Abstract
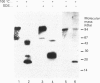
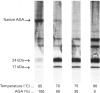
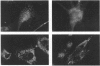
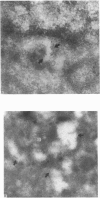
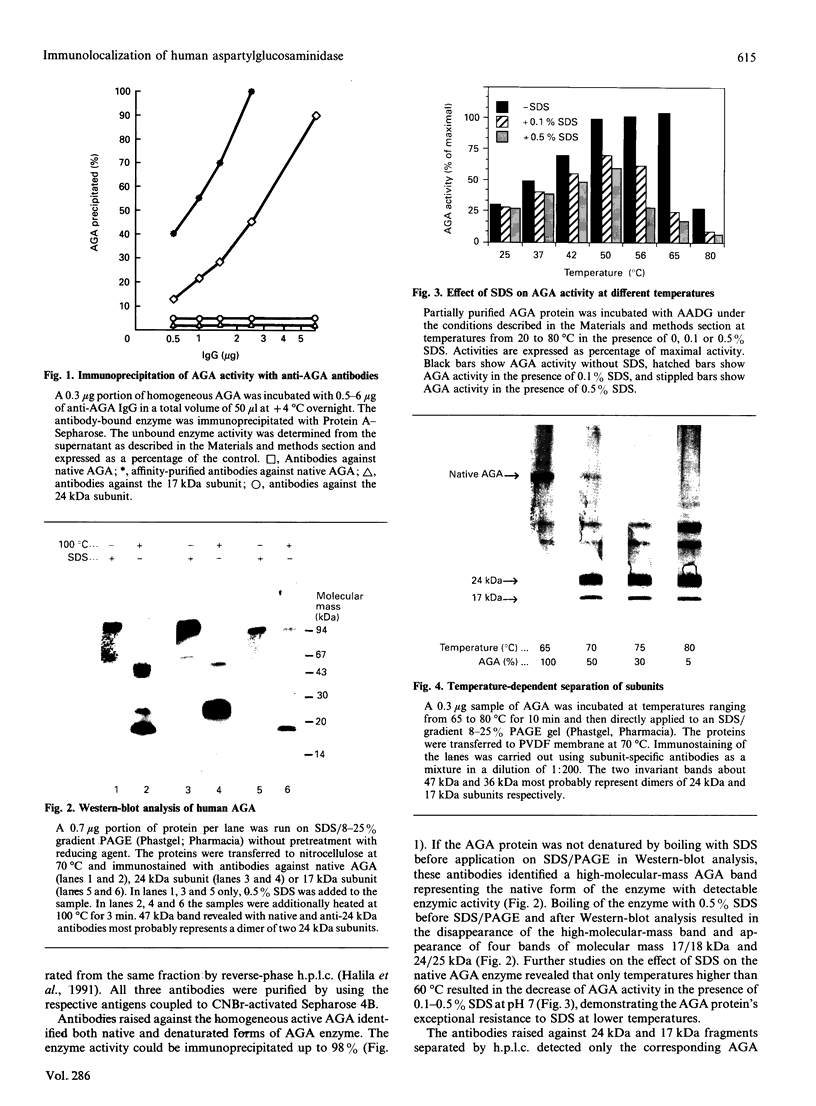
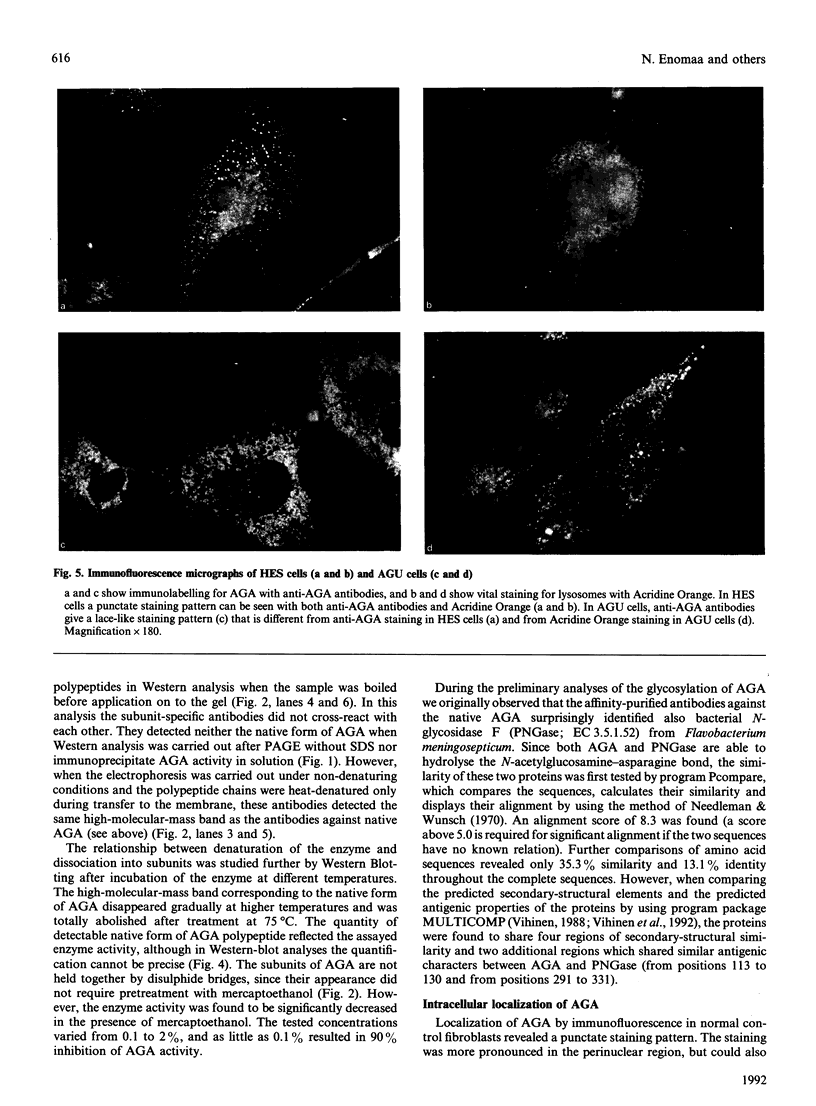
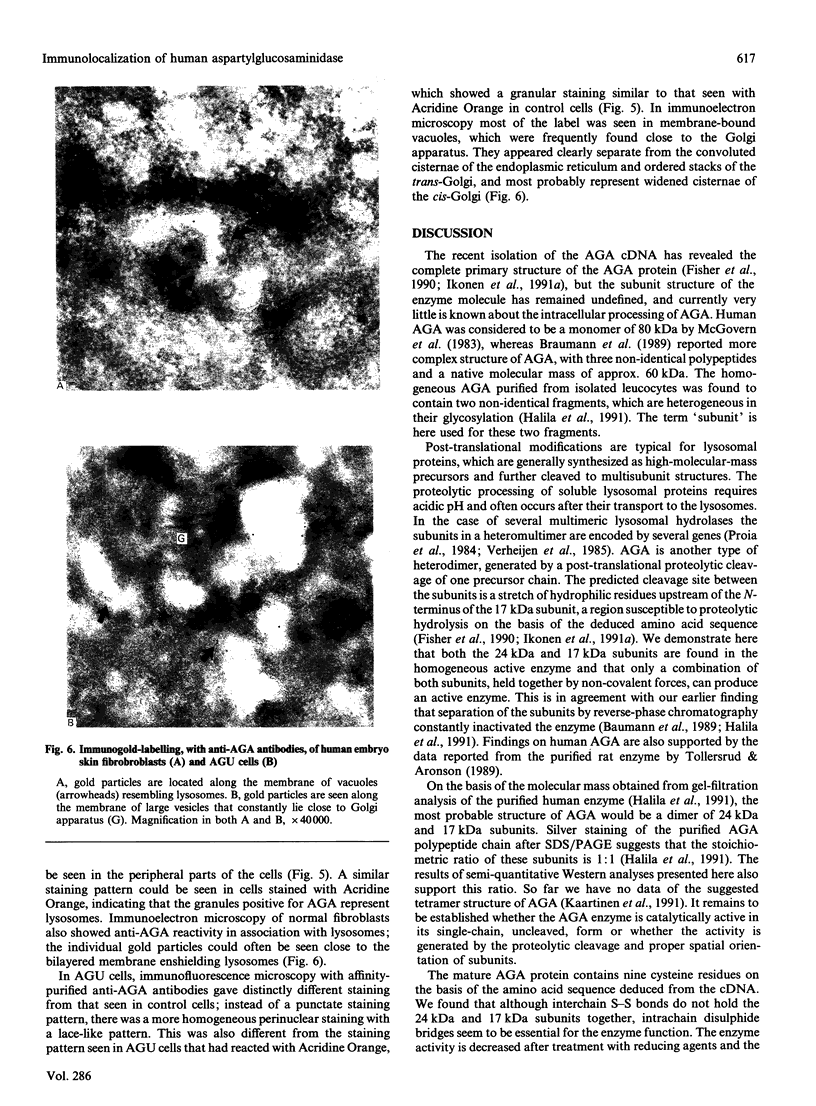
Aspartylglucosaminidase (AGA, EC 3.5.1.26) is an essential enzyme in the degradation of asparagine-linked glycoproteins. In man, deficient activity of this enzyme leads to aspartylglucosaminuria (AGU), a recessively inherited lysosomal storage disease. Here we used affinity-purified polyclonal antibodies against the native AGA and its denatured subunits to establish the molecular structure and intracellular location of the enzyme in normal and AGU fibroblasts. Inactivation of the enzyme was found to coincide with the dissociation of the heterodimeric enzyme complex into subunits. Although the subunits were not linked by covalent forces, the intrapolypeptide disulphide bridges were found to be essential for the normal function of AGA. AGA was localized into lysosomes in control fibroblasts by both immunofluorescence microscopy and immuno-electron microscopy, whereas in AGU cells the location of antigen was different, suggesting that, owing to the mutation, a missing disulphide bridge, most of the enzyme molecules get retarded in the cis-Golgi region and most probably face intracellular degradation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann M., Peltonen L., Aula P., Kalkkinen N. Isolation of a human hepatic 60 kDa aspartylglucosaminidase consisting of three non-identical polypeptides. Biochem J. 1989 Aug 15;262(1):189–194. doi: 10.1042/bj2620189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Fisher K. J., Tollersrud O. K., Aronson N. N., Jr Cloning and sequence analysis of a cDNA for human glycosylasparaginase. A single gene encodes the subunits of this lysosomal amidase. FEBS Lett. 1990 Sep 3;269(2):440–444. doi: 10.1016/0014-5793(90)81211-6. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Halila R., Baumann M., Ikonen E., Enomaa N., Peltonen L. Human leucocyte aspartylglucosaminidase. Evidence for two different subunits in a more complex native structure. Biochem J. 1991 May 15;276(Pt 1):251–256. doi: 10.1042/bj2760251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Baumann M., Grön K., Syvänen A. C., Enomaa N., Halila R., Aula P., Peltonen L. Aspartylglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J. 1991 Jan;10(1):51–58. doi: 10.1002/j.1460-2075.1991.tb07920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Enomaa N., Ulmanen I., Peltonen L. In vitro mutagenesis helps to unravel the biological consequences of aspartylglucosaminuria mutation. Genomics. 1991 Sep;11(1):206–211. doi: 10.1016/0888-7543(91)90120-4. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kaartinen V., Williams J. C., Tomich J., Yates J. R., 3rd, Hood L. E., Mononen I. Glycosaparaginase from human leukocytes. Inactivation and covalent modification with diazo-oxonorvaline. J Biol Chem. 1991 Mar 25;266(9):5860–5869. [PubMed] [Google Scholar]
- Le A., Graham K. S., Sifers R. N. Intracellular degradation of the transport-impaired human PiZ alpha 1-antitrypsin variant. Biochemical mapping of the degradative event among compartments of the secretory pathway. J Biol Chem. 1990 Aug 15;265(23):14001–14007. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Tappel A. L. Beta-aspartylglucosylamine amido hydrolase of rat liver and kidney. J Biol Chem. 1967 Oct 25;242(20):4568–4576. [PubMed] [Google Scholar]
- Makino M., Kojima T., Yamashina I. Enzymatic cleavage of glycopeptides. Biochem Biophys Res Commun. 1966 Sep 22;24(6):961–966. doi: 10.1016/0006-291x(66)90344-5. [DOI] [PubMed] [Google Scholar]
- Maury C. P. Accumulation of glycoprotein-derived metabolites in neural and visceral tissue in aspartylglycosaminuria. J Lab Clin Med. 1980 Nov;96(5):838–844. [PubMed] [Google Scholar]
- McGovern M. M., Aula P., Desnick R. J. Purification and properties of human hepatic aspartylglucosaminidase. J Biol Chem. 1983 Sep 10;258(17):10743–10747. [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Pakula A. A., Sauer R. T. Genetic analysis of protein stability and function. Annu Rev Genet. 1989;23:289–310. doi: 10.1146/annurev.ge.23.120189.001445. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Proia R. L., Neufeld E. F. Synthesis of beta-hexosaminidase in cell-free translation and in intact fibroblasts: an insoluble precursor alpha chain in a rare form of Tay-Sachs disease. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6360–6364. doi: 10.1073/pnas.79.20.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Syvänen A. C., Ikonen E., Manninen T., Bengtström M., Söderlund H., Aula P., Peltonen L. Convenient and quantitative determination of the frequency of a mutant allele using solid-phase minisequencing: application to aspartylglucosaminuria in Finland. Genomics. 1992 Mar;12(3):590–595. doi: 10.1016/0888-7543(92)90452-x. [DOI] [PubMed] [Google Scholar]
- Tan-Wilson A. L., Reichlin M., Noble R. W. Isolation and characterization of low and high affinity goat antibodies directed to single antigenic sites on human hemoglobin. Immunochemistry. 1976 Nov;13(11):921–927. doi: 10.1016/0019-2791(76)90236-6. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The isolation and structure of the core oligosaccharide sequences of IgM. Biochemistry. 1975 Dec 16;14(25):5516–5523. doi: 10.1021/bi00696a021. [DOI] [PubMed] [Google Scholar]
- Tollersrud O. K., Aronson N. N., Jr Purification and characterization of rat liver glycosylasparaginase. Biochem J. 1989 May 15;260(1):101–108. doi: 10.1042/bj2600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen F. W., Palmeri S., Hoogeveen A. T., Galjaard H. Human placental neuraminidase. Activation, stabilization and association with beta-galactosidase and its protective protein. Eur J Biochem. 1985 Jun 3;149(2):315–321. doi: 10.1111/j.1432-1033.1985.tb08928.x. [DOI] [PubMed] [Google Scholar]
- Vihinen M. An algorithm for simultaneous comparison of several sequences. Comput Appl Biosci. 1988 Mar;4(1):89–92. doi: 10.1093/bioinformatics/4.1.89. [DOI] [PubMed] [Google Scholar]
- Wendland M., von Figura K., Pohlmann R. Mutational analysis of disulfide bridges in the Mr 46,000 mannose 6-phosphate receptor. Localization and role for ligand binding. J Biol Chem. 1991 Apr 15;266(11):7132–7136. [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]