Abstract
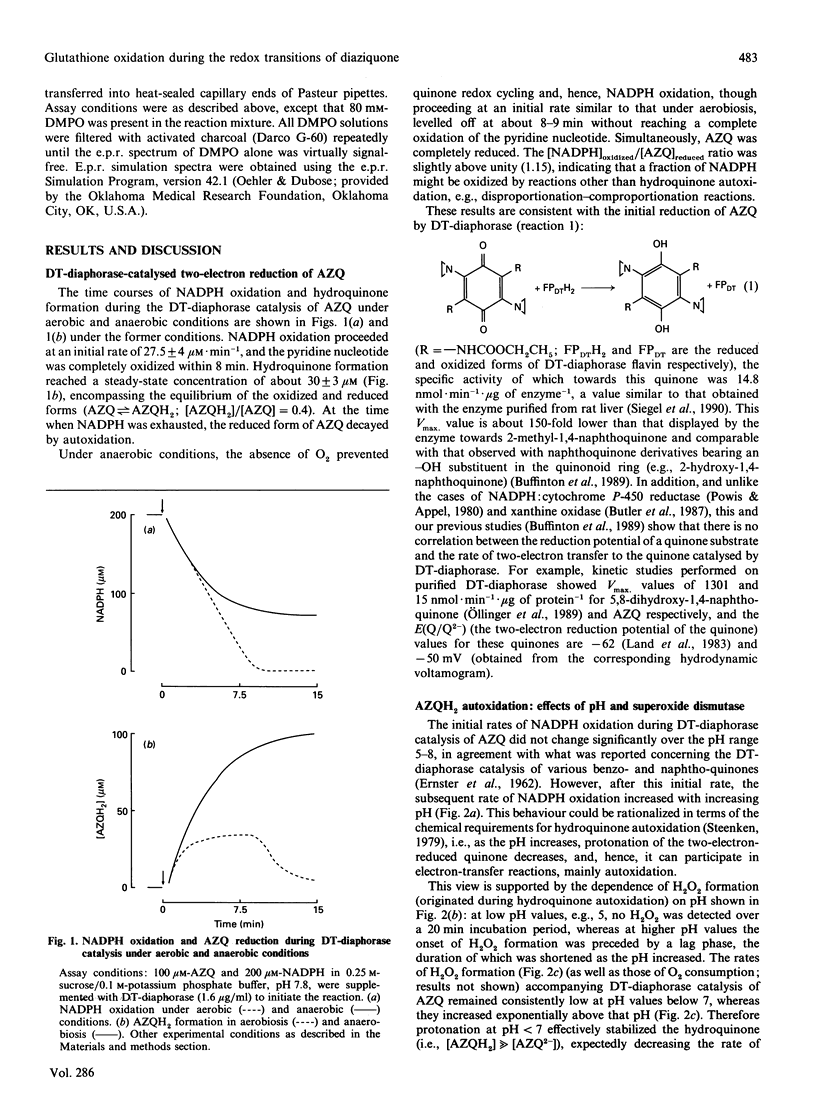
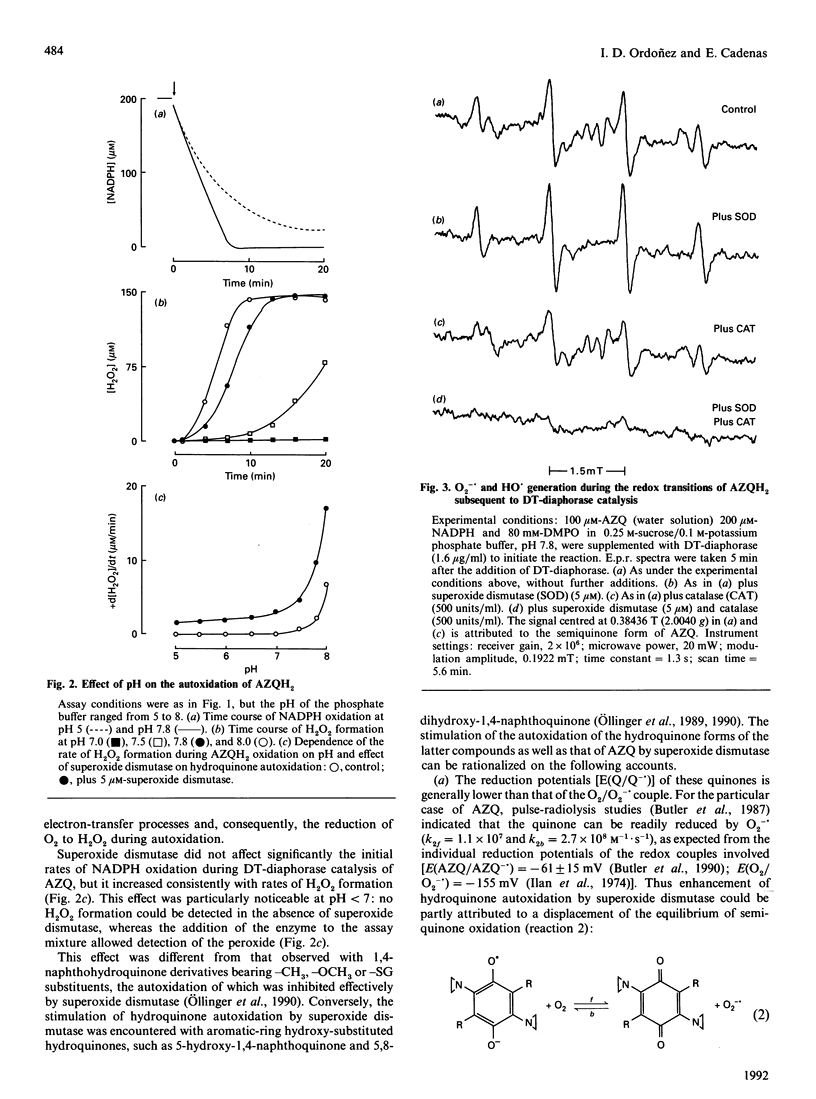
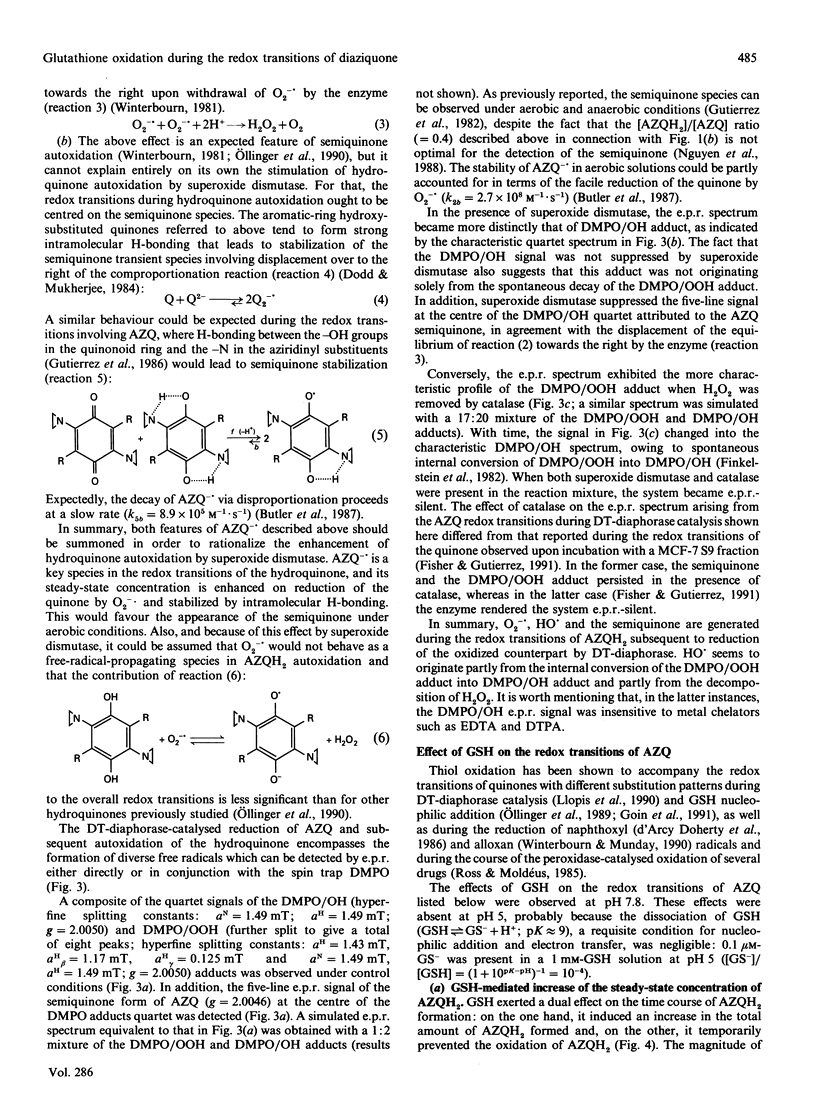
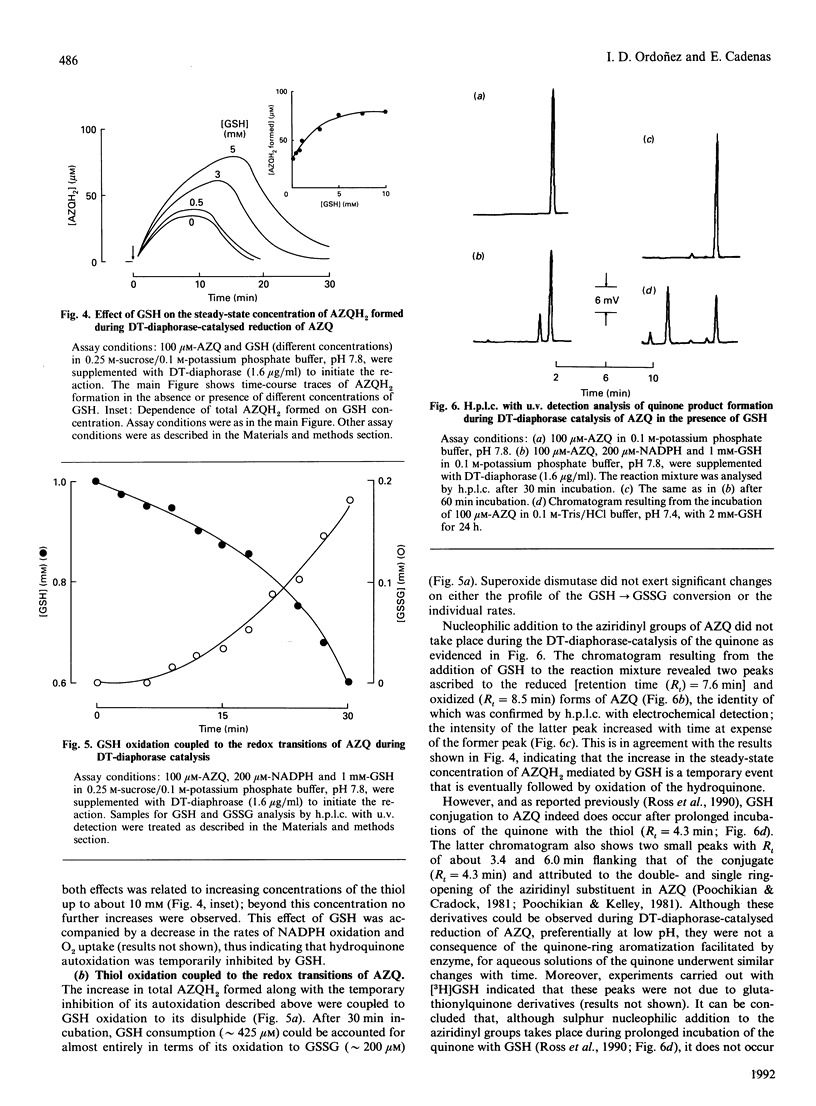
DT-diaphorase [NAD(P)H:quinone oxidoreductase; EC 1.6.99.2] catalysed the two-electron reduction of the anti-tumour quinone 2,5-bis-(1-aziridinyl)-3,6-bis(ethoxycarbonylamino)-1,4-benzoquino ne (AZQ) to the hydroquinone form (AZQH2). Although DT-diaphorase catalysis of AZQ was not significantly affected by pH, the hydroquinone product was effectively stabilized by protonation at pH values below 7, whereas, above that pH, hyroquinone autoxidation, evaluated in terms of H2O2 production, increased exponentially. The autoxidation of AZQH2 entailed the formation of diverse radicals, such as O2-.,HO., and the semiquinone form of AZQ (AZQ-.), which contributed to different extents to the e.p.r. spectrum. Superoxide dismutase enhanced the autoxidation of AZQH2 and suppressed the e.p.r. signal ascribed to AZQ-., in agreement with a displacement of the equilibrium of the semiquinone autoxidation reaction (AZQ-.+O2 in equilibrium with AZQ+O2-.) upon enzymic withdrawal of O2-.. GSH increased the steady-state concentration of AZQH2 formed during DT-diaphorase catalysis and inhibited temporarily its autoxidation. This effect was accompanied by oxidation of the thiol to the disulphide within a process involving glutathionyl radical (GS.) formation, the relative contribution of which to the e.p.r. spectrum was enhanced by increasing GSH concentrations. GS. formation in this experimental model can be rationalized as originating from the reaction of GSH with AZQ-., rather than with O2-. or HO., for thiol oxidation was not affected significantly by superoxide dismutase, and GS. formation was insensitive to catalase. In addition, GSH suppressed the e.p.r. signal attributed to AZQ-.. No glutathionyl-quinone conjugate was detected during the DT-diaphorase-catalysed reduction of AZQ; although the chemical requirements for alkylation were partly fulfilled (quinone ring aromatization and acid-assisted aziridinyl ring opening), the negligible dissociation of GSH (GS(-)+H+ in equilibrium with GSH) at low pH prevented any nucleophilic addition to occur. Therefore the redox transitions of AZQ during DT-diaphorase catalysis seemed to be centred on the semiquinone species, the fate of which was inversely affected by catalytic amounts of superoxide dismutase and large amounts of GSH: the former enhanced AZQ-. autoxidation and the latter favoured AZQ-. reduction. Accordingly, superoxide dismutase and GSH suppressed the semiquinone e.p.r. signal. These results are discussed in terms of three interdependent redox transitions (comprising one-electron transfer reactions involving the quinone, oxygen and the thiol) and the thermodynamic and kinetic properties of the reactions involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdella B. R., Fisher J. A chemical perspective on the anthracycline antitumor antibiotics. Environ Health Perspect. 1985 Dec;64:4–18. doi: 10.1289/ehp.85644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic Biol Med. 1989;7(4):435–477. doi: 10.1016/0891-5849(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Brunmark A., Cadenas E. Reductive addition of glutathione to p-benzoquinone, 2-hydroxy-p-benzoquinone, and p-benzoquinone epoxides. Effect of the hydroxy- and glutathionyl substituents on p-benzohydroquinone autoxidation. Chem Biol Interact. 1988;68(3-4):273–298. doi: 10.1016/0009-2797(88)90021-x. [DOI] [PubMed] [Google Scholar]
- Buffinton G. D., Ollinger K., Brunmark A., Cadenas E. DT-diaphorase-catalysed reduction of 1,4-naphthoquinone derivatives and glutathionyl-quinone conjugates. Effect of substituents on autoxidation rates. Biochem J. 1989 Jan 15;257(2):561–571. doi: 10.1042/bj2570561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J., Dzielendziak A., Lea J. S., Ward T. H., Hoey B. M. Contrasting cytotoxic mechanisms of similar antitumour diaziridinylbenzoquinones. Free Radic Res Commun. 1990;8(4-6):231–239. doi: 10.3109/10715769009053356. [DOI] [PubMed] [Google Scholar]
- Butler J., Hoey B. M., Lea J. S. The reduction of anti-tumour diaziridinyl benzoquinones. Biochim Biophys Acta. 1987 Aug 13;925(2):144–149. doi: 10.1016/0304-4165(87)90103-6. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Ernster L. Quinoid compounds: high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1990;186:180–196. doi: 10.1016/0076-6879(90)86108-8. [DOI] [PubMed] [Google Scholar]
- Dodd N. J., Mukherjee T. Free radical formation from anthracycline antitumour agents and model systems--I. Model naphthoquinones and anthraquinones. Biochem Pharmacol. 1984 Feb 1;33(3):379–385. doi: 10.1016/0006-2952(84)90229-6. [DOI] [PubMed] [Google Scholar]
- Driebergen R. J., Holthuis J. J., Hulshoff A., Postma-Kelder S. J., Verboom W., Reinhoudt D. N., Lelieveld P. Electrochemistry of potential bioreductive alkylating quinones: its use in the development of new aziridinylquinones. Anticancer Res. 1986 Jul-Aug;6(4):605–619. [PubMed] [Google Scholar]
- Dzielendziak A., Butler J., Hoey B. M., Lea J. S., Ward T. H. Comparison of the structural and cytotoxic activity of novel 2,5-bis(carboethoxyamino)-3,6-diaziridinyl-1,4-benzoquinone analogues. Cancer Res. 1990 Apr 1;50(7):2003–2008. [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Fariss M. W., Reed D. J. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Fisher G. R., Gutierrez P. L. The reductive metabolism of diaziquone (AZQ) in the S9 fraction of MCF-7 cells: free radical formation and NAD(P)H: quinone-acceptor oxidoreductase (DT-diaphorase) activity. Free Radic Biol Med. 1991;10(6):359–370. doi: 10.1016/0891-5849(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Goin J., Gibson D. D., McCay P. B., Cadenas E. Glutathionyl- and hydroxyl radical formation coupled to the redox transitions of 1,4-naphthoquinone bioreductive alkylating agents during glutathione two-electron reductive addition. Arch Biochem Biophys. 1991 Aug 1;288(2):386–396. doi: 10.1016/0003-9861(91)90211-z. [DOI] [PubMed] [Google Scholar]
- Gutierrez P. L., Biswal S., Nardino R., Biswal N. Reductive activation of diaziquone and possible involvement of free radicals and the hydroquinone dianion. Cancer Res. 1986 Nov;46(11):5779–5785. [PubMed] [Google Scholar]
- Gutierrez P. L., Friedman R. D., Bachur N. R. Biochemical activation of AZQ [3,6-diaziridinyl-2,5-bis(carboethoxyamino)-1,4-benzoquinone] to its free radical species. Cancer Treat Rep. 1982 Feb;66(2):339–342. [PubMed] [Google Scholar]
- Gutierrez P. L. Mechanism(s) of bioreductive activation. The example of diaziquone (AZQ). Free Radic Biol Med. 1989;6(4):405–445. doi: 10.1016/0891-5849(89)90087-7. [DOI] [PubMed] [Google Scholar]
- Janzen E. G., Stronks H. J., Dubose C. M., Poyer J. L., McCay P. B. Chemistry and biology of spin-trapping radicals associated with halocarbon metabolism in vitro and in vivo. Environ Health Perspect. 1985 Dec;64:151–170. doi: 10.1289/ehp.8564151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. H., Driscoll J. S. Potential central nervous system antitumor agents. Aziridinylbenzoquinones. 1. J Med Chem. 1976 Feb;19(2):313–317. doi: 10.1021/jm00224a022. [DOI] [PubMed] [Google Scholar]
- Lind C., Cadenas E., Hochstein P., Ernster L. DT-diaphorase: purification, properties, and function. Methods Enzymol. 1990;186:287–301. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- Llopis J., Ernster L., Cadenas E. Effect of glutathione on the redox transitions of naphthohydroquinone derivatives formed during DT-diaphorase catalysis. Free Radic Res Commun. 1990;8(4-6):271–285. doi: 10.3109/10715769009053360. [DOI] [PubMed] [Google Scholar]
- Lusthof K. J., de Mol N. J., Janssen L. H., Prins B., Verboom W., Reinhoudt D. N. Interactions between potential anti-tumour 2,5-bis(1-aziridinyl)-1,4-benzoquinone derivatives and glutathione: reductive activation, conjugation and DNA damage. Anticancer Drug Des. 1990 Aug;5(3):283–290. [PubMed] [Google Scholar]
- Mason R. P., Rao D. N. Thiyl free radical metabolites of thiol drugs, glutathione, and proteins. Methods Enzymol. 1990;186:318–329. doi: 10.1016/0076-6879(90)86125-f. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I., Rojas A., Smith K. A., Soranson J. A., Shrieve D. C., Jones N. R., Bremner J. C. Glutathione depletion in tissues after administration of buthionine sulphoximine. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1261–1264. doi: 10.1016/0360-3016(84)90329-8. [DOI] [PubMed] [Google Scholar]
- Moore H. W. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977 Aug 5;197(4303):527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- Nguyen B., Biswal S., Gutierrez P. L. The influence of diaziquone free radicals on the in vitro activity of diaziquone. Xenobiotica. 1988 May;18(5):593–602. doi: 10.3109/00498258809041696. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J., Kaul H. K., Rauth A. M. Differential cytotoxicity of diaziquone toward Chinese hamster ovary cells under hypoxic and aerobic exposure conditions. Cancer Res. 1990 Mar 1;50(5):1516–1520. [PubMed] [Google Scholar]
- Ollinger K., Buffinton G. D., Ernster L., Cadenas E. Effect of superoxide dismutase on the autoxidation of substituted hydro- and semi-naphthoquinones. Chem Biol Interact. 1990;73(1):53–76. doi: 10.1016/0009-2797(90)90108-y. [DOI] [PubMed] [Google Scholar]
- Ollinger K., Llopis J., Cadenas E. Study of the redox properties of naphthazarin (5,8-dihydroxy-1,4-naphthoquinone) and its glutathionyl conjugate in biological reactions: one- and two-electron enzymatic reduction. Arch Biochem Biophys. 1989 Dec;275(2):514–530. doi: 10.1016/0003-9861(89)90398-6. [DOI] [PubMed] [Google Scholar]
- Poochikian G. K., Cradock J. C. 2,5-Diaziridinyl-3,6-bis(carboethoxyamino)-1,4-benzoquinone I: Kinetics in aqueous solutions by high-performance liquid chromatography. J Pharm Sci. 1981 Feb;70(2):159–162. doi: 10.1002/jps.2600700211. [DOI] [PubMed] [Google Scholar]
- Poochikian G. K., Kelley J. A. 2,5-Diaziridinyl-3,6-bis(carboethoxyamino)-1,4-benzoquinone II: Isolation and characterization of degradation products. J Pharm Sci. 1981 Feb;70(2):162–167. doi: 10.1002/jps.2600700212. [DOI] [PubMed] [Google Scholar]
- Powis G., Appel P. L. Relationship of the single-electron reduction potential of quinones to their reduction by flavoproteins. Biochem Pharmacol. 1980 Oct 1;29(19):2567–2572. doi: 10.1016/0006-2952(80)90068-4. [DOI] [PubMed] [Google Scholar]
- Powis G. Free radical formation by antitumor quinones. Free Radic Biol Med. 1989;6(1):63–101. doi: 10.1016/0891-5849(89)90162-7. [DOI] [PubMed] [Google Scholar]
- Quintiliani M., Badiello R., Tamba M., Esfandi A., Gorin G. Radiolysis of glutathione in oxygen-containing solutions of pH7. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Aug;32(2):195–202. doi: 10.1080/09553007714550891. [DOI] [PubMed] [Google Scholar]
- Ross D., Moldeus P. Generation of reactive species and fate of thiols during peroxidase-catalyzed metabolic activation of aromatic amines and phenols. Environ Health Perspect. 1985 Dec;64:253–257. doi: 10.1289/ehp.8564253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D., Siegel D., Gibson N. W., Pacheco D., Thomas D. J., Reasor M., Wierda D. Activation and deactivation of quinones catalyzed by DT-diaphorase. Evidence for bioreductive activation of diaziquone (AZQ) in human tumor cells and detoxification of benzene metabolites in bone marrow stroma. Free Radic Res Commun. 1990;8(4-6):373–381. doi: 10.3109/10715769009053371. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C. The role of mitomycin antibiotics in the chemotherapy of solid tumors. Biochem Pharmacol. 1986 Jan 1;35(1):67–69. doi: 10.1016/0006-2952(86)90559-9. [DOI] [PubMed] [Google Scholar]
- Siegel D., Gibson N. W., Preusch P. C., Ross D. Metabolism of diaziquone by NAD(P)H:(quinone acceptor) oxidoreductase (DT-diaphorase): role in diaziquone-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res. 1990 Nov 15;50(22):7293–7300. [PubMed] [Google Scholar]
- Szmigiero L., Erickson L. C., Ewig R. A., Kohn K. W. DNA strand scission and cross-linking by diaziridinylbenzoquinone (diaziquone) in human cells and relation to cell killing. Cancer Res. 1984 Oct;44(10):4447–4452. [PubMed] [Google Scholar]
- Szmigiero L., Kohn K. W. Mechanisms of DNA strand breakage and interstrand cross-linking by diaziridinylbenzoquinone (diaziquone) in isolated nuclei from human cells. Cancer Res. 1984 Oct;44(10):4453–4457. [PubMed] [Google Scholar]
- Wardman P. Bioreductive activation of quinones: redox properties and thiol reactivity. Free Radic Res Commun. 1990;8(4-6):219–229. doi: 10.3109/10715769009053355. [DOI] [PubMed] [Google Scholar]
- Wilson I., Wardman P., Lin T. S., Sartorelli A. C. Reactivity of thiols towards derivatives of 2- and 6-methyl-1,4-naphthoquinone bioreductive alkylating agents. Chem Biol Interact. 1987 Mar;61(3):229–240. doi: 10.1016/0009-2797(87)90003-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Cowden W. B., Sutton H. C. Auto-oxidation of dialuric acid, divicine and isouramil. Superoxide dependent and independent mechanisms. Biochem Pharmacol. 1989 Feb 15;38(4):611–618. doi: 10.1016/0006-2952(89)90206-2. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Munday R. Concerted action of reduced glutathione and superoxide dismutase in preventing redox cycling of dihydroxypyrimidines, and their role in antioxidant defence. Free Radic Res Commun. 1990;8(4-6):287–293. doi: 10.3109/10715769009053361. [DOI] [PubMed] [Google Scholar]
- d'Arcy Doherty M., Wilson I., Wardman P., Basra J., Patterson L. H., Cohen G. M. Peroxidase activation of 1-naphthol to naphthoxy or naphthoxy-derived radicals and their reaction with glutathione. Chem Biol Interact. 1986 May;58(2):199–215. doi: 10.1016/s0009-2797(86)80098-9. [DOI] [PubMed] [Google Scholar]


