Abstract
Plant-derived phytochemicals from medicinal plants are becoming increasingly attractive natural sources of antimicrobial and antiviral agents due to their therapeutic value, mechanism of action, level of toxicity and bioavailability. The continued emergence of more immune-evasive strains and the rate of resistance to current antiviral drugs have created a need to identify new antiviral agents against SARS-CoV-2. This study investigated the antiviral potential of balsaminol, a bioactive compound from Momordica balsamina, and its inhibitory activities against SARS-CoV-2 receptor proteins. In this study, three Food and Drug Administration (FDA) COVID-19 approved drugs namely; nirmatrelvir, ritonavir and remdesivir were used as positive control. Molecular docking was performed to determine the predominant binding mode (most negative Gibbs free energy of binding/ΔG) and inhibitory activity of balsaminol against SARS-CoV-2 receptor proteins. The pharmacokinetics, toxicity, physicochemical and drug-like properties of balsaminol were evaluated to determine its potential as an active oral drug candidate as well as its non-toxicity in humans. The results show that balsaminol E has the highest binding affinity to the SARS CoV-2 papain-like protease (7CMD) with a free binding energy of − 8.7 kcal/mol, followed by balsaminol A interacting with the spike receptor binding domain (6VW1) with − 8.5 kcal/mol and balsaminol C had a binding energy of − 8.1 kcal/mol with the main protease (6LU7) comparable to the standard drugs namely ritonavir, nirmatrelvir and remdesivir. However, the ADMET and drug-like profile of balsaminol F favours it as a better potential drug candidate and inhibitor of the docked SARS-CoV-2 receptor proteins. Further preclinical studies are therefore recommended.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-024-00241-0.
Keywords: SARS-CoV-2 receptor proteins, In silico, Balsaminol, Binding energy, Pharmacokinetics
Introduction
The SARS-CoV-2 virus was first detected in a wholesale seafood market in Wuhan, Hubei province, China, in December 2019. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) brought the world to a standstill, overwhelmed health facilities around the world and plunged global economies into a severe recession. Today, the world is still struggling to recover from the devastating impact of the pandemic, with new variants emerging. The SARS-CoV-2 virus causes severe pneumonia, a severe acute respiratory syndrome, leading to significant morbidity and mortality worldwide (Low et al. 2022; Yadav et al. 2021). The increased transmissibility, re-infection potential, disease severity and ongoing genetic mutations of SARS-CoV-2 have caused over 6.5 million deaths and 600 million cases worldwide, making it a public health emergency of international concern (Low et al. 2022). SARS-CoV-2 has been reported to cause mild to severe respiratory symptoms in immunocompromised patients and is asymptomatic in non-immunocompromised individuals (Yan et al. 2022). These observations underscore the need for continued testing, active monitoring, surveillance, control, antiviral treatment and, most importantly, vaccination. The evolution of SARS-CoV-2 with iteration on the spike glycoprotein gave rise to different variants of concern (VOC) such as alpha, beta, delta, gamma and omicron, which are currently variants under surveillance (VUM) (Mohsin and Mahmud 2022; Petersen et al. 2022). The emergence of the Omicron variant and its substrains BA.4 and BA.5, and other more immune-evasive strains such as BQ.1, BQ.1.1, BF.7, XBB and XBB.1, has drawn the attention of the World Health Organization (WHO), public health experts, scientists and governments to the urgency of alternative treatments (Offit 2023).
SARS-CoV-2 is a positive single-stranded RNA (+ ssRNA) spherically enveloped by a symmetric lipid bilayer nucleocapsid that also contains structural and non-structural proteins. The viral particle is composed of four structural proteins, the spike S, membrane M, envelope E and nucleocapsid N proteins (Nelson et al. 2020; Wong and Saier 2021). These structural proteins play essential roles in the host immune response, virion assembly, viral transcription, activation of the endoplasmic reticulum stress response and follow a signal sequence that facilitates their translocation to the endoplasmic reticulum. Non-structural proteins range from nsp1 to nsp10 and nsp12 to nsp16 (Thomas 2020; Wu et al. 2020). These nonstructural proteins perform a variety of functions, including protein cleavage, degradation of host mRNAs, viral replication-transcription, methylation to the 5' cap structure of viral mRNAs, and many others (Encinar et al. 2020; Yadav et al. 2021).
The SARS-CoV-2 life cycle involves viral cellular invasion, orchestrated by the spike S glycoprotein binding the host cell receptor ACE2 (angiotensin-converting enzyme 2) via its SARS-CoV-2 receptor binding domain of the S1 subunit to form the spike S glycoprotein-host cell receptor-ACE2 complex (Fehr et al. 2016; Nakagawa et al. 2018). This is followed by protease-mediated cleavage at the S1/S2 site by the transmembrane protease serine 2 (TMPRSS2), leading to membrane fusion, which facilitates viral nucleocapsid entry into the host cell cytosol (Li et al. 2003; Yadav et al. 2021). Following release of the viral nucleocapsid into the host cell cytosol, the polyproteins pp1a and pp1b produce 16 nsps through an autoproteolytic process, which subsequently forms the replication-transcription complex (RTC) for viral RNA synthesis, releasing multiple copies of viral RNA (Letko et al. 2020). The viral RNA (-ssRNA) serves as an intermediate template on which polymerase acts to produce multiple 5' nested sets of negative sense sgRNAs, which are subsequently used as templates to form a 3' nested set of positive sense sgRNAs, which then interact with the host ribosome to synthesize accessory and structural proteins for the SARS-CoV-2 (Kleine-Weber et al. 2018; Wang et al. 2020). This replication, post-translation mechanism and maturation steps in the cytosol of the host cell are tightly coordinated by the cysteine proteinase in nsp 5, more recently termed main proteinase (Mpro) or 3 cysteine-like proteinase (3CLpro) and papain-like protease (PLpro) (Cheng et al. 2021; V'Kovski et al. 2021). The main proteinase is a highly conserved 3-domain cysteine protease responsible for processing the C-terminus of nsp 4 to nsp 16 while activating RIG-I and MAVS through the K63-linked ubiquitination process, thereby allowing SARS-CoV-2 virus to escape neutralizing antibodies (Astuti et al. 2020). In contrast, the papain-like proteinase (PLpro), among other highly conserved multi-domain proteins, is designed to cleave ISGylated substrates (Naqvi et al. 2020; Yan et al. 2022). Notably, the immune escape mechanism orchestrated by nsp 3 via ubiquitin-like domain 1 depends on the ability of PLpro to target ISG15 modifications. Importantly, the immune escape mechanisms of nsp 3 and nsp 5 serve as attractive targets for antiviral drug design.
Recent studies have shown that in silico investigations are a rapid and cost-effective medium for screening plant-derived bioactive compounds with therapeutic activity using a computational biology approach to discover new drugs and inhibitors (small molecules or ligands) against potential molecular targets of the SARS-CoV-2 virus (Bultum et al. 2022). In some cases, certain drugs have been repurposed using a virtual screening approach to target SARS-CoV-2 viral proteases, with Paxlovid (nirmatrelvir-ritonavir), molnupiravir and remdesivir being approved by the Food and Drug Administration (FDA) for the treatment of hospitalised COVID-19 patients, particularly adults and children (Kushari et al. 2023; Umadevi et al. 2020). In addition, studies have demonstrated the potential of phytochemicals such as polyphenols (flavonoids), triterpenes and phytosterols, terpenoids, polysaccharides, capsaicinoids, carotenoids and tocopherols, alkaloids, saponins and glucosinolates, among others. Quercetin, piperin, curcumin, reserpine, aloe-emodin, homonataloin and many others (Mujwar and Harwansh 2022; Rutwick Surya and Praveen 2021; Umadevi et al. 2020). Most of these phytochemicals possess medicinal properties such as antiviral, antioxidant, antimicrobial, anti-inflammatory, immunomodulatory and anticarcinogenic (Chtita et al. 2022; Prasanth et al. 2023).
The aim of this study was to investigate the antiviral potential of phytochemicals from Momordica balsamina. Momordica balsamina is a perennial plant, commonly known as balsam apple or African pumpkin, that grows in tropical Africa, India and Asia (Mashiane et al. 2021). It is a member of the Cucurbitaceae family, a creeper that grows on building walls and trees and is rich in medicinal and nutritious phytochemicals (Mashiane et al. 2021). In Africa, it is used in traditional medicine to treat diabetes (anti-hyperglycaemic and renoprotective), malaria (anti-malarial), reverse multidrug resistance in cancer (anticarcinogenic), and has antiviral, antibacterial, anti-inflammatory, antioxidant and analgesic effects, among others (Jibril et al. 2018; Kaur et al. 2013; Ludidia et al. 2019; Ramalhete et al. 2022). The whole vegetative parts of Momordica balsamina have been reported to have medicinal properties either as infusion, decoction, herbal tea or sauce (Managa et al. 2020; Subramaniam et al. 2017). In the present study, we evaluated six balsaminol sets (with different oxidation patterns), namely balsaminol A, balsaminol B, balsaminol C, balsaminol D, balsaminol E and balsaminol F from Momordica balsamina, as potential drugs against SARS-CoV-2 virus. These plant-derived phytoconstituents, along with the Food and Drug Administration (FDA) COVID-19 approved drugs Paxlovid (nirmatrelvir-ritonavir) and remdesivir as positive controls, were screened against 3 cysteine-like proteases (3CLpro) or main proteases, papain-like proteases (PLpro) and spike S glycoprotein receptor binding domain using molecular docking studies. The molecular interactions between balsaminol phytochemicals and SARS-CoV-2 receptors results revealed a favourable free binding energy (ΔG ≥ 6.7 kcal/mol). Balsaminol E complexes with the most favaorable binding affinity against papain-like protease and balsaminol F with the most favoured pharmacokinetic properties were simulated for 100 ns to analyze the stability.
Materials and methods
Phytochemical retrieval
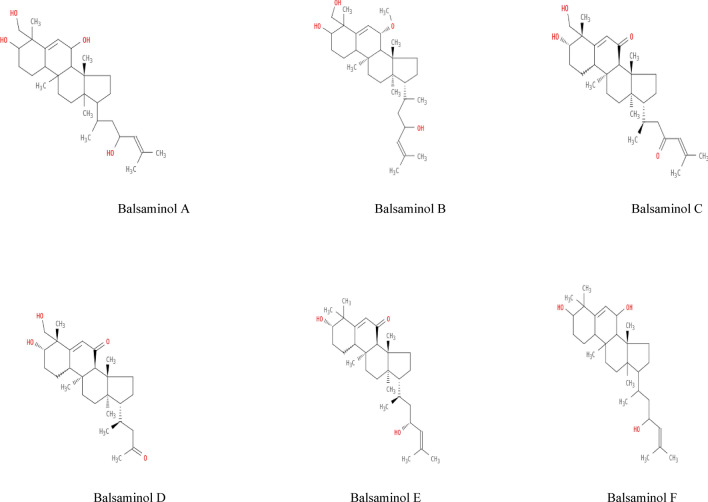
Chemical, structural and canonical Simplified Molecular Input Line Entry System (SMILES) data for the ligand molecules were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and the ChEMBL database (ebi.ac.uk). Using the canonical SMILES, the Protein Data Bank (PDB), Structural Data File (SDF) and MOL files of the compounds were obtained using the Swiss Target Prediction tool (Swiss Target Prediction) and their structures were predicted (Daina et al. 2017). The phytochemical compounds from Momordica balsamina (Fig. 1 with different oxidation patterns) are: Balsaminol A, Balsaminol B, Balsaminol C, Balsaminol D, Balsaminol E and Balsaminol F. Paxlovid (nirmatrelvir-ritonavir), molnupiravir and remdesivir (Wishart et al. 2018) were used as positive controls.
Fig. 1.
Showing 2D structures of balsaminol bioactive compounds from Momordica balsamina
Target receptor preparation
The crystal structures of SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) were used as target receptors in this study. The 3D structures were obtained from the Protein Data Bank (PDB) (http://www.rcsb.org/pdb) (Burley et al. 2023). Water molecules and other unwanted complexes or ligands were removed from the downloaded protease to avoid unwanted molecular interactions and to ensure that no molecule interfered with the potential binding site of the target protease during the docking simulation.
The SARS-CoV-2 main protease (PDB ID: 6LU7), also known as 3-Cysteine-like protease (3CLpro), is a non-structural protein 5 (nsp5) that cleaves viral polyproteins translated from viral RNA, resulting in nsp5 to nsp16. The receptor binding domain (PDB ID: 6VW1) of the SARS-CoV-2 spike protein is known to recognise and bind to the host receptor angiotensin converting enzyme (ACE2) and fuse into the membrane of respiratory epithelial cells. The papain-like protease (PDB ID: 7CMD), a non-structural protein 3 (nsp3) required for cleavage of two viral polyproteins (pp1a and pp1ab) and the ubiquitin-like protein ISG15, evades host antiviral immune responses (Lubin et al. 2023; Yadav et al. 2021).
Tertiary structure prediction, refinement and validation
The three-dimensional model structures of the receptor proteins were constructed using the online tool GalaxyWeb (Seok et al. 2013). This was done based on the conserved coordinates between the target proteins and the templates. The quaternary structural annotation of the model was used to model the target sequences in their oligomeric forms. The method (Bertoni et al. 2017) was based on a supervised machine learning algorithm, Support Vector Machines (SVM), which combines interface conservation, structural clustering and other template features to provide a quaternary structure quality estimate (QSQE). The protein structures of SARS-CoV-2 main protease (PDB ID: 6LU7), Spike Receptor Binding Domain (PDB ID: 6VW1) and Papain-like protease (PDB ID: 7CMD) were subjected to in-depth analysis of model quality and potential function using the SWISS model structure assessment server and Galaxyweb model structure refinement (Heo et al. 2013; Lee et al. 2016; Naveed et al. 2022). Torsion angles of phi-psi amino acid residues in protein structures were analysed using the Ramachandran plot, and model quality was assessed using Molprobity version 4.4. The z-score of SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) was validated using the ProSAweb server (https://prosa.services.came.sbg.ac.at/prosa.php).
Receptor protein binding cavity prediction
The binding pocket, ligand interactions and all amino acids in the active site of SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) were determined using the CB-Dock2 web server (http://clab.labshare.cn/cb-dock/php/index.php) (Liu et al. 2022) and Biovia Discovery Studio (2019). The information collected was compared and validated with the experimental data previously reported for the SARS-CoV-2 Mpro active site complexed with the native N3 ligand (Liu et al. 2020).
Molecular docking
Molecular docking was performed using the CB-Dock2 web server (http://clab.labshare.cn/cb-dock/php/ index.php), a protein–ligand blind docking server that integrates cavity detection, docking and homologous template matching. CB-Dock automatically identifies the binding sites, calculates the cavity centre (XYX coordinates) and cavity size (XYZ dimension), adjusts the docking box size according to the query ligand, then performs the molecular docking with AutoDock Vina and arranges them according to their Vina scores (Liu et al. 2022). The crystal structures of SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) were used for the molecular docking. The 3D and 2D poses of the protein–ligand complexes were then analysed and visualised using Discovery Studio Visualizer 21.1.0.20298.
Assessment of ADMET and drug likeness
Absorption, distribution, metabolism, excretion and toxicity (ADMET) properties of the selected compounds were predicted using the Swiss ADME (http://www.swissadme.ch/) and ADMET SAR2 web servers (http://lmmd.ecust.edu.cn/admetsar2/), while drug-like properties were evaluated using the AI Drug Lab online tool (Daina et al. 2017; Tian et al. 2022; Yang et al. 2019).
Molecular dynamics
The balsaminol with the highest binding affinity was selected for the molecular dynamics (MD) simulation study. The molecular dynamics (MD) simulation was performed using BioBB-Wfs server (https://mmb.irbbarcelona.org/biobb-wfs) with AMBER99SB force field (Bayarri et al. 2022). The protein and its complexes were solvated in octahedron boxes using the TIP3P model. All structures were neutralised by the addition of sodium or chlorine counterions at 0.05. All systems were then equilibrated for NVT and NPT for 1 ns each. NVT equilibration was performed at 300 K using a leap frog integrator and NPT equilibration was performed at 1.0 bar. The 500 ps MD simulation was performed and a total of 10,000 frames of each system were stored at 10 ps intervals. After 500 ps of simulation, the trajectory was analysed using parameters such as root mean square deviation (RMSD), radius of gyration (Rg), GROMACS Energies.
Results
Tertiary structure prediction, refinement and validation
The prediction of the 3D structures of SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) as shown in Figs. 2A, 3A and 4A was achieved using the SWISS-MODEL server (https://swissmodel.expasy.org/interactive). The initial predicted 3D structure with SWISS-MODEL Structure Assessment (https://swissmodel.expasy.org/assess) shows a Ramachandran plot of 97.04% residues in the most favoured region for 6LU7, 97.67% residues for 6SVB and 98.11% residues in the most favoured region for 7CMD (Figs. 2B, 3B and 4B). On refinement using the GalaxyWeb server, model 1 was selected as the best model with the following parameters GDT-HA (0.9992), RMSD (0.185), MolProbity (1.39), clash score (7.05), poor rotamers (0.00%) and Ramachandran plot (98.36%) for 6LU7; GDT-HA (0.9988), RMSD (0.182), MolProbity (1.38), clash score (6.81), poor rotamers (0. 53%) and Ramachandran plot (98.60%) for 6VW1; and on the other hand for 7CMD, GDT-HA (0.9929), RMSD (0.243), MolProbity (1.19), clash score (3.99), poor rotamers (0.72%) and Ramachandran plot (98.11%) (Supporting Information T1). Validation of the predicted 3D structure with SWISS-MODEL Structure Assessment (https://swissmodel.expasy.org/assess) shows a Ramachandran plot of 98.36% residues in the most favoured region, QMEAN (-0.44) and torsion (0.0) for 6LU7. In a new light, we had 98.60% residues in the most favoured region, QMEAN (-1.73) and torsion (-0.86) for 6VW1, while we had 98.11% residues in the most favoured region, QMEAN (0.41) and torsion (0.47) for 7CMD. In addition, the SARS-CoV-2 main protease (PDB ID: 6LU7), spike receptor binding domain (PDB ID: 6VW1) and papain-like protease (PDB ID: 7CMD) constructs also fall within the range of native proteins with Z scores of -7.54, -5.54 and -8.44 using the ProSAweb server (Figs. 2C, 3C and 4C).
Fig. 2.
SARS-CoV-2 main protease (PDB ID: 6LU7) 3D structure predicted A model quality assessment, B Ramachandran plot (98.36%) residues in favored region, C Z-graph NMR spectroscopy (dark blue) and X-ray crystallography with main protease spotted as a black dot (light blue) (z-score = − 7.54), (D) ProSA web plot of residue scores
Fig. 3.
SARS-CoV-2 Spike Receptor Binding Domain (PDB ID: 6VW1) 3D structure predicted A model quality assessment, B Ramachandran plot (98.60%) residues in favored region, C Z-graph NMR spectroscopy (dark blue) and X-ray crystallography with Spike Receptor Binding Domain spotted as a black dot (light blue) (z-score = -5.54), D ProSA web plot of residue scores
Fig. 4.
SARS-CoV-2 Papain-like Protease (PDB ID: 7CMD) 3D structure predicted A model quality assessment, B Ramachandran plot (98.11%) residues in favored region, C Z-graph NMR spectroscopy (dark blue) and X-ray crystallography with Papain-like Protease spotted as a black dot (light blue) (z-score = -8.44), D ProSA web plot of residue scores
Receptor protein cavity prediction
The protein binding cavities were determined using the CB-Dock Cavity Detection Guide blind docking tool by calculating the cavity volumes, centres and cavity sizes of the top N (n = 5) best cavities. The prediction of the main SARS-CoV-2 protease (PDB ID: 6LU7) yielded 5 cavities or pockets, with the top-ranked cavity having a cavity volume of 425 Å3, cavity centre (-20x, 51y, 27z) and cavity size (8x, 11y, 7z). The spike receptor binding domain (PDB ID: 6VW1) had the highest ranked binding pocket with a cavity volume of 300 Å3, cavity centre (-64x, 8y, -12z) and cavity size (12x, 11y, 4z), while the papain-like protease (PDB ID: 7CMD) had the highest ranked cavity volume of 535 Å3, cavity centre (-3x, 93y, 17z) and cavity size (15x, 10y, 8z) (Supporting Information T2).
Molecular docking
Based on the molecular docking results of the investigation, all six balsaminol phytochemicals from Momordica balsamina and the positive control drugs showed negative binding energy on the target proteins. A structural conformation study of the protein–ligand complexes was carried out to identify the ligand-amino acid residue interactions (conventional hydrogen bonding, carbon-hydrogen bonding, van der Waals forces and hydrophobic interactions) and the protein-drug surface conformation. The binding energies, interacting amino acid residues, docking centre, inhibition constant (Ki) μM and docking size between balsaminol phytochemicals from Momordica balsamina and the receptor proteins are presented in Tables 1, 2 and 3. The investigation showed that balsaminol E has the highest binding affinity to the SARS CoV-2 papain-like protease (7CMD) with a free binding energy of − 8.7 kcal/mol, followed by balsaminol A interacting with the spike receptor binding domain (6VW1) to produce a binding energy of − 8.5 kcal/mol and balsaminol C had a binding energy of − 8.1 kcal/mol with the main protease (6LU7) comparable to the standard drugs namely ritonavir, nirmatrelvir and remdesivir used as positive controls. The 3D and 2D pose views of the interactions between the balsaminol phytochemicals from Momordica balsamina and the receptor proteins are shown in Figs. 5, 6 and 7.
| 1 |
| 2 |
Table 1.
Binding Energies (ΔG), ligand interaction with amino acid residues and inhibition constant of balsaminol phytochemicals from Momordica balsamina against Main protease (6LU7)
| Ligand | Binding energy (ΔG) Vina Score kcal/mol |
Cavity volume (Å3) | Contact Residues and Ligand interaction | Inhibition constant (Ki) μM |
|---|---|---|---|---|
| Ritonavira | – 7.4 | 258 |
Conventional hydrogen bond: ASN142, GLU166, GLN189 Alkyl interaction: MET49, CYS145, PRO168 Pi-Pi T-shaped: HIS41 Van der Waals: THR24 THR25 THR26 LEU27 CYS44 SER46 GLU47 LEU50 TYR54 PHE140 LEU141 GLY143 SER144 HIS163 HIS164 MET165 LEU167 THR169 GLY170 HIS172 ASP187 ARG188 THR190 ALA191 GLN192 |
3.77 |
| Nirmatrelvirb | – 7.9 | 258 |
Hydrogen bond: HIS164, GLU166, GLN189 Carbon H-bond: LEU167 PRO168 Pi-Alkyl interaction: MET165 Pi-sigma: HIS41 Halogen: GLU166, THR190 Van der Waals: THR24 THR25 THR26 LEU27 CYS44 THR45 SER46 GLU47 MET49 PRO52 TYR54 PHE140 LEU141 ASN142 GLY143 SER144 CYS145 HIS163 HIS172 PHE181 VAL186 ASP187 ARG188 GLN192 |
1.61 |
| Remdesivir | – 7.4 | 258 |
Hydrogen bond: THR45, THR190 Unfavorable donor-donor: GLU166 Carbon H-bond: MET165 Alkyl interaction: MET49, MET165 Pi-sigma: MET49 Pi-sulfur: MET165 Van der Waals: THR24 THR25 THR26 LEU27 HIS41 CYS44 SER46 GLU47 PRO52 TYR54 PHE140 LEU141 ASN142 GLY143 SER144 CYS145 HIS163 HIS164 LEU167 PRO168 HIS172 PHE181 VAL186 ASP187 ARG188 GLN189 GLN192 |
3.76 |
| Balsaminol A | – 7.8 | 258 |
Hydrogen bond: THR24, SER46, GLU166 Alkyl interaction: HIS41, CYS145, HIS163, MET165 Van der Waals: THR25 THR26 LEU27 CYS44 THR45 MET49 PRO52 TYR54 PHE140 LEU141 ASN142 GLY143 SER144 HIS164 LEU167 PRO168 HIS172 ASP187 ARG188 GLN189 THR190 ALA191 GLN192 |
1.91 |
| Balsaminol B | – 7.5 | 258 |
Hydrogen bond: LEU141, THR190 Alkyl interaction: HIS163, PRO168, HIS172 Van der Waals: THR24 THR25 THR26 LEU27 HIS41 CYS44 THR45 SER46 MET49 TYR54 PHE140 ASN142 GLY143 SER144 CYS145 HIS164 MET165 GLU166 LEU167 ASP187 ARG188 GLN189 ALA191 GLN192 |
3.18 |
| Balsaminol C | – 8.1 | 258 |
Hydrogen bond: THR25 Carbon H-bond: THR45 Alkyl interaction: CYS145, HIS163, MET165 Van der Waals: THR24 THR26 LEU27 HIS41 CYS44 SER46 MET49 LEU50 PHE140 LEU141 ASN142 GLY143 SER144 HIS164 GLU166 LEU167 PRO168 THR169 GLY170 HIS172 VAL186 ASP187 ARG188 GLN189 THR190 ALA191 GLN192 |
1.17 |
| Balsaminol D | – 7.1 | 258 |
Hydrogen bond: CYS145 Alkyl interaction: HIS41 Van der Waals: THR24 THR25 THR26 LEU27 CYS44 THR45 SER46 MET49 LEU50 PHE140 LEU141 ASN142 GLY143 SER144 HIS163 HIS164 MET165 GLU166 LEU167 PRO168 HIS172 ARG188 GLN189 THR190 ALA191 GLN192 |
6.26 |
| Balsaminol E | – 7.4 | 258 |
Alkyl interaction: LEU27, HIS41, CYS145, HIS163 Van der Waals: THR24 THR25 THR26 CYS44 THR45 SER46 MET49 PHE140 LEU141 ASN142 GLY143 SER144 HIS164 MET165 GLU166 LEU167 PRO168 HIS172 GLN189 THR190 ALA191 GLN192 |
3.76 |
| Balsaminol F | – 6.7 | 258 |
Hydrogen bond: LEU141 Carbon H-bond: ASN142 Alkyl interaction: HIS41, CYS145 Van der Waals: THR24 THR25 THR26 LEU27 CYS44 THR45 SER46 MET49 PHE140 GLY143 SER144 HIS163 HIS164 MET165 GLU166 LEU167 PRO168 HIS172 GLN189 THR190 ALA191 GLN192 |
0.12 |
‘a, b and c’ = positive control
Table 2.
Binding Energies (ΔG), ligand interaction with amino acid residues and inhibition constant of balsaminol phytochemicals from Momordica balsamina against Receptor Binding Domain (6VW1)
| Ligand | Binding Energy (ΔG) Vina Score kcal/mol |
Cavity Volume (Å3) | Contact Residues and Ligand interaction | Inhibition constant (Ki) μM |
|---|---|---|---|---|
| Ritonavira | – 7.2 | 1874 |
Conventional hydrogen bond: ARG518, GLN442 Carbon hydrogen bond: GLU406 Alkyl interaction: PHE274, PRO346, ALA413 Pi-Pi T-shaped: HIS374 Pi-sigma: PHE274, LEU370, THR371 Salt bridge: GLU406 Van der Waals: GLU145 PRO146 ASN149 GLU150 ALA153 GLY268 ASP269 TRP271 ARG273 THR276 ASN277 HIS345 ASP367 ASP368 GLU375 GLU402 SER409 LEU410 LYS441 THR445 ILE446 LEU503 HIS505 TYR515 |
5.29 |
| Nirmatrelvirb | – 7.9 | 1874 |
Conventional hydrogen bond: TYR127, ASN149, GLU150, ARG273 Carbon hydrogen bond: HIS505 Alkyl interaction: PHE274 Van der Waals: LEU144 GLU145 PRO146 ALA153 LEU267 GLY268 ASP269 MET270 TRP271 PHE274 TRP275 THR276 ASN277 HIS345 PRO346 ASP367 ASP368 LEU370 THR371 HIS374 GLU375 GLU406 SER409 LEU410 ALA413 LYS441 GLN442 THR445 ILE446 LEU503 PHE504 TYR515 ARG518 |
1.61 |
| Remdesivirc | – 7.6 | 1874 |
Conventional hydrogen bond: GLU406, SER409, ARG518 Unfavorable donor: LYS441 Alkyl interaction: PHE274, PRO346, LEU370, ALA413 Pi-sigma: PHE274 Pi-cation: HIS374, GLU375, GLU402,GLU406 Van der Waals LEU144 GLU145 ASN149 GLU150 ALA153 GLY268 ASP269 TRP271 ARG273 THR276 ASN277 HIS345 MET366 ASP367 ASP368 THR371 HIS374 HIS378 GLY405 LEU410 PHE438 LYS441 GLN442 ALA443 THR445 ILE446 HIS505 TYR515 |
2.68 |
| Balsaminol A | – 8.5 | 1874 |
Conventional hydrogen bond: GLU402, LYS441, THR445 Alkyl interaction: PHE274, LEU370, HIS374 Unfavorable acceptor- acceptor: GLN442 Van der Waals: TYR127 LEU144 GLU145 PRO146 GLY147 ASN149 GLU150 ILE151 MET152 ALA153 ASN154 GLY268 ASP269 MET270 TRP271 ARG273 THR276 ASN277 HIS345 PRO346 THR347 MET366 ASP367 THR371 GLU375 GLU406 SER409 LEU410 ALA413 PHE438 ILE446 LEU503 PHE504 HIS505 TYR515 ARG518 |
58.59 |
| Balsaminol B | – 8.4 | 1874 |
Alkyl interaction: PHE274, LEU370, ILE446 Van der Waals: GLU145 PRO146 ASN149 GLU150 ALA153 GLY268 ASP269 MET270 TRP271 ARG273 THR276 ASN277 HIS345 PRO346 MET360 ASP367 ASP368 THR371 HIS374 GLU375 HIS378 GLU402 GLU406 SER409 LEU410 ALA413 LYS441 GLN442 THR445 LEU503 HIS505 TYR515 ARG518 |
69.45 |
| Balsaminol C | – 8.3 | 1874 |
Conventional hydrogen bond: GLU406, ARG518 Alkyl interaction: PRO346, HIS374, ILE446 Van der Waals: GLU145 PRO146 ASN149 GLU150 ALA153 GLY268 ASP269 TRP271 ARG273 PHE274 TRP275 THR276 ASN277 HIS345 ASP367 LEU370 THR371 GLU375 GLU402 SER409 LEU410 ALA413 LYS441 GLN442 THR445 HIS505 TYR515 |
82.33 |
| Balsaminol D | – 7.6 | 1874 |
Conventional hydrogen bond: ARG273, ASN277, GLU402, ARG518 Alkyl interaction: PHE274, TYR515 Van der Waals: GLU145 ASN149 ALA153 GLY268 ASP269 MET270 TRP271 THR276 HIS345 PRO346 ASP367 LEU370 THR371 HIS374 GLU375 HIS378 GLU406 SER409 LEU410 ALA413 LYS441 GLN442 THR445 ILE446 LEU503 HIS505 |
2.68 |
| Balsaminol E | – 8.3 | 1874 |
Conventional hydrogen bond: ARG273 Alkyl interaction: PHE274, LEU370; LEU410, ALA413, TYR515 Van der Waals: GLU145 ASN149 GLU150 MET152 ALA153 GLY268 ASP269 TRP271 ARG273 PHE274 THR276 ASN277 HIS345 PRO346 ASP367 THR371 HIS374 GLU375 HIS378 GLU402 GLU406 SER409 PHE438 LYS441 GLN442 THR445 ILE446 HIS505 TYR515 ARG518 |
82.33 |
| Balsaminol F | – 8.0 | 1874 |
Conventional hydrogen bond: PRO346 Pi-donor hydrogen bond: HIS345 Alkyl interaction: HIS345, PRO346, LEU370, HIS374, ILE446 Pi-sgma: PHE274 Van der Waals: GLU145 ASN149 GLU150 ALA153 GLY268 ASP269 MET270 TRP271 ARG273 TRP275 THR276 ASN277 THR347 ASP367 THR371 GLU375 GLU402 GLU406 SER409 LEU410 ALA413 LYS441 GLN442 THR445 TYR515 ARG518 |
1.37 |
‘a, b and c’ = positive control
Table 3.
Binding Energies (ΔG), ligand interaction with amino acid residues and inhibition constant of balsaminol phytochemicals from Momordica balsamina against Papain-like Protease (nsp3) (7CMD)
| Ligand | Binding energy (ΔG) Vina score kcal/mol |
Cavity volume (Å3) | Contact residues and ligand interaction | Inhibition constant (Ki) μM |
|---|---|---|---|---|
| Ritonavira | – 8.0 | 1495 |
Conventional hydrogen bond: LYS94, LYS105, LYS217 Carbon hydrogen bond: LYS92, SER212 Alkyl interaction: LYS92, LYS217, TYR25 Unfavorable positive-positive: LYS105 Pi-cation: LYS94 Pi-anion: GLU214 Van der Waals: Chain A—R213 GLU214 LYS218 TYR251 GLU252 LEU253 LYS254 HIS255 GLY256 THR257 PHE258 THR259 VAL303 PHE304 TYR305 LYS306 GLU307 SER309 TYR310 THR311 THR312 THR313 ILE314. Chain C—ASN88 HIS89 TRP93 TRP106 ALA107 ASP108 ASN109 THR158 VAL159 GLY160 |
1.37 |
| Nirmatrelvirb | – 8.9 | 1495 |
Conventional hydrogen bond: LYS94, LYS105, ASP108, LYS217, THR257 Carbon hydrogen bond: HIS89, TRP93, ASP108 Alkyl interaction: TYR213, TYR310 Halogen: LYS105, TRP106 Van der Waals: Chain A—SER212 GLU214 LYS217 LYS218 GLY256 THR257 PHE258 THR259 TYR305 LYS306 GLU307 ASN308 SER309 THR311 THR312 THR313 ILE314. Chain C—ASN88 HIS89 LYS92 LYS94 ALA107 ASN109 GLY160 |
29.99 |
| Remdesivirc | – 8.4 | 1495 |
Conventional hydrogen bond: LYS217 Carbon hydrogen bond: GLY256, THR257 Alkyl interaction: LYS105, ALA107 Unfavorable donor-donor: ASN109 Pi-anion: ASP108, GLU214, GLU307 Van der Waals: Chain A—LEU211 SER212 TYR213 GLU214 TYR251 LYS254 PHE258 THR259 SER278 PHE304 TYR305 LYS306 ASN308 SER309 TYR310 THR311 THR312 THR313 Chain C—ASN88 HIS89 THR90 LYS92 TRP93 LYS94 TRP106 VAL159 GLY160 GLU161 |
69.46 |
| Balsaminol A | – 8.5 | 1495 |
Conventional hydrogen bond: LYS306 Carbon hydrogen bond: THR257 Alkyl interaction: HIS89, LYS92, TRP106, TYR305 Unfavorable donor-donor: LYS306 Van der Waals: Chain A—SER212 TYR213 GLU214 GLN215 LYS217 LYS218 TYR251 GLU252 LYS254 GLY256 PHE258 THR259 PHE304 TYR305 GLU307 SER309 TYR310 THR311 THR312 THR313 ILE314. Chain C—TRP93 LYS94 LYS105 ALA107 ASP108 ASN109 ASN110 VAL159 GLY160 |
58.59 |
| Balsaminol B | – 8.1 | 1495 |
Conventional hydrogen bond: TRP106 Alkyl interaction: LYS92, TRP93, ALA107, LYS217, LYS218 Van der Waals: Chain A—TYR213 GLU214 THR257 TYR305 LYS306 GLU307 ASN308 SER309 TYR310 THR311 THR312 THR313 ILE314 Chain C—ASN88 HIS89 LYS94 LYS105 ASP108 VAL159 |
1.16 |
| Balsaminol C | – 8.3 | 1495 |
Conventional hydrogen bond: LYS217, THR311 Alkyl interaction: LYS94, TRP106, TYR305, TYR310 Van der Waals: Chain A—TYR213 GLU214 LYS218 GLY256 THR257 PHE258 LYS306 GLU307 SER309 THR312 THR313. Chain C—HIS89 LYS92 TRP93 LYS105 ALA107 ASP108 VAL159 GLY160 |
82.33 |
| Balsaminol D | – 7.7 | 1495 |
Conventional hydrogen bond: LYS94, GLU307 Carbon hydrogen bond: ALA107, TYR213, TYR310 Alkyl interaction: ALA107, Van der Waals: Chain A—SER212 GLU214 GLN215 LYS217 LYS218 GLU252 LEU253 LYS254 HIS255 THR257 PHE258 TYR305 LYS306 ASN308 SER309 THR311 THR312 THR313 ILE314. Chain C—ASN88 HIS89 LYS92 TRP93 ILE104 LYS105 TRP106 ALA107 ASP108 ASN109 ASN110 THR158 VAL159 |
2.28 |
| Balsaminol E | – 8.7 | 1495 |
Conventional hydrogen bond: TRP106, SER309 Alkyl interaction: ALA107, LYS217, LYS218, TYR310 Unfavorable donor-donor: THR313 Van der Waals: Chain A—SER212 TYR213 GLU214 GLN215 GLY219 TYR251 GLU252 LYS254 GLY256 THR257 TYR305 LYS306 GLU307 ASN308 THR311 THR312 ILE314. Chain C—ASN88 HIS89 LYS92 TRP93 LYS94 LYS105 ASP108 ASN109 VAL159 GLY160 |
41.71 |
| Balsaminol F | – 8.0 | 1495 |
Conventional hydrogen bond: SER309 Alkyl interaction: LYS94, LYS105, TRP106, ALA107, LYS217, TYR310, ILE314 Van der Waals: Chain A—TYR213 GLU214 LYS218 THR257 TYR305 LYS306 GLU307 ASN308 SER309 THR311 THR312 THR313 Chain C—ASN88 HIS89 LYS92 TRP93 ASP108 VAL159 |
1.37 |
‘a, b and c’ = positive control
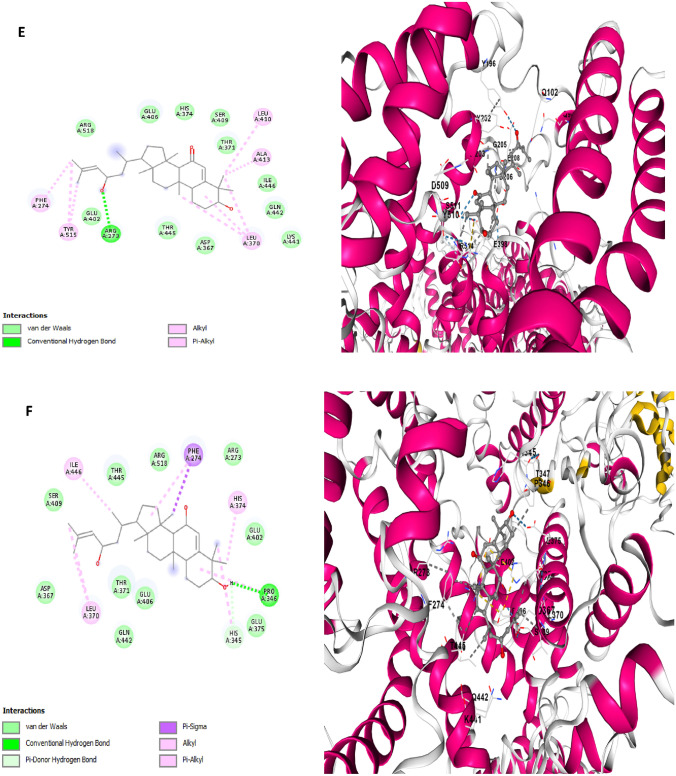
Fig. 5.
Two dimensional (2D) pose views and three dimensional (3D) pose views molecular docking interactions between Main protease (6LU7) and A Balsaminol A, B Balsaminol B, C Balsaminol C, D Balsaminol D, E Balsaminol E, F Balsaminol F while Ritonavir Nirmatrelvir, and Remdesivir (were used as positive control drugs)
Fig. 6.
Two dimensional (2D) pose views and three dimensional (3D) pose views molecular docking interactions between receptor binding domain of spike glycoprotein (6WV1) and A Balsaminol A, B Balsaminol B, C Balsaminol C, D Balsaminol D, E Balsaminol E, F Balsaminol F while Ritonavir, Nirmatrelvir and Remdesivir (were used as positive control drugs)
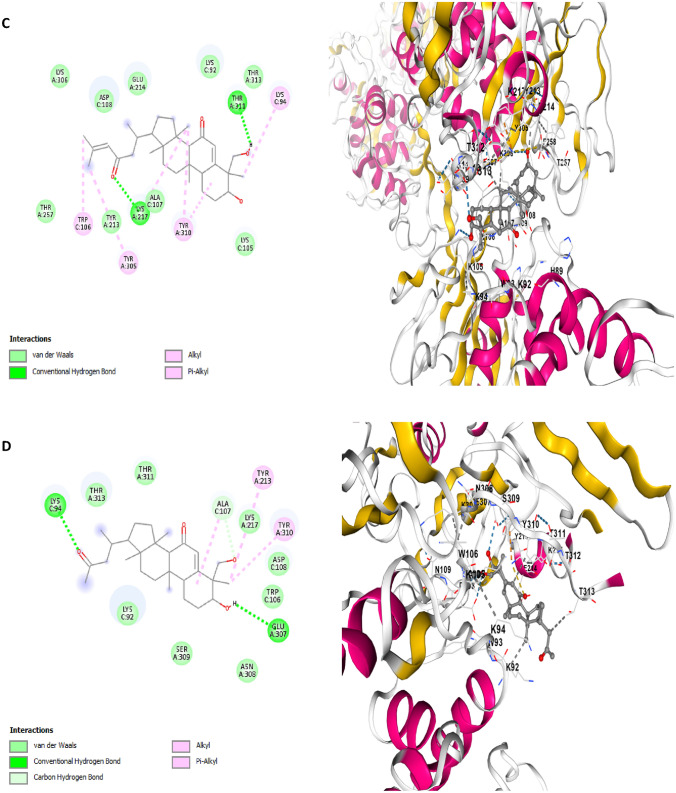
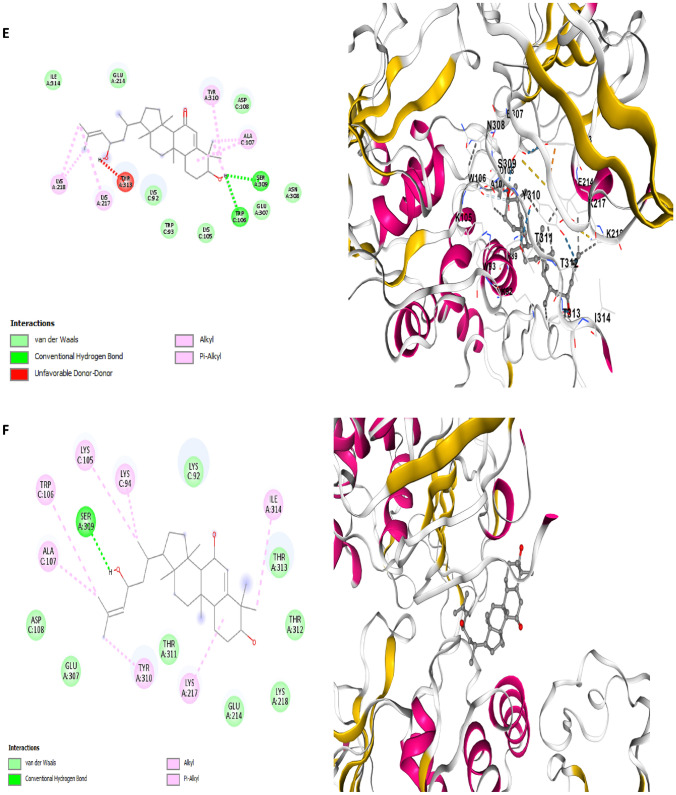
Fig. 7.
Two dimensional (2D) pose views and three dimensional (3D) pose views molecular docking interactions between papain-like protease (7CMD) and A Balsaminol A, B Balsaminol B, C Balsaminol C, D Balsaminol D, E Balsaminol E, F Balsaminol F, while Ritonavir, Nirmatrelvir and Remdesivir ( were used as positive control drugs)
ΔG = Binding energy
Ki = Inhibition Constant
R = 1.987 × 10–3 kcalmol−1 K−1 (Gas constant)
T = 298.15 K (absolute temperature)
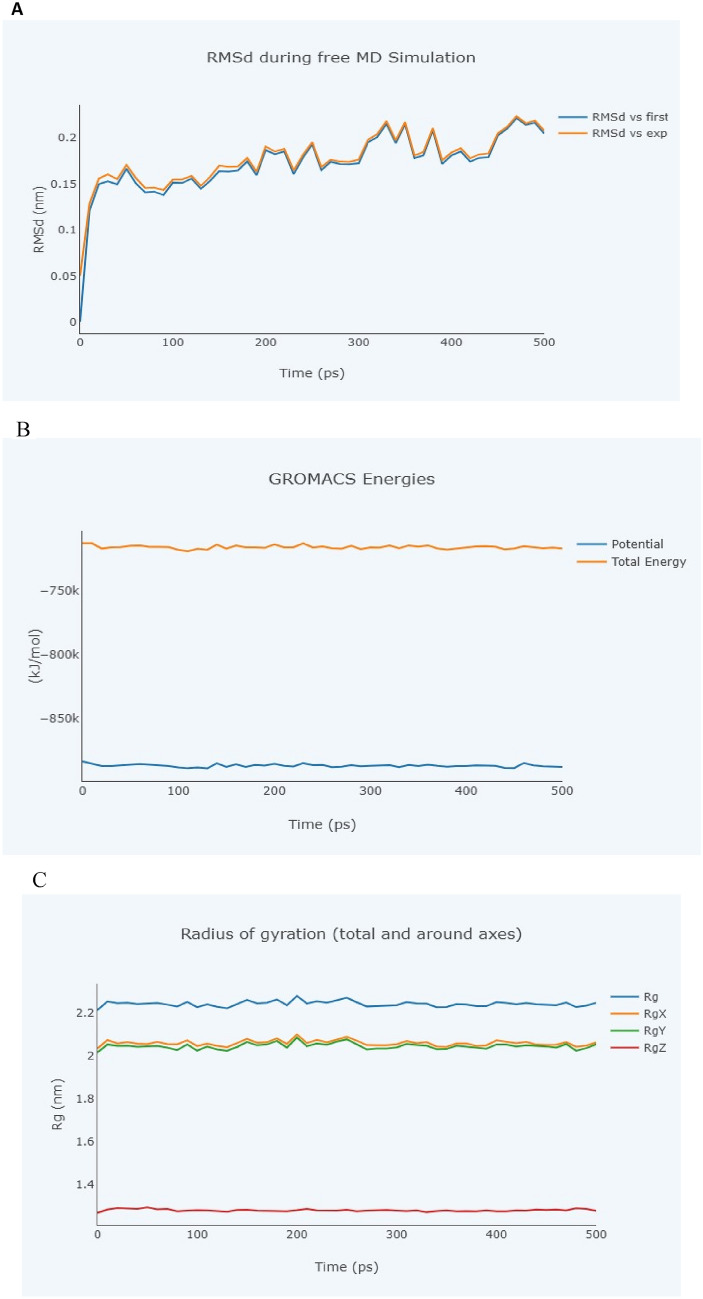
Molecular dynamics simulation
The molecular dynamic behaviours of balsaminol E and balsaminol F complexes were evaluated using BioBB-Wfs server (https://mmb.irbbarcelona.org/biobb-wfs). The results of the study utilized the MD simulation method to generate snapshots of the complexes over 500 ps simulation time. Figures 8A–C and 9A–C depict the root mean square deviations (RSMD) of balsaminol E with papain-like protease and balsaminol F with main protease complexes at 0, 100, 200, 300, 400 and 500 ps to study the stable hydrogen bonds between protein and balsaminol complexes. The number of H-bonds and free energy of interaction between main protease, papain-like protease and the balsaminol molecules play very essential roles for stabilization of the complexes. The total energy of the complexes from the GROMACS energies plot showed a significant free binding energy compared to the potential energy which corroborate with the report of Mahdian et al. 2021.
Fig. 8.
Molecular dynamics simulation of SARS-CoV-2 papain-like protease (PDB: 7CMD) and balsaminol F. A Root mean square deviation. B GROMACS Energies. C Radius of gyration
Fig. 9.
Molecular dynamics simulation of SARS-CoV-2 main protease (PDB: 6LU7) and balsaminol F. A Root mean square deviation. B GROMACS Energies. C Radius of gyration
Assessment of ADMET and drug-likeness
The Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) properties of balsaminol phytochemicals from Momordica balsamina were analyzed to assess the physicochemical, pharmacokinetic and drug likeness potential for drug discovery and development. The physicochemical and Lipinski's rule of five for drug likeness results revealed sufficient characteristics for all the balsaminol phytochemicals, indicating their potential as drug candidates for therapeutic medication as shown in Table 4 and Fig. 8. Their molecular weights (MW) were ≤ 500 g/mol, hydrogen bond donors (HBDs) ≤ 5, hydrogen bond acceptors (HDAs) ≤ 10, TPSA 1402 and octanol–water partition coefficients (LogP) ≤ 5, except for balsaminol B, balsaminol E and balsaminol F. Based on Lipinski's rule, which allows only one violation, all balsaminol phytochemicals fulfilled the rule of five compared to three positive control drugs, confirming their potential as drug candidates (Table 4). The pharmacokinetic profile showed that all balsaminol phytochemicals exhibited high gastrointestinal absorption capacity for oral administration. A bioavailability score greater than zero indicates that the balsaminol phytochemicals have substantial bioavailability and cross the cell membrane efficiently (Lounkine et al. 2012). However, the balsaminol phytochemicals do not cross the blood–brain barrier. The permeability glycoprotein can interact with xenobiotics and other therapeutic agents during drug metabolism to limit cellular uptake by acting as a transmembrane efflux pump, pumping its substrates from the inside to the outside of the cell. Of all the balsaminol phytochemicals, only balsaminol A, balsaminol C and balsaminol D were found to be P-glycoprotein substrates (Table 5). In addition, all balsaminol phytochemicals were moderately soluble and non-inhibitory to cytochrome P450 enzymes, except for balsaminol C, which acts as an inhibitor of the CYP3A4 isoenzyme. Finally, the toxicity profiles of the balsaminol phytochemicals balsaminol C, balsaminol D and balsaminol E showed hepatoxicity; balsaminol D showed both carcinogenicity and Ames mutagenesis; and balsaminol A, balsaminol B and balsaminol F showed non-hepatoxicity, non-carcinogenicity, non-mutagenesis and non-nephrotoxicity when compared to the three positive control drugs, demonstrating their desirable properties as potential therapeutic agents as shown in Table 6.
Table 4.
Physicochemical properties and Lipinski’s rule of five for drug-likeness analysis of balsaminol phytochemicals from Momordica balsamina
| S/No | Ligand | Molecular weight (g/mol) | Lipophilicity (log P) | Hydrogen bond donors | Hydrogen bond acceptors | TPSA (Å2) | ROTB | Number of Lipinski’s rule violations |
|---|---|---|---|---|---|---|---|---|
| 1 | Ritonavir | 720.94 | 5.19 | 4 | 7 | 202.26 | 22 | 2 |
| 2 | Nirmatrelvir | 499.53 | 1.89 | 3 | 8 | 131.40 | 11 | 0 |
| 3 | Remdesivir | 602.58 | 1.54 | 4 | 12 | 213.36 | 14 | 2 |
| 4 | Balsaminol A | 474.72 | 4.95 | 4 | 4 | 80.92 | 5 | 1 |
| 5 | Balsaminol B | 488.74 | 5.46 | 2 | 4 | 69.92 | 6 | 1 |
| 6 | Balsaminol C | 470.68 | 5.13 | 2 | 4 | 74.60 | 5 | 0 |
| 7 | Balsaminol D | 430.62 | 4.27 | 2 | 4 | 74.60 | 4 | 0 |
| 8 | Balsaminol E | 456.70 | 5.73 | 2 | 3 | 57.53 | 4 | 1 |
| 9 | Balsaminol F | 458.72 | 5.63 | 3 | 3 | 60.69 | 4 | 1 |
TPSA topological polar surface area, ROTB number of rotatable bonds
Table 5.
Pharmacokinetics of balsaminol phytochemicals from Momordica balsamina
| S/No | Ligand | Water solubility Silicos-IT | GI absorption | BBB permeability | Bioavailability Score | P-gp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ritonavir | Insoluble | Low | No | 0.17 | Yes | No | No | No | No | Yes |
| 2 | Nirmatrelvir | Soluble | High | No | 0.55 | Yes | No | No | No | No | Yes |
| 3 | Remdesivir | Moderately soluble | Low | No | 0.17 | Yes | No | No | No | No | Yes |
| 4 | Balsaminol A | Moderately soluble | High | No | 0.55 | Yes | No | No | No | No | No |
| 5 | Balsaminol B | Moderately soluble | High | No | 0.55 | No | No | No | No | No | No |
| 6 | Balsaminol C | Moderately soluble | High | No | 0.55 | Yes | No | No | No | No | Yes |
| 7 | Balsaminol D | Moderately soluble | High | No | 0.55 | Yes | No | No | No | No | No |
| 8 | Balsaminol E | Moderately soluble | High | No | 0.55 | No | No | No | No | No | No |
| 9 | Balsaminol F | Moderately soluble | High | No | 0.55 | No | No | No | No | No | No |
Blood–brain barrier (BBB) permeability, P-glycoprotein (P-gp) and cytochrome P450 (CPY) isoenzymes
Table 6.
Toxicity profile of balsaminol phytochemicals from Momordica balsamina
| S/No | Ligand | Hepatotoxicity | Carcinogenicity | Ames mutagenesis | Nephrotoxicity | Caco-2 Caco-2 Caco-2 Caco-2 Caco-2 Caco-2 | LLD50 (mg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Ritonavir | Yes | No | No | Yes | No | 2.51 |
| 2 | Nirmatrelvir | Yes | No | No | Yes | No | 1.74 |
| 3 | Remdesivir | Yes | No | No | Yes | No | 2.20 |
| 4 | Balsaminol A | No | No | No | No | No | 2.23 |
| 5 | Balsaminol B | No | No | No | No | No | 2.43 |
| 6 | Balsaminol C | Yes | No | No | No | Yes | 2.23 |
| 7 | Balsaminol D | Yes | Yes | Yes | No | Yes | 2.10 |
| 8 | Balsaminol E | Yes | No | No | No | Yes | 2.75 |
| 9 | Balsaminol F | No | No | No | No | No | 2.75 |
Caco-2—Cancer coli
Discussion
In the past and still today, alternative medicine based on the use of herbs has attracted a lot of attention from researchers due to its incredible effectiveness in treating diseases. Molecular docking is one of the most frequently used methods in hit identification in drug discovery to deliver a molecule with therapeutic activity against a receptor target. It helps in identifying potential drug candidates by predicting the binding affinity of ligands within the binding pocket of a specific therapeutic drug target or receptor of interest based on interactions of molecular residues present in the binding pocket. The active site of the target receptors were first identified using the co-crystal ligands to predict the bind pockets residues. The crystal structure of SARS-CoV-2 main protease (PDB ID: 6LU7) with N3 co-crystal ligand depicted amino acid residues HIS41, MET49, MET165, GLU166, LEU167, GLN189, THR190 participated in stabilizing the protein–ligand interactions. The crystal structure of the receptor binding domain of spike glycoprotein (PDB ID: 6VW1) with NAG ligand showed stable interaction involving MET366, GLU406, PHE438, LYS441, GLN442, LEU410, ALA413 while that of the crystal structure of papain-like protease (7CMD) with TTT, the amino acid residue involved in stabilizing the molecular interactions are TRP106, ALA107, ASP108, LYS217, TYR305, GLU307, ASN308 respectively.
The binding affinity of the balsaminol phytochemicals against SARS-CoV-2 main protease (PDB ID: 6LU7), receptor binding domain of spike glycoprotein (PDB ID: 6VW1) and papain-like protease (7CMD) reflects their inhibitory activity which is a very effective strategy to modulate enzyme activity and disrupt disease related pathways. Molecular interaction studies revealed negative binding energies for all balsaminol phytochemicals on the target proteins. SARS-CoV-2 main protease (PDB ID: 6LU7) molecular interactions with balsaminol phytochemicals produced binding energy range between -8.1 kcal/mol and -6.7 kcal/mol. The stabilizing interactions between Balsaminol C and SARS-CoV-2 main protease (PDB ID: 6LU7) amino acid residues formed one strong hydrogen bond with THR25, one carbon H-bond with THR45, three alkyl interaction with CYS145, HIS163, MET16 and Van der Waals interactions with THR24 THR26 LEU27 HIS41 CYS44 SER46 MET49 LEU50 PHE140 LEU141 ASN142 GLY143 SER144 HIS164 GLU166 LEU167 PRO168 THR169 GLY170 HIS172 VAL186 ASP187 ARG188 GLN189 THR190 ALA191 GLN192. The hydrogen bonds facilitate the stabilization of protein–ligand binding by introducing hydrogen bond donor or acceptors as well as other no-covalent intermolecular interactions involving alkyl interactions and van der Waals forces. This molecular interaction produced the highest binding energy of − 8.1 kcal/mol with an inhibition constant of 1.17 μM (Fig. 5C) compared to ritonavir, Nirmatrelvir and Remdesivir which were used as standard drugs (positive controls) (Mujwar and Harwansh 2022). It is interesting to note that based on the molecular analysis of the investigation, the amino acids reported above for Balsaminol C (Table 1) are also predicted in the active site of SARS-CoV-2 main protease (PDB ID: 6LU7) interaction with the co-crystal N3 inhibitor and the standard drugs used in the analysis which include ritonavir, Nirmatrelvir and Remdesivir. This implies that Balsaminol C shares the same pocket and interacts effectively with the active site of the target receptor and can potentially serve as a good inhibitor of SARS-CoV-2 main protease for drug design and development. Balsaminol C also showed good binding affinity to SARS-CoV-2 main protease (PDB ID: 6LU7) compared to ritonavir with a binding energy value of − 7.4 kcal/mol, nirmatrelvir with a binding energy of − 7.9 kcal/mol and remdesivir with a binding energy of − 7.4 kcal/mol as shown in Table 1 (Kushari et al. 2023; Umadevi et al. 2020). Interestingly, the binding energy value for balsaminol A on SARS-CoV-2 main protease (PDB ID: 6LU7) was − 7.8 kcal/mol, showing three hydrogen bonds with THR24, SER46, GLU166 and four alkyl interaction with HIS41, CYS145, HIS163, MET165 (Fig. 5A).
The molecular interactions between balsaminol phytochemicals and the receptor binding domain of spike glycoprotein (PDB ID: 6VW1) produced a binding energy range between − 8.5 kcal/mol and − 7.6 kcal/mol. It is obvious from (Table 2) that Balsaminol A had the highest binding energy with -8.5 kcal/mol and Balsaminol D with − 7.6 kcal/mol had the lowest binding energy against the receptor binding domain of spike glycoprotein (PDB ID: 6VW1) (Chikhale et al. 2021). The binding affinity between Balsaminol A and receptor binding domain of spike glycoprotein (PDB ID: 6VW1) formed one hydrogen bond with hydrogen bond with GLU402, LYS441, THR445, three alkyl interaction with PHE274, LEU370, HIS374, one unfavorable acceptor- acceptor with GLN44 and Van der Waals forces with GLU145 PRO146 ASN149 GLU150 ALA153 GLY268 ASP269 MET270 TRP271 ARG273 THR276 ASN277 HIS345 PRO346 MET360 ASP367 ASP368 THR371 HIS374 GLU375 HIS378 GLU402 GLU406 SER409 LEU410 ALA413 LYS441 GLN442 THR445 LEU503 HIS505 TYR515 ARG518 with the release of -8.5 kcal/mol which corroborate with the report of Toor et al. 2021 (Table 2). Predicting the binding site and evaluating the molecular interactions of the balsaminol in the active pocket of receptor binding domain of spike glycoprotein (PDB ID: 6VW1) plays a key role in drug design with an inhibitory constant 58.59 μM. The analysis of the active site between receptor binding domain of spike glycoprotein (PDB ID: 6VW1) and its co-crystal ligand showed strong similarities with that of Balsaminol A. Furthermore, Balsaminol A shared the same binding pocket with ritonavir, Nirmatrelvir and Remdesivir which were used as standard drug with a prominent molecular interaction observed by LYS441 and GLU406.
The mode of interaction between balsaminol phytochemicals and papain-like protease (PDB ID: 7CMD) produced binding energy that range between − 8.7 and − 7.7 kcal/mol (Table 3). It can be seen, that the balsaminol phytochemicals showed reasonable binding energy compared to the standard drugs with balsaminol E having the highest binding energy of − 8.7 kcal/mol and balsaminol D with the least binding energy of − 7.7 kcal/mol. The interaction mode between balsaminol E and papain-like protease (7CMD) apparently produced a binding energy of − 8.7 kcal/mol along with an inhibitory constant of 41.71 μM while forming two conventional hydrogen bonds with TRP106, SER309, four alkyl interactions with ALA107, LYS217, LYS218 TYR310 and one unfavorable donor-donor interaction with THR313 while the remaining interactions involving Van der Waal forces was observed with the following amino acids residues: TYR213 GLU214 LYS218 THR257 TYR305 LYS306 GLU307 ASN308 SER309 THR311 THR312 THR313, ASN88 HIS89 LYS92 TRP93 ASP108 and VAL15 (Fig. 7E). The interacting amino acids TRP106 and SER309 were seen to be fully involved in the formation of conventional hydrogen bonds and ALA107 forming akyl interaction with balsaminol E which corroborates with similar interactions observed by the co-crystal inhibitor (TTT) of papain-like protease (PDB ID: 7CMD) crystal structure (Osipiuk et al. 2021; Bolcato et al. 2020). Balsaminol E was observed to bind papain-like protease at same binding pocked with the co-crystal ligand and the standard drugs. This further validates the probable potential of balsaminol E as drug candidate against SARS-CoV-2.
The results of the MD simulation generate snapshots of the complexes over 500 ps simulation time as shown in Figs. 8A and 9A. It depicts the root mean square deviations (RSMD) of balsaminol E with papain-like protease and balsaminol F with main protease complexes at 0, 100, 200, 300, 400 and 500 ps. The investigation showed significant stabilization of the complexes along with the total energy of the complexes from the GROMACS energies plot for both balsaminol E with papain-like protease and balsaminol F with main protease. This result also corroborate with the findings of Mujwar and Harwansh (2022) were mormodicin was observed to favourably exert inhibitory activity on SARS-CoV-2 main protease.
It is interesting to note that, all the six different oxidation patterns of balsaminol phytochemicals studied, balsaminol F showed a very good pharmacokinetic properties than the others. Similarly, the physicochemical properties and toxicity profile of balsaminol F were remarkable compared to the other balsaminol phytochemicals. Apparently, the molecular interactions between the studied balsaminol phytochemicals and the various SARS-CoV-2 target receptors shared the same binding pockets (composition of amino acids residues) with co-crystal inhibitors as well as the standard drug (positive controls) on the active sites SARS-CoV-2 target receptors. This implies that balsaminol interacts effectively with the target receptor and could be explore for drug design purposes as a potential inhibitor.
Conclusion
In this study, balsaminol C showed a strong binding affinity with the main protease of SARS-CoV-2 at − 8.1 kcal/mol, and balsaminol A binds to the receptor binding domain of the spike glycoprotein at − 8.5 kcal/mol. Similarly, balsaminol E achieved the most favaorable binding affinity with papain-like protease at − 8.7 kcal/mol. The molecular interactions between balsaminol phytochemicals and SARS-CoV-2 receptors results revealed a favourable free binding energy (ΔG ≥ 6.7 kcal/mol). As for the main protease receptor, the binding energy range between − 8.1 and − 6.7 kcal/mol, receptor binding domain of spike glycoprotein binding energy range between − 8.5 and − 7.6 kcal/mol whereas for papain-like protease binding energy range between − 8.7 and − 7.7 kcal/mol. Interestingly, these binding affinities in a way underscore balsaminol inhibitory activities against SARS-CoV-2 molecular disease mechanism in drug design and discovery. In addition, the pharmacokinetic properties of the phytochemical balsaminol F from Momordica balsamina were exceptional, showing it to be orally bioavailable with excellent human gastrointestinal absorption (GIA), non-carcinogenic, non-AMES toxic, blood–brain barrier (BBB) impermeable, non-inhibitor of CYP1A2, CYP2C19, CYP3A4, CYP2C9, CYP2D6, CYP3A4 cytochrome P450 enzymes and non-substrate to P-glycoprotein (P-gp). Therefore, our findings present balsaminol F as a potentially promising drug candidate against SARS-CoV-2 for further controlled experimental research in animal models and cell lines.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This article is dedicated to all the research scientists and healthcare workers across the world that had contributed in vaccine development, drug design and development.
Abbreviations
- ADMET
Absorption: distribution: metabolism: excretion: toxicity
- ACE2
Angiotensin-converting enzyme 2
- COVID-19
Coronavirus diseases 19
- 3CLpro
3 Cysteine-like proteinase
- ChEMBL
Chemical biology database European molecular biology laboratory
- CYP
Cytochrome P
- E
Envelope
- FDA
Food and drug administration
- GDT
Global distance test
- HBAs
Hydrogen bond acceptors
- HBD
Hydrogen bond donor
- ISG15
Interferon stimulated gene 15
- N3
N-[(5-METHYLISOXAZOL-3-YL)CARBONYL]ALANYL-L-VALYL-N ~ 1 ~ -((1R,2Z)-4-(BENZYLOXY)-4-OXO-1-{[(3R)-2-OXOPYRROLIDIN-3 YL]METHYL}BUT-2-ENYL)-L-LEUCINAMIDE
- NAG
2-Acetamido-2-deoxy-beta-D-glucopyranose
- NAG
N: Nucleocapsid protein
- Mpro
Main proteinase
- M
Membrane
- mRNAs
Messenger ribonucleic acids
- MAVS
Mitochondrial antiviral-signaling protein
- MW
Molecular weights
- nsp
Nonstructural proteins
- + ssRNA
Positive single stranded RNA
- PLpro
Papain-like protease
- pp
Polyproteins
- PDB
Protein Data Bank
- PubChem
Chemical information database
- QMEAN
Qualitative model energy analysis
- QSQE
Quaternary structure quality estimate
- RBD
Receptor binding domain
- RNA
Ribonucleic acids
- RTC
Replication-transcription complex
- RIG-I
Retinoic acid-inducible gene I
- RMSD
Root mean standard deviation
- RCSB
Research collaborator for bioinformatics
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- S
Spike
- ssRNA
Single stranded RNA
- sgRNAs
Single guide RNA
- SMILES
Simplified molecular input line entry system
- SDF
Structural data file
- SVM
Support vector machines
- TTT
5-Amino-2-methyl-N-[(1R)-1-naphthalen-1-ylethyl]benzamide
- TPSA
Topological polar surface area
- TMPRSS2
Transmembrane protease serine 2
- VOC
Variant of concern
- VUM
Variant under monitoring
- WHO
World Health Organization
Author contributions
DDG, AM, JSM and RA designed the research, performed virtual screening. DDG, AM, JSM, RA, AJD, ROB, MH, SSE, RIO, MS revised the manuscript. DDG, AM, JSM and RA performed the bioinformatics analysis, molecular dynamics simulation, analyzed the virtual screening experiment data and drafted the manuscript. All authors have read and approved the final manuscript.
Data availability
All data has been included inside the manuscript.
Declarations
Conflict of interest
The authors claim that the researchers in this study have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Astuti IY (2020) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr 14(4):407–412. 10.1016/j.dsx.2020.04.020 10.1016/j.dsx.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarri G, Andrio P, Hospital A, Orozco M, Gelpí JL (2022) BioExcel Building Blocks Workflows (BioBB-Wfs), an integrated web-based platform for biomolecular simulations. Nucleic Acids Res 50(W1):W99–W107. 10.1093/nar/gkac380 10.1093/nar/gkac380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni M, Kiefer F, Biasini M, Bordoli L, Schwede T (2017) Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci Rep 7(1):10480. 10.1038/s41598-017-09654-8 10.1038/s41598-017-09654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcato G, Bissaro M, Pavan M, Sturlese M, Moro S (2020) Targeting the coronavirus SARS-CoV-2: computational insights into the mechanism of action of the protease inhibitors lopinavir, ritonavir and nelfinavir. Sci Rep 10(1):20927. 10.1038/s41598-020-77700-z 10.1038/s41598-020-77700-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultum LE, Tolossa GB, Kim G, Kwon O, Lee D (2022) In silico activity and ADMET profiling of phytochemicals from Ethiopian indigenous aloes using pharmacophore models. Sci Rep 12(1):22221. 10.1038/s41598-022-26446-x 10.1038/s41598-022-26446-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley SK, Bhikadiya C, Bi C, Bittrich S, Chao H, Chen L et al (2023) RCSB Protein Data Bank (Rcsb.org): delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Res 51(D1):D488–D508. 10.1093/nar/gkac1077 10.1093/nar/gkac1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang X, Chen Y, Wang D, Zhang D, Yan S et al (2021) Dynamic landscape mapping of humoral immunity to SARS-CoV-2 identifies non-structural protein antibodies associated with the survival of critical COVID-19 patients. Signal Transduct Target Ther 6(1):304. 10.1038/s41392-021-00718-w 10.1038/s41392-021-00718-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale RV, Sinha SK, Patil RB, Prasad SK, Shakya A, Gurav N et al (2021) In-silico investigation of phytochemicals from Asparagus racemosus as plausible antiviral agent in COVID-19. J Biomol Struct Dyn 39(14):5033–5047. 10.1080/07391102.2020.1784289 10.1080/07391102.2020.1784289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtita S, Fouedjou RT, Belaidi S, Djoumbissie LA, Ouassaf M, Qais FA et al (2022) In silico investigation of phytoconstituents from Cameroonian medicinal plants towards COVID-19 treatment. Struct Chem 33(5):1799–1813. 10.1007/s11224-022-01939-7 10.1007/s11224-022-01939-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassault Systèmes BIOVIA (2019) Discovery Studio Modeling Environment, Release 2019. Dassault Systèmes, San Diego, CA, USA [Google Scholar]
- Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 10.1038/srep42717 10.1038/srep42717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinar JA, Menendez JA (2020) Potential drugs targeting early innate immune evasion of SARS-Coronavirus 2 via 2’-O-methylation of viral RNA. Viruses 12(5):525. 10.3390/v12050525 10.3390/v12050525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr AR, Channappanavar R, Jankevicius G, Fett C, Zhao J, Athmer J et al (2016) The conserved Coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome Coronavirus infection. Mbio. 10.1128/mBio.01721-16 10.1128/mBio.01721-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo L, Park H, Seok C (2013) GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res 41:W384. 10.1093/nar/gkt458 10.1093/nar/gkt458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibril A, Musa U, Saidu B, Ajape A, Maina I, Jimoh A et al (2018) Effects of balsam apple (Momordica balsamina) leaf extract on blood glucose level and the haematological parameters of japaneese quails. Int J Livest Res 8(02):66–105. 10.5455/ijlr.20170817041629 10.5455/ijlr.20170817041629 [DOI] [Google Scholar]
- Kaur I, Puri M, Ahmed Z, Blanchet FP, Mangeat B, Piguet V (2013) Inhibition of HIV-1 replication by balsamin, a ribosome inactivating protein of Momordica balsamina. PLoS ONE 8(9):e73780. 10.1371/journal.pone.0073780 10.1371/journal.pone.0073780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Weber H, Elzayat MT, Hoffmann M, Pöhlmann S (2018) Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci Rep 8(1):16597. 10.1038/s41598-018-34859-w 10.1038/s41598-018-34859-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushari S, Hazarika I, Laloo D, Kumar S, Kalita JM, Sarma H (2023) An integrated computational approach towards the screening of active plant metabolites as potential inhibitors of SARS-CoV-2: an overview. Struct Chem 34(3):1073–1104. 10.1007/s11224-022-02066-z 10.1007/s11224-022-02066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Heo L, Seok C (2016) Effective protein model structure refinement by loop modeling and overall relaxation: refinement with loop modeling and MD relaxation. Proteins 84:293–301. 10.1002/prot.24858 10.1002/prot.24858 [DOI] [PubMed] [Google Scholar]
- Letko M, Marzi A, Munster V (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5(4):562–569. 10.1038/s41564-020-0688-y 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA et al (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426(6965):450–454. 10.1038/nature02145 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Grimm M, Dai WT, Hou MC, Xiao ZX, Cao Y (2020) CB-Dock: a web server for cavity detection-guided protein-ligand blind docking. Acta Pharmacol Sin 41(1):138–144. 10.1038/s41401-019-0228-6 10.1038/s41401-019-0228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang X, Gan J, Chen S, Xiao Z-X, Cao Y (2022) CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res 50(W1):W159–W164. 10.1093/nar/gkac394 10.1093/nar/gkac394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounkine E, Keiser MJ, Whitebread S, Mikhailov D, Hamon J, Jenkins JL et al (2012) Large-scale prediction and testing of drug activity on side-effect targets. Nature 486(7403):361–367. 10.1038/nature11159 10.1038/nature11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low ZY, Zabidi NZ, Yip AJW, Puniyamurti A, Chow VTK, Lal SK (2022) SARS-CoV-2 non-structural proteins and their roles in host immune evasion. Viruses 14(9):1991. 10.3390/v14091991 10.3390/v14091991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Martinusen SG, Zardecki C, Olivas C, Bacorn M, Balogun M et al (2023) A comprehensive survey of coronaviral main protease active site diversity in 3D: Identifying and analyzing drug discovery targets in search of broad specificity inhibitors for the next coronavirus pandemic. bioRxivorg. 10.1101/2023.01.30.526101 10.1101/2023.01.30.526101 [DOI] [Google Scholar]
- Ludidi A, Baloyi MC, Khathi A, Sibiya NH, Ngubane PS (2019) The effects of Momordica balsamina methanolic extract on haematological function in streptozotocin-induced diabetic rats: Effects on selected markers. Biomed Pharmacother 116(108925):108925. 10.1016/j.biopha.2019.108925 10.1016/j.biopha.2019.108925 [DOI] [PubMed] [Google Scholar]
- Mahdian S, Zarrabi M, Panahi Y, Dabbagh S (2021) Repurposing FDA-approved drugs to fight COVID-19 using in silico methods: Targeting SARS-CoV-2 RdRp enzyme and host cell receptors (ACE2, CD147) through virtual screening and molecular dynamic simulations. Inform Med Unlocked. 10.1016/j.imu.2021.100541 10.1016/j.imu.2021.100541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managa MG, Shai J, Thi Phan AD, Sultanbawa Y, Sivakumar D (2020) Impact of household cooking techniques on African nightshade and Chinese cabbage on phenolic compounds, antinutrients, in vitro antioxidant, and β-glucosidase activity. Front Nutr 7:580550. 10.3389/fnut.2020.580550 10.3389/fnut.2020.580550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiane P, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D (2021) Cooking African pumpkin leaves (momordicabalsamina L.) by stir-frying improved bioactivity and bioaccessibility of metabolites-metabolomic and chemometric approaches. Foods 10(11):2890. 10.3390/foods10112890 10.3390/foods10112890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin M, Mahmud S (2022) Omicron SARS-CoV-2 variant of concern: A review on its transmissibility, immune evasion, reinfection, and severity. Medicine (baltimore) 101(19):e29165. 10.1097/MD.0000000000029165 10.1097/MD.0000000000029165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujwar S, Harwansh RK (2022) In silico bioprospecting of taraxerol as a main protease inhibitor of SARS-CoV-2 to develop therapy against COVID-19. Struct Chem 33(5):1517–1528. 10.1007/s11224-022-01943-x 10.1007/s11224-022-01943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Narayanan K, Wada M, Makino S (2018) Inhibition of stress granule formation by Middle East respiratory syndrome Coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J Virol. 10.1128/JVI.00902-18 10.1128/JVI.00902-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A et al (1866) (2020) Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis 10:165878. 10.1016/j.bbadis.2020.165878 10.1016/j.bbadis.2020.165878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M, Sheraz M, Amin A, Waseem M, Aziz T, Khan AA et al (2022) Designing a novel peptide-based multi-Epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant Providencia heimbachae. Vaccines 10(8):1300. 10.3390/vaccines10081300 10.3390/vaccines10081300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CW, Ardern Z, Goldberg TL, Meng C, Kuo C-H, Ludwig C et al (2020) Dynamically evolving novel overlapping gene as a factor in the SARS-CoV-2 pandemic. Elife. 10.7554/eLife.59633 10.7554/eLife.59633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit PA (2023) Bivalent covid-19 vaccines - a cautionary tale. N Engl J Med 388(6):481–483. 10.1056/NEJMp2215780 10.1056/NEJMp2215780 [DOI] [PubMed] [Google Scholar]
- Osipiuk J, Azizi S-A, Dvorkin S, Endres M, Jedrzejczak R, Jones KA et al (2021) Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat Commun 12(1):743. 10.1038/s41467-021-21060-3 10.1038/s41467-021-21060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E, Ntoumi F, Hui DS, Abubakar A, Kramer LD, Obiero C et al (2022) Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529) - highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int J Infect Dis 114:268–272. 10.1016/j.ijid.2021.11.040 10.1016/j.ijid.2021.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth DSNBK, Singh G, Panda SP, Achanti S, Soni H, Chaudhuri TK et al (2023) In silico screening of plant-derived anti-virals from Shorea hemsleyana (king) king ex foxw against SARS CoV-2 main protease. Chem Afr 6(1):345–366. 10.1007/s42250-022-00521-2 10.1007/s42250-022-00521-2 [DOI] [Google Scholar]
- Ramalhete C, Gonçalves BMF, Barbosa F, Duarte N, Ferreira M-JU (2022) Momordica balsamina: phytochemistry and pharmacological potential of a gifted species. Phytochem Rev 21(2):617–646. 10.1007/s11101-022-09802-7 10.1007/s11101-022-09802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutwick Surya U, Praveen N (2021) A molecular docking study of SARS-CoV-2 main protease against phytochemicals of Boerhavia diffusa Linn. for novel COVID-19 drug discovery. Vir Dis 32(1):46–54. 10.1007/s13337-021-00683-6 10.1007/s13337-021-00683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Rosdi MHB, Kuppusamy UR (2017) Customized cooking methods enhance antioxidant, antiglycemic, and insulin-like properties of Momordica charantia and Moringa oleifera. J Food Qual. 10.1155/2017/9561325 10.1155/2017/9561325 [DOI] [Google Scholar]
- Thomas S (2020) The structure of the membrane protein of SARS-CoV-2 resembles the sugar transporter SemiSWEET. Pathog Immun 5(1):342–363. 10.20411/pai.v5i1.377 10.20411/pai.v5i1.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Ketkar R, Tao P (2022) ADMETboost: a web server for accurate ADMET prediction. J Mol Model 28(12):408. 10.1007/s00894-022-05373-8 10.1007/s00894-022-05373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor HG, Banerjee DI, Lipsa Rath S, Darji SA (2021) Computational drug re-purposing targeting the spike glycoprotein of SARS-CoV-2 as an effective strategy to neutralize COVID-19. Eur J Pharmacol 890(173720):173720. 10.1016/j.ejphar.2020.173720 10.1016/j.ejphar.2020.173720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umadevi P, Manivannan S, Fayad AM, Shelvy S (2020) In silico analysis of phytochemicals as potential inhibitors of proteases involved in SARS-CoV-2 infection. J Biomol Struct Dyn 40(11):5053–5059. 10.1080/07391102.2020.1866669 10.1080/07391102.2020.1866669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vkovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19(3):155–170. 10.1038/s41579-020-00468-6 10.1038/s41579-020-00468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A et al (2020) Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell 182(2):417-428.e13. 10.1016/j.cell.2020.05.034 10.1016/j.cell.2020.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46(D1):D1074–D1082. 10.1093/nar/gkx1037 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NA, Saier MH Jr (2021) The SARS-Coronavirus infection cycle: a survey of viral membrane proteins, their functional interactions and pathogenesis. Int J Mol Sci 22(3):1308. 10.3390/ijms22031308 10.3390/ijms22031308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P et al (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27(3):325–328. 10.1016/j.chom.2020.02.001 10.1016/j.chom.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Chaudhary JK, Jain N, Chaudhary PK, Khanra S, Dhamija P et al (2021) Role of structural and non-structural proteins and therapeutic targets of SARS-CoV-2 for COVID-19. Cells 10(4):821. 10.3390/cells10040821 10.3390/cells10040821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Zheng Y, Zeng X, He B, Cheng W (2022) Structural biology of SARS-CoV-2: open the door for novel therapies. Signal Transduct Target Ther 7(1):26. 10.1038/s41392-022-00884-5 10.1038/s41392-022-00884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z et al (2019) admetSAR 20: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 35(6):1067–1069. 10.1093/bioinformatics/bty707 10.1093/bioinformatics/bty707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data has been included inside the manuscript.