Abstract
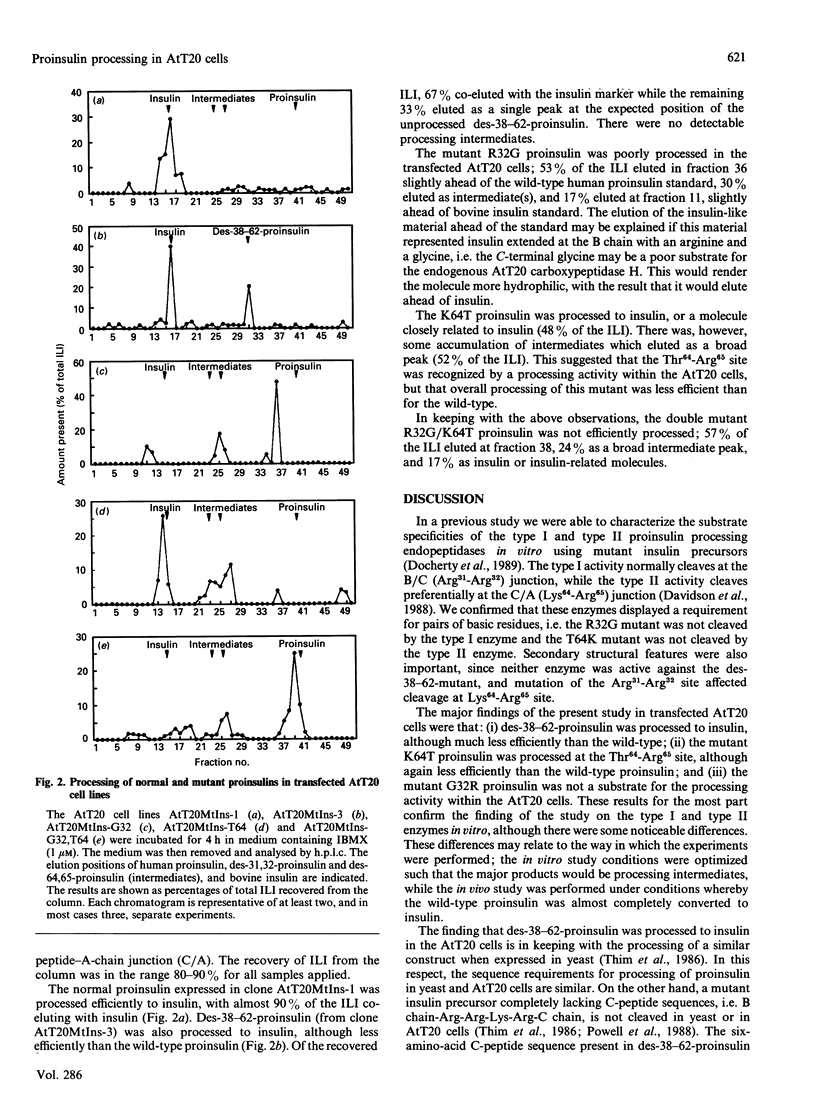
To investigate the sequence requirements for proteolytic processing of prohormones at pairs of basic amino acids, normal and mutant proinsulins were expressed in the mouse pituitary corticotrophic cell line AtT20. The extent of processing was determined by h.p.l.c. analysis of insulin-like immunoreactivity secreted into the media of transfected cells. In this model system, normal proinsulin was efficiently processed to insulin. The mutant des-38-62-proinsulin, in which all but six amino acids of the C-peptide were deleted, was also processed to insulin but less efficiently than the wild-type. The mutant Lys64-Arg65 to Thr64-Arg65 was partially processed to insulin, while the mutant Arg31-Arg32 to Arg31-Gly32 was not processed at either site. These results indicate: (i) that a six-amino-acid spacer between the two pairs of basic amino acids in proinsulin is sufficient to permit processing at both sites; (ii) that the endoproteinase responsible for cleavage at the Lys64-Arg65 site will also recognize Thr64-Arg65; (iii) that the endoproteinase responsible for cleavage at the Arg31-Arg32 site will not recognize Arg31-Gly32; and (iv) that the change Arg31-Arg32 to Arg31-Gly32 affects processing at the Lys64-Arg65 site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bek E., Berry R. Prohormonal cleavage sites are associated with omega loops. Biochemistry. 1990 Jan 9;29(1):178–183. doi: 10.1021/bi00453a024. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Dickerson I. M., Dixon J. E., Mains R. E. Biosynthesis and posttranslational processing of site-directed endoproteolytic cleavage mutants of pro-neuropeptide Y in mouse pituitary cells. J Biol Chem. 1990 Feb 15;265(5):2462–2469. [PubMed] [Google Scholar]
- Dickerson I. M., Dixon J. E., Mains R. E. Transfected human neuropeptide Y cDNA expression in mouse pituitary cells. Inducible high expression, peptide characterization, and secretion. J Biol Chem. 1987 Oct 5;262(28):13646–13653. [PubMed] [Google Scholar]
- Docherty K., Rhodes C. J., Taylor N. A., Shennan K. I., Hutton J. C. Proinsulin endopeptidase substrate specificities defined by site-directed mutagenesis of proinsulin. J Biol Chem. 1989 Nov 5;264(31):18335–18339. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Ferber S., Gross D. J., Villa-Komaroff L., Danehy F., Vollenweider F., Meyer K., Loeken M. R., Kahn C. R., Halban P. A. Heterogeneity of expression and secretion of native and mutant [AspB10]insulin in AtT20 cells. Mol Endocrinol. 1991 Mar;5(3):319–326. doi: 10.1210/mend-5-3-319. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J., Pekar A. H. Physical studies on proinsulin. A comparison of the titration behavior of the tyrosine residues in insulin and proinsulin. Biochemistry. 1972 Dec 19;11(26):4926–4931. doi: 10.1021/bi00776a008. [DOI] [PubMed] [Google Scholar]
- Gomez S., Boileau G., Zollinger L., Nault C., Rholam M., Cohen P. Site-specific mutagenesis identifies amino acid residues critical in prohormone processing. EMBO J. 1989 Oct;8(10):2911–2916. doi: 10.1002/j.1460-2075.1989.tb08440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gross D. J., Villa-Komaroff L., Kahn C. R., Weir G. C., Halban P. A. Deletion of a highly conserved tetrapeptide sequence of the proinsulin connecting peptide (C-peptide) inhibits proinsulin to insulin conversion by transfected pituitary corticotroph (AtT20) cells. J Biol Chem. 1989 Dec 25;264(36):21486–21490. [PubMed] [Google Scholar]
- Hutton J. C. Subtilisin-like proteinases involved in the activation of proproteins of the eukaryotic secretory pathway. Curr Opin Cell Biol. 1990 Dec;2(6):1131–1142. doi: 10.1016/0955-0674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Lapps W., Eng J., Stern A. S., Gubler U. Expression of porcine cholecystokinin cDNA in a murine neuroendocrine cell line. Proteolytic processing, sulfation, and regulated secretion of cholecystokinin peptides. J Biol Chem. 1988 Sep 15;263(26):13456–13462. [PubMed] [Google Scholar]
- Nagahama M., Nakayama K., Murakami K. Sequence requirements for prohormone processing in mouse pituitary AtT-20 cells. Analysis using prorenins as model substrates. Eur J Biochem. 1991 Apr 10;197(1):135–140. doi: 10.1111/j.1432-1033.1991.tb15891.x. [DOI] [PubMed] [Google Scholar]
- Noel G., Keutmann H. T., Mains R. E. Investigation of the structural requirements for peptide precursor processing in AtT-20 cells using site-directed mutagenesis of proadrenocorticotropin/endorphin. Mol Endocrinol. 1991 Mar;5(3):404–413. doi: 10.1210/mend-5-3-404. [DOI] [PubMed] [Google Scholar]
- Oda K., Ikeda M., Tsuji E., Sohda M., Takami N., Misumi Y., Ikehara Y. Sequence requirements for proteolytic cleavage of precursors with paired basic amino acids. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1181–1186. doi: 10.1016/0006-291x(91)91696-a. [DOI] [PubMed] [Google Scholar]
- Powell S. K., Orci L., Craik C. S., Moore H. P. Efficient targeting to storage granules of human proinsulins with altered propeptide domain. J Cell Biol. 1988 Jun;106(6):1843–1851. doi: 10.1083/jcb.106.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn D., Orci L., Ravazzola M., Moore H. P. Intracellular transport and sorting of mutant human proinsulins that fail to form hexamers. J Cell Biol. 1991 Jun;113(5):987–996. doi: 10.1083/jcb.113.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett. 1986 Oct 20;207(1):1–6. doi: 10.1016/0014-5793(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W. The processing of peptide precursors. 'Proline-directed arginyl cleavage' and other monobasic processing mechanisms. FEBS Lett. 1986 May 5;200(1):1–10. doi: 10.1016/0014-5793(86)80500-2. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shakur Y., Shennan K. I., Taylor N. A., Docherty K. A major C-peptide deletion prevents secretion of a mutant human proinsulin from transfected monkey kidney cells. J Mol Endocrinol. 1989 Sep;3(2):155–162. doi: 10.1677/jme.0.0030155. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Snell C. R., Smyth D. G. Proinsulin: a proposed three-dimensional structure. J Biol Chem. 1975 Aug 25;250(16):6291–6295. [PubMed] [Google Scholar]
- Thim L., Hansen M. T., Norris K., Hoegh I., Boel E., Forstrom J., Ammerer G., Fiil N. P. Secretion and processing of insulin precursors in yeast. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6766–6770. doi: 10.1073/pnas.83.18.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne B. A., Thomas G. An in vivo characterization of the cleavage site specificity of the insulin cell prohormone processing enzymes. J Biol Chem. 1990 May 25;265(15):8436–8443. [PubMed] [Google Scholar]
- Weiss M. A., Frank B. H., Khait I., Pekar A., Heiney R., Shoelson S. E., Neuringer L. J. NMR and photo-CIDNP studies of human proinsulin and prohormone processing intermediates with application to endopeptidase recognition. Biochemistry. 1990 Sep 11;29(36):8389–8401. doi: 10.1021/bi00488a028. [DOI] [PubMed] [Google Scholar]


