Of those selecting an FEV1/DLCO threshold <30%, 40% were adherent to the recommendation of cardiopulmonary exercise testing. For those selecting an FEV1/DLCO threshold <60%, 30% were adherent to the recommendation of low technology modality such as a 6 minute walk test, stair climbing test or shuttle walk test. Less than a quarter of respondents were adherent to the recommendation of referring patients with borderline lung function for pulmonary rehabilitation.
Table 4.
Adherence of survey respondents to ACCP physiologic evaluation for lung cancer resection guidelines
| Guideline Component | Adherence |
|---|---|
|
| |
| Order spirometry | 98% |
| Assess for cardiac risk factors / inquire about functional status / calculate ThRCRI composite | 98% |
| Refer patients with cardiovascular risk factors to a cardiologist | 52% |
| Order DLCO testing | 84% |
| Calculate ppoFEV1 | 76% |
| Calculate ppoDLCO | 57% |
| If the ppoFEV1 and/or ppoDLCO is <30%, the patient is further evaluated via cardiopulmonary exercise testing (CPET) | 40% |
| If the ppoFEV1 and/or ppoDLCO is <60%, the patient is further evaluated via either 6 minute walk test, stair climbing test, or shuttle walk test | 30% |
| Refer patients with borderline lung function to pulmonary rehabilitation | 21% |
ThRCRI: Thoracic Revised Cardiac Risk Index; ppoFEV1: predicted postoperative forced expiratory volume in 1 second; ppoDLCO: predicted postoperative diffusion capacity of the lungs for carbon monoxide
Comment
Our survey of thoracic surgeons’ preoperative evaluation strategies revealed heterogeneity in practice patterns. The majority of surgeons agreed on the importance of preoperative spirometry and DLCO testing, though fewer agreed upon calculating predicted postoperative values. The interpretation of PFT results and the subsequent testing varied among surgeons. According to the ACCP guidelines, all patients should undergo spirometry and DLCO testing with calculation of ppoFEV1 and ppoDLCO (Grade 1B recommendation) [12]. All patients with a ppoFEV1 or ppoDLCO ≥60% are deemed low risk and do not require further evaluation before surgery (Grade 1C recommendation). Those with ppoFEV1 or ppoDLCO values <60% or ≥30% are recommended to undergo low technology exercise testing with a stair climb of >22 meters or a shuttle walk test or >400 meters, and if successful are deemed low operative risk (Grade 1C recommendation). It is recommended that patients with ppoFEV1 or ppoDLCO values <30% or having failed a low technology test undergo CPET (Grade 1B and 1C recommendations, respectively). A VO2 max of 10–20 ml/kg/minute is considered moderate operative risk, while those with a VO2 max of <10 ml/kg/minute are considered high operative risk and nonoperative therapies should be considered (Grade 1C recommendation). These algorithms were predicated upon a plethora of previous retrospective analyses and modeling to determine appropriate ppoFEV1 or ppoDLCO cut-off values [4–7,13]. Only 9.9% of respondents chose a ppoFEV1 or ppoDLCO of <60% as their cut-off for further testing, with most using a cut-off of <40%. If patients are below this threshold, most surgeons forgo any low technology exercise testing and elect for CPET. There is some difficulty in evaluating these survey responses, as those who use a ppoFEV1 or ppoDLCO of <30% as their threshold would be ACCP guideline concordant if they then subsequently perform CPET for all of their patients below the threshold. However, only 40% of those respondents choosing a threshold of <30% then recommended further evaluation with CPET. Similarly, only 30% of surgeons using the ppoFEV1 or ppoDLCO threshold of <60% followed ACCP guidelines with further evaluation by low technology exercise testing. As such, spirometry and DLCO testing are used nearly universally by thoracic surgeons but their interpretation and implementation into the preoperative evaluation varies widely and is rarely ACCP guideline concordant.
CT surgeons more commonly use a ppoFEV1 or ppoDLCO threshold of <60%, while GT surgeons use <40%. GT surgeons may have developed more personalized interpretations of PFTs based on their experiences, or are more comfortable operating on patients with lower pulmonary function without further testing. Additionally, more experienced surgeons were less likely to calculate ppoDLCO and more frequently used a threshold that varied by surgical approach. Clinical decisions based on past experience may play an important role for more experienced surgeons. Select patients with marginal pulmonary function may be adequate surgical candidates with no increase in postoperative morbidity or mortality compared to their counterparts with better pulmonary function [14–16]. Some purport that a minimally invasive approach allows for safer pulmonary resection in these borderline patients, but there is conflicting evidence for this argument [17,18]. A short-coming of our survey is that it may fail to capture those surgical practices where a preoperative clinic visit includes an informal test such as stair climbing. Thus, it is possible that formal low technology exercise tests such as the stair climb and shuttle walk are infrequently if ever used when more informal tests or even clinical judgement alone are employed by the surgeon, and any patients who appear frail in the office or have borderline PFTs are referred for CPET.
Very few respondents routinely refer patients with borderline pulmonary function to preoperative or postoperative pulmonary rehabilitation. Preoperative pulmonary rehabilitation improves preoperative PFTs and recovery of PFTs postoperatively [19], reduces the odds of a pulmonary complication after lobectomy [19–22], and can decrease length of stay [21]. These programs can be completed preoperatively without delaying surgery [23]. CT and private practice surgeons more frequently refer patients for pulmonary rehabilitation. Perhaps a lack of access to quality rehabilitation programs or insurance coverage makes such programs less readily available to certain thoracic surgery programs.
With regard to preoperative cardiac evaluation, most respondents adhered to ACCP guidelines by referring those patients with abnormal stress tests or cardiovascular disease risk factors for further evaluation by a cardiologist. CT surgeons were more likely to refer all patients for cardiac clearance by a cardiologist prior to surgery, suggesting an alternative preoperative clinic workflow or relationship to cardiologist offices among CT surgeons. Very few respondents adhered to the ACCP recommendation of calculating the ThRCRI, despite evidence that it is highly predictive of perioperative cardiac complications in patients undergoing lung resection [24–26].
Overall, few respondents were highly adherent to the ACCP guidelines, with less than half providing survey responses that were 50% concordant with ACCP guidelines. Given such limited guideline adherence in a cohort of STS GTSD participating surgeons with collective excellent operative outcomes, a perioperative mortality rate of 1.4% and major morbidity rate of 9.1%, seems counterintuitive [2]. Further study is needed to link guideline adherence to postoperative morbidity and mortality to determine whether guideline adherence impacts outcomes. There may be components of the ACCP guidelines that are considered essential by thoracic surgeons and others less important and perhaps even unnecessary. While this survey demonstrates significant non-adherence to ACCP guidelines it is important to assess whether specific components of the guidelines commonly underutilized by surgeons have an impact on postoperative outcomes. To this end, future investigations might include a qualitative analysis of our respondents’ survey answers and opinions on the questions as they relate to the ACCP guidelines. Such qualitative analyses could shed light on issues of importance for future guideline revisions. Moreover, this survey does not capture whether thoracic surgeons are inappropriately excluding patients from surgery or doing a sublobar resection when a lobectomy may be the better operation in certain patients. In other words, does strict guideline adherence lead to improved outcomes because of “over selection”? This could be elucidated by gathering guideline adherence data and comparing it not only to outcomes of those undergoing surgery but also those who were either not selected for surgery or underwent a lesser resection.
This study has several limitations. The survey data limits analysis beyond that of trends and practices, and the anonymity of the survey prevents direct correlation between practice and patient outcomes. There is certainly risk of selection bias for respondents to the survey, as seen by the disproportionate distribution of older, male, private practice and CT surgeons in the non-respondent subgroup. There is wide variability in response rates to internet based surveys, and it is not uncommon for response rates of health professionals to be <20% [27]. Though individual surgeons received reminder emails at specified intervals, providing a financial incentive and outreach to cardiothoracic surgery programs to encourage surgeon participation may have improved the response rate. Additionally, respondents were not blinded to the identity of the researchers, such that a selection bias due to increased responsiveness by respondents who personally know or know of the authors may have occurred. However, we ascertained the demographic data of the non-respondents to compare them to those of the respondents to understand whether this survey was generalizable. Finally, there is a lack of generalizability of this STS GSTD contributing cohort of surgeons to the broader population of surgeons practicing thoracic surgery.
Despite these limitations, this study provides evidence that there is wide heterogeneity in cardiopulmonary assessment practice patterns prior to lung resection. Further study of guideline adherence linked to postoperative morbidity and mortality is warranted to determine whether adherence impacts outcomes. This may inform future cardiopulmonary assessment guideline revisions.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121–7. doi: 10.1016/j.lungcan.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fernandez FG, Kosinski AS, Burfeind W, Decamp MM, Seder C, Marshall B, et al. STS Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370–7. doi: 10.1016/j.athoracsur.2016.02.098.STS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cancer Stat Facts: Lung and Bronchus Cancer. Natl Cancer Inst Surveillance, Epidemiol End Results Progr 2018. https://seer.cancer.gov/statfacts/html/lungb.html (accessed September 21, 2018).
- [4].Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardio-Thoracic Surg 2008. doi: 10.1016/j.ejcts.2008.07.037. [DOI] [PubMed] [Google Scholar]
- [5].Licker MJ, Widikker I, Robert J, Frey J-G, Spiliopoulos A, Ellenberger C, et al. Operative Mortality and Respiratory Complications After Lung Resection for Cancer: Impact of Chronic Obstructive Pulmonary Disease and Time Trends. Ann Thorac Surg 2006. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- [6].Magdeleinat P, Seguin A, Alifano M, Boubia S, Regnard J-F. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099–105. doi: 10.1016/j.ejcts.2005.01.034. [DOI] [PubMed] [Google Scholar]
- [7].Brunelli A, Al Refai M, Monteverde M, Sabbatini A, Xiumé F, Fianchini A. Predictors of early morbidity after major lung resection in patients with and without airflow limitation. Ann. Thorac. Surg, vol. 74, 2002, p. 999–1003. doi: 10.1016/S0003-4975(02)03852-3. [DOI] [PubMed] [Google Scholar]
- [8].Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- [9].Sawabata N, Nagayasu T, Kadota Y, Goto T, Horio H, Mori T, et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by guideline committee of the Japanese Association for Chest Surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:14–21. doi: 10.1007/s11748-014-0475-x. [DOI] [PubMed] [Google Scholar]
- [10].Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemoradiotherapy). Eur Respir J 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- [11].Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery. Chest 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- [12].Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery. Chest 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- [13].Brunelli A, Xiumé F, Refai M, Salati M, Di Nunzio L, Pompili C, et al. Peak oxygen consumption measured during the stair-climbing test in lung resection candidates. Respiration 2010;80:207–11. doi: 10.1159/000279331. [DOI] [PubMed] [Google Scholar]
- [14].Taylor MD, LaPar DJ, Isbell JM, Kozower BD, Lau CL, Jones DR. Marginal pulmonary function should not preclude lobectomy in selected patients with non–small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:738–46. doi: 10.1016/j.jtcvs.2013.09.064. [DOI] [PubMed] [Google Scholar]
- [15].Paul Subroto, Andrews Weston, Nasar Abu, Port Jeffrey, Lee Paul, Stiles Brendon, Altorki N. Outcomes of lobectomy in patients with severely compromised lung function (predicted postoperative diffusing capacity of the lung for carbon monoxide % ≤ 40%). Ann Am Thorac Soc 2013;10:616–21. [DOI] [PubMed] [Google Scholar]
- [16].Almquist D, Khanal N, Smith L, Ganti AK. Preoperative Pulmonary Function Tests (PFTs) and Outcomes from Resected Early Stage Non-small Cell Lung Cancer (NSCLC). Anticancer Res 2018;38:2903–7. doi: 10.21873/anticanres.12537. [DOI] [PubMed] [Google Scholar]
- [17].Burt BM, Kosinski AS, Shrager JB, Onaitis MW, Weigel T. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19–28. doi: 10.1016/j.jtcvs.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [18].Zhang R, Lee SM, Wigfield C, Vigneswaran WT, Ferguson MK. Lung Function Predicts Pulmonary Complications Regardless of the Surgical Approach. Ann Thorac Surg 2015;99:1761–7. doi: 10.1016/j.athoracsur.2015.01.030. [DOI] [PubMed] [Google Scholar]
- [19].Saito H, Hatakeyama K, Konno H, Matsunaga T, Shimada Y, Minamiya Y. Impact of pulmonary rehabilitation on postoperative complications in patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cancer 2017;8:451–60. doi: 10.1111/1759-7714.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bradley A, Marshall A, Stonehewer L, Reaper L, Parker K, Bevan-Smith E, et al. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg 2013;44:e266–71. doi: 10.1093/ejcts/ezt381. [DOI] [PubMed] [Google Scholar]
- [21].Gao K, Yu PM, Su JH, He CQ, Liu LX, Bin Zhou Y, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: A study for 342 cases. Thorac Cancer 2015;6:443–9. doi: 10.1111/1759-7714.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Licker M, Karenovics W, Diaper J, Frésard I, Triponez F, Ellenberger C, et al. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J Thorac Oncol 2017;12:323–33. doi: 10.1016/j.jtho.2016.09.125. [DOI] [PubMed] [Google Scholar]
- [23].Tarumi S, Yokomise H, Gotoh M, Kasai Y, Matsuura N, Chang SS, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg 2015;149:569–73. doi: 10.1016/j.jtcvs.2014.09.123. [DOI] [PubMed] [Google Scholar]
- [24].Thomas DC, Blasberg JD, Arnold BN, Rosen JE, Salazar MC, Detterbeck FC, et al. Validating the Thoracic Revised Cardiac Risk Index Following Lung Resection. Ann Thorac Surg 2017;104:389–94. doi: 10.1016/j.athoracsur.2017.02.006. [DOI] [PubMed] [Google Scholar]
- [25].Brunelli A, Ferguson MK, Salati M, Vigneswaran WT, Jimenez MF, Varela G. Thoracic Revised Cardiac Risk Index Is Associated With Prognosis After Resection for Stage I Lung Cancer. Ann. Thorac. Surg, vol. 100, 2015, p. 195–200. doi: 10.1016/j.athoracsur.2015.03.103. [DOI] [PubMed] [Google Scholar]
- [26].Brunelli A, Cassivi SD, Fibla J, Halgren LA, Wigle DA, Allen MS, et al. External validation of the recalibrated thoracic revised cardiac risk index for predicting the risk of major cardiac complications after lung resection. Ann Thorac Surg 2011;92:445–8. doi: 10.1016/j.athoracsur.2011.03.095. [DOI] [PubMed] [Google Scholar]
- [27].Dykema J, Jones NR, Piché T, Stevenson J. Surveying Clinicians by Web: Current Issues in Design and Administration. Eval Heal Prof 2013. doi: 10.1177/0163278713496630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.