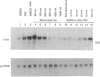
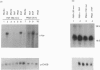
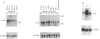Abstract
In A-431 cells, platelet-activating factor (PAF) induces the expression of c-fos and TIS-1 genes in both the absence and the presence of cycloheximide in a structurally specific and receptor-coupled manner. We have now investigated the molecular mechanisms of this response, particularly in relation to the role of protein kinases. Pretreatment of cells with genistein or methyl-2,5-dihydroxycinnamate (tyrosine kinase inhibitors) or staurosporine (a protein kinase C inhibitor) for 20 min abolished the c-fos expression induced by PAF. Interestingly, when genistein was added 90 s after addition of PAF, no inhibition was observed. Similarly, staurosporine did not inhibit c-fos expression when added 8 min after PAF addition to the cells. These inhibitions were dose-dependent (IC50 for staurosporine was 180 nM, and for genistein 50 microM). Simultaneous addition of PAF and phorbol 12-myristate 13-acetate (PMA) did not give a synergistic effect on c-fos expression. Pretreatment of cells with PMA had no effect on [3H]PAF binding, but abolished the PAF-induced gene expression. PAF-stimulated gene expression was desensitized if cells were pretreated with PAF. Interestingly, epidermal growth factor was able to stimulate c-fos expression in PAF-desensitized cells, and thus indicated involvement of distinct mechanisms for the two stimuli. Forskolin, an activator of adenylate cyclase, did not induce c-fos expression and had no effect on the PAF response. Exposure of cells to PAF for as little as 1 min, followed by its removal, was sufficient to activate the gene expression and demonstrated the rapidity and the exquisite nature of the signalling involved in this process. It is concluded that activation of PAF receptor (a proposed G-protein-coupled receptor) causes rapid production of signals which induce the expression of c-fos gene and that this is mediated via tyrosine kinase and protein kinase C.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. Converging and diverging pathways in polypeptide hormone action. Clin Res. 1989 Oct;37(4):554–563. [PubMed] [Google Scholar]
- Carter R. H., Park D. J., Rhee S. G., Fearon D. T. Tyrosine phosphorylation of phospholipase C induced by membrane immunoglobulin in B lymphocytes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2745–2749. doi: 10.1073/pnas.88.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Dhar A., Paul A. K., Shukla S. D. Platelet-activating factor stimulation of tyrosine kinase and its relationship to phospholipase C in rabbit platelets: studies with genistein and monoclonal antibody to phosphotyrosine. Mol Pharmacol. 1990 Apr;37(4):519–525. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Georgopoulos K., Greenberg M. E., Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988 Dec 2;55(5):917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Lee W. M., Snyderman R. Chemoattractant-induced activation of c-fos gene expression in human monocytes. J Exp Med. 1987 Jun 1;165(6):1524–1538. doi: 10.1084/jem.165.6.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kanei-Ishii C., Ishii S. Dual enhancer activities of the cyclic-AMP responsive element with cell type and promoter specificity. Nucleic Acids Res. 1989 Feb 25;17(4):1521–1536. doi: 10.1093/nar/17.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Martinson E. A., Trilivas I., Brown J. H. Rapid protein kinase C-dependent activation of phospholipase D leads to delayed 1,2-diglyceride accumulation. J Biol Chem. 1990 Dec 25;265(36):22282–22287. [PubMed] [Google Scholar]
- Mazer B., Domenico J., Sawami H., Gelfand E. W. Platelet-activating factor induces an increase in intracellular calcium and expression of regulatory genes in human B lymphoblastoid cells. J Immunol. 1991 Mar 15;146(6):1914–1920. [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- Morrison W. J., Dhar A., Shukla S. D. Staurosporine potentiates platelet activating factor stimulated phospholipase C activity in rabbit platelets but does not block desensitization by platelet activating factor. Life Sci. 1989;45(4):333–339. doi: 10.1016/0024-3205(89)90143-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nakano H., Kobayashi E., Takahashi I., Tamaoki T., Kuzuu Y., Iba H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 1987 May;40(5):706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Powis G. Signalling targets for anticancer drug development. Trends Pharmacol Sci. 1991 May;12(5):188–194. doi: 10.1016/0165-6147(91)90545-4. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Platelet-activating factor. J Biol Chem. 1990 Oct 15;265(29):17381–17384. [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. W. Bombesin induction of c-fos and c-myc proto-oncogenes in Swiss 3T3 cells: significance for the mitogenic response. J Cell Physiol. 1987 May;131(2):218–225. doi: 10.1002/jcp.1041310211. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Verma I. M. Regulation of proto-oncogene fos: a paradigm for early response genes. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):749–760. doi: 10.1101/sqb.1988.053.01.085. [DOI] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Halenda S. P. Phospholipase D in cell signalling and its relationship to phospholipase C. Life Sci. 1991;48(9):851–866. doi: 10.1016/0024-3205(91)90031-6. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Inositol phospholipid turnover in PAF transmembrane signalling. Lipids. 1991 Dec;26(12):1028–1033. doi: 10.1007/BF02536496. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Platelet activating factor-stimulated formation of inositol triphosphate in platelets and its regulation by various agents including Ca2+, indomethacin, CV-3988, and forskolin. Arch Biochem Biophys. 1985 Aug 1;240(2):674–681. doi: 10.1016/0003-9861(85)90075-x. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992 Mar;6(6):2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- Spangler R., Bailey S. C., Sytkowski A. J. Erythropoietin increases c-myc mRNA by a protein kinase C-dependent pathway. J Biol Chem. 1991 Jan 15;266(2):681–684. [PubMed] [Google Scholar]
- Squinto S. P., Block A. L., Braquet P., Bazan N. G. Platelet-activating factor stimulates a fos/jun/AP-1 transcriptional signaling system in human neuroblastoma cells. J Neurosci Res. 1989 Dec;24(4):558–566. doi: 10.1002/jnr.490240414. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Berk B. C., Izumo S., Tsuda T., Alexander R. W., Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem. 1989 Jan 5;264(1):526–530. [PubMed] [Google Scholar]
- Trejo J., Brown J. H. c-fos and c-jun are induced by muscarinic receptor activation of protein kinase C but are differentially regulated by intracellular calcium. J Biol Chem. 1991 Apr 25;266(12):7876–7882. [PubMed] [Google Scholar]
- Tripathi Y. B., Kandala J. C., Guntaka R. V., Lim R. W., Shukla S. D. Platelet activating factor induces expression of early response genes c-fos and TIS-1 in human epidermoid carcinoma A-431 cells. Life Sci. 1991;49(23):1761–1767. doi: 10.1016/0024-3205(91)90319-7. [DOI] [PubMed] [Google Scholar]