Abstract
This study compared the power of the novel inflammatory markers systemic immune inflammation index (SII) and the system inflammation response index (SIRI) versus the classical hematological indices neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and platelet counts in distinguishing between major depressive disorder (MDD) with and without suicide attempts and distinguishing the non-response to selective serotonin reuptake inhibitor (SSRI) treatment. A total of 139 young adult MDD patients and 54 healthy controls (HC) were included. We found that, in comparison to HC, baseline NLR, PLR, SII, and SIRI were significantly higher in MDD patients, but only NLR and SII had area under the ROC curve (AUC) values greater than 0.7. MDD patients with suicide attempts (SA) showed significantly higher baseline MLR and SIRI, and a tendency to increase NLR compared to those without SA. In terms of AUC, sensitivity, and specificity, NLR was better than MLR, SIRI, SII, and PLR in distinguishing SA. Non-responders to SSRI treatment showed a significant increase in baseline platelet count and PLR compared to responders with an AUC greater than 0.7. These findings highlight the potential benefit of combining novel and classical hematological indices in predicting depression, suicide attempts and treatment response.
Keywords: Depression outcomes, Neutrophil-to-lymphocyte ratio (NLR), Platelet-to-lymphocyte ratio (PLR), Platelet count, Systemic immune-inflammation index (SII), Systemic inflammation response index (SIRI)
Subject terms: Immunology, Psychology, Biomarkers, Diseases, Medical research
Introduction
Growing evidence supports the hypothesis that the development and progression of depression are related to an inflammatory process1–4. Numerous studies have consistently demonstrated elevated levels of peripheral and central inflammatory cytokines and acute-phase proteins in individuals with depression. Systematic reviews and meta-analyses have reinforced the significance of the connection between inflammation and depression and highlighted the potential role of inflammatory mediators, particularly C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), in their blood stream in the development and progression of depression5–9. These inflammatory mediators, which are markers of inflammation in the body, have emerged as one of the first biomarkers of the disease.
Recently, an increasing number of studies have shown that some people with depression have higher numbers of certain immune cells, such as whicte blood cells (WBC), monocytes, and neutrophils, which can contribute to an inflammatory state. A systematic review and meta-analysis revealed elevated absolute counts of WBC, neutrophils, and monocytes in patients with depression compared to controls10. Hematological parameters, including the neutrophil-to-lymphocyte ratio (NLR), the monocyte-to-lymphocyte ratio (MLR), and the platelet-to-lymphocyte ratio (PLR), can reflect the systemic inflammatory response.
Depression has been attributed to an increased chance of death, with suicide being a major cause. Identifying individuals with depression at risk for suicide attempts (SA) and predicting the efficacy of treatment are major challenges in Psychiatry. Elevated NLR, MLR, and PLR have been suggested as potential biomarkers for the inflammation progression and the severity of depression in major depressive disorder (MDD). A higher NLR has been associated with a higher severity of depression11–13 and a higher suicide risk in patients with depression11,13,14. Recently, we and others have demonstrated that higher MLR, PLR, and platelet count are linked to more severe depression and a high suicide risk14–16. In addition, treatment with selective serotonin reuptake inhibitors (SSRIs) has a greater effect on monocyte percentage and MLR than NLR or PLR. Higher baseline platelet counts have been associated with nonresponse to SSRI treatment, suggesting high baseline platelet counts as a predictor of treatment outcomes17. Systematic reviews and meta-analyses have confirmed the association of these inflammatory markers with depression and suicidal behaviors, suggesting their potential as indicators or predictors4,18–22. In a recent meta-analysis, Neupane et al.21 concluded that inflammatory abnormalities in suicide over and above the findings in depression could indicate that inflammatory biomarkers may serve as a predictor of imminent risk of suicidal behavior. Despite these findings, the diagnostic performance of these inflammatory markers in classifying or diagnosing, risk stratifying, subtyping the patient population with MDD related to inflammation or immune dysregulation, or distinguishing between MDD patients with and without suicidal behavior remains uncertain.
Systemic immune-inflammation index (SII) and systemic inflammation response index (SIRI) are two novel inflammatory indexes and convenient measurements based on peripheral blood neutrophils, monocyte counts, platelet counts, and lymphocyte counts. The SII (calculated as neutrophil count × platelet count/lymphocyte count) was first proposed as a powerful prognostic indicator of poor outcome in patients with hepatocellular carcinoma in 2014 by Hu et al. 23. Similarly, SIRI (calculated as neutrophil count × monocyte count/lymphocyte) was first described by Qi et al.24. Given that SII brings together three inflammatory peripheral cell counts, including neutrophil, platelet, and lymphocyte counts, into an equation and SIRI incorporates neutrophil, monocyte, and lymphocyte counts into an equation, they should offer a more comprehensive reflection of the inflammatory state.
Several studies confirmed that a higher SII was significantly associated with an increased risk for depression in patients with diabetes mellitus25, stroke26, and tuberculosis27. In addition, a study by Li et al.28 demonstrated that high levels of SII and SIRI were associated with an increased risk of depression. However, to our knowledge, there is no study comparing the prognostic values of these indices with clinical outcomes in Psychiatry, including suicide attempts and the response to treatment among patients with MDD.
We hypothesized that baseline SII and/or SIRI might be a better prognostic indicator for depression and poor clinical outcomes of the disease, such as suicide attempts and nonresponse to SSRI treatment, compared to monocyte count, platelet count, NLR, MLR, and PLR. This study aims to evaluate and compare the predictive performance of baseline SII and SIRI against baseline MLR, NLR, PLR, and platelet counts in diagnosing depression and suicide attempts in MDD sample, as well as in predicting the response to SSRI treatment among young adults diagnosed with MDD.
Methods
Study design and participants
This was a retrospective cohort study involving university students who were diagnosed with MDD from the psychiatry clinic of Walailak University Hospital in Nakhon Si Thammarat, southern Thailand. The sample included 139 young adults with MDD and 54 matched healthy control subjects (HC). To take part in the study, all young adults diagnosed with MDD who had never taken antidepressants or any depressive treatment were approached for participation.
The participants were university students involved in a project initiated by the Division of Student Affairs called “Clearance for Depression", which aimed to improve awareness of depression and suicide prevention among university students. The students were screened for and measured the severity of depressive symptoms using the self-reported Patient Health Questionnaire (PHQ-9) scale. Students who scored seven or higher on the PHQ-9 scale, indicating a high likelihood of depression, were then referred to the outpatient psychiatric unit, where the diagnosis of MDD was confirmed by a qualified psychiatrist.
Major depressive disorder (MDD) diagnosis and suicidal behavior assessment
MDD diagnosis was made by a licensed psychiatrist using the criteria specified in the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Both new onset and recurrent episodes of MDD were included.
Suicidal behaviors and suicidality severity were assessed by a psychiatrist during the diagnostic or therapeutic interviews using the 8Q scale, a Thai version of the suicidality module of the Mini International Neuropsychiatric Interview (M.I.N.I.) 5.0.0. This 8Q scale has demonstrated excellent psychometric properties, including a Kappa value of 0.86, sensitivity of 0.96, specificity of 0.91, a positive predictive value of 0.90, a negative predictive value of 0.97, and an efficiency of 0.9329. Based on suicidal behaviors, the MDD patients were categorized into: (1) MDD with suicide attempts in the past month (MDD with SA) and (2) MDD without suicide attempts in the past month (MDD without SA). Based on suicidality severity assessed through the 8Q Scale, the patients were categorized into: (1) no suicide risk (score = 0), (2) low suicide risk (score = 1–16), and (3) high suicide risk (score ≥ 17), as previously described16.
Each MDD patient was prescribed a SSRI treatment (fluoxetine, 20 mg/day, or sertraline, 50 mg/day). The MDD patients were followed up to monitor their responsiveness to SSRI after 12 weeks of treatment.
The young adults diagnosed with MDD were then screened through medical records to confirm their eligibility, followed by a personal invitation that was extended by a member of the research team during a routine clinical visit. Participants were provided with a detailed explanation of the study's aims, procedures, and the potential implications of the study findings for understanding MDD. They were also informed of their rights as study participants, including their right to withdraw at any point without any consequences to their ongoing clinical care. Written informed consent was obtained from all individuals who agreed to participate. Baseline data were collected between November 2020 and April 2021.
Inclusion and exclusion criteria
Inclusion criteria
All young adults diagnosed with MDD, aged 18–24 years, antidepressant-naive, and receiving outpatient care at the university hospital were included in the study. Healthy control subjects (HC) with age and sex matching were also included. HC were selected from university students who did not have MDD.
Exclusion criteria
Participants, both MDD subjects and HC, were excluded from the study if they met or reported any of the following conditions related to inflammatory processes: (1) had physical comorbidities; (2) were febrile or exhibited other signs of recent infections; (3) had chronic physical illnesses or inflammatory diseases; (4) were using anti-inflammatory or immunosuppressive drugs; (5) were diagnosed with other mood disorders or mental illnesses; (6) had immune system or hematological system disorders; (7) were pregnant or had been pregnant within the past six months; and (8) were using substances, alcohol, or smoking. Furthermore, they were excluded if their medical records did not contain information pertaining to the exclusion criteria.
Blood sample collection and inflammatory index measurement
A complete blood count (CBC) with differential analysis was performed for each participant at the date of diagnosis, before initiating MDD-specific treatment, and after completing the 12-week treatment protocol. A routine CBC with differential was analyzed from a 3-ml EDTA blood sample within an hour of its collection in the Walailak University Hospital Central Laboratory using an automated analyzer. MLR, NLR, PLR, SII, and SIRI were calculated. SII was calculated by (N × P)/L (N, P, and L represent neutrophil counts, platelet counts, and lymphocyte counts, respectively). SIRI was calculated by (N × M)/L. Other inflammatory indexes (MLR, NLR, and PLR) were measured according to the previous study15–17.
Monitoring response to SSRI treatment
The treatment outcomes were evaluated by a psychiatrist. Based on response to treatment, the patients were classified into: (1) responders and (2) nonresponders, as previously described. Briefly, a subject is identified as a responder if there is a minimum 50% decrease in the severity of depressive symptoms from the starting point, as determined by scores on the PHQ-9 scale. Conversely, a "nonresponder" is defined as someone who does not exhibit an adequate response to a sufficient dose of an antidepressant over the course of treatment17,30. After 12 weeks of SSRI treatment, venous blood was drawn to access changes in the CBC parameters, inflammatory ratios and indexes (NLR, MLR, PLR, and platelet count, SII, and SIRI). The follow-up sample comprised 45 MDD patients who completed the 12-week treatment protocol; the remaining were drop-outs mainly because of the COVID-19 situation in southern Thailand during their follow-up period.
Statistics
Statistical analyses were performed with SPSS software (version 22.0, Chicago, IL, USA). Descriptive data are shown as mean ± SD and as frequencies and percentages. For categorical data, analyses were performed using chi-squared tests. For continuous data with a normal distribution, the t-test, or ANOVA, was used. Kruskal–Wallis or Mann–Whitney U were used for continuous data with a non-normal distribution. The receiver operating curve (ROC) analysis was performed to assess the power of NLR, MLR, PLR, SII and SIRI in distinguishing MDD from HC and classifying MDD with and without suicide attempts. Furthermore, we used the baseline biomarker levels to identify the response to SSRI treatment in the sample using ROC curve analysis. The area under the ROC curve (AUC), optimal cut-off value, sensitivity, and specificity were determined by ROC curve analysis. Statistical significance was set at p < 0.05 for all analyses.
Ethical approval
This research adhered to the Declaration of Helsinki and its updates31, and it was approved by the institutional ethics committee of Walailak University (WUEC-20-064-02).
Results
Participant demographics
This study included 139 MDD patients and 54 age- and sex-matched healthy controls; all of them were young adults aged between 18 and 24 years. The characteristics and baseline complete blood count (CBC) parameters of the participants are detailed in Tables 1 and 2. There were no significant differences in terms of body mass index (BMI) between MDD patients and HC. A majority of the participants were female, 69.78% for MDD patients and 64.81% for HC. Most MDD participants (91.37%) had their first depressive episode at the beginning of the study while 80 (57.55%) of them were evaluated as having some level of suicide risk.
Table 1.
Characteristics, baseline complete blood parameters, and inflammatory markers of the participants.
| Characteristics | MDD patients (n = 139) | Healthy controls (n = 54) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.688 | ||
| Male | 42 (30.22) | 19 (35.19) | |
| Female | 97 (69.78) | 35 (64.81) | |
| Age (years) | 0.916 | ||
| Mean ± SD | 20.44 ± 1.13 | 20.42 ± 1.32 | |
| BMI | 0.643 | ||
| Mean ± SD | 22.69 ± 6.07 | 23.03 ± 3.92 | |
| State of depressive episode, n (%) | – | ||
| First episode | 127 (91.37) | – | |
| Recurrent depression | 12 (8.63) | – | |
| Suicide risk score (8Q score), n (%) | < 0.0001* | ||
| No suicide risk (score = 0) | 59 (42.45) | 54 (100.00) | |
| Low suicide risk (score = 1–16) | 56 (40.29) | 0 (0.00) | |
| High suicide risk (score ≥ 17) | 24 (17.26) | 0 (0.00) | |
| WBC count (× 109 cells/L) | 7.91 ± 2.15 | 6.91 ± 1.45 | 0.002* |
| Neutrophil (%) | 60.56 ± 6.06 | 54.52 ± 4.82 | < 0.0001* |
| Neutrophil count (× 109 cells/L) | 4.82 ± 1.54 | 3.77 ± 0.85 | < 0.0001* |
| Lymphocyte (%) | 32.09 ± 5.53 | 37.20 ± 3.84 | < 0.0001* |
| Lymphocyte count (× 109 cells/L) | 2.51 ± 0.72 | 2.57 ± 0.61 | 0.585 |
| Monocyte (%) | 5.33 ± 2.74 | 5.69 ± 2.13 | 0.342 |
| Monocyte count (× 109 cells/L) | 0.41 ± 0.23 | 0.39 ± 0.16 | 0.476 |
| Platelet count (× 109 cells/L) | 290.27 ± 67.31 | 263.37 ± 43.55 | 0.001* |
| NLR | 2.00 ± 0.72 | 1.49 ± 0.26 | < 0.0001* |
| MLR | 0.172 ± 0.096 | 0.156 ± 0.061 | 0.162 |
| PLR | 122.78 ± 39.22 | 106.19 ± 23.16 | < 0.0001* |
| SII (109 cells/L) | 573.66 ± 221.46 | 389.40 ± 78.25 | < 0.0001* |
| SIRI (109 cells/L) | 0.81 ± 0.51 | 0.58 ± 0.24 | < 0.0001* |
Data are shown as mean ± SD, unless otherwise noted. *P < 0.05.
MDD major depressive disorder, n number of patients, BMI body mass index, 8Q score depression scale from the 8Q Scale, WBC white blood cell, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Table 2.
Characteristics, baseline complete blood parameters, and inflammatory markers in MDD patients with or without suicide attempts.
| Characteristics | MDD with SA (n = 43) | MDD without SA (n = 96) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.229 | ||
| Male | 16 (37.21) | 26 (27.08) | |
| Female | 27 (62.79) | 70 (72.92) | |
| Age (years) | 0.705 | ||
| Mean ± SD | 20.49 ± 1.47 | 20.40 ± 1.26 | |
| BMI | 0.323 | ||
| Mean ± SD | 23.82 ± 5.57 | 22.69 6.27 | |
| State of depressive episode, n (%) | 0.135 | ||
| First episode | 37 (86.05) | 90 (93.75) | |
| Recurrent depression | 6 (13.95) | 6 (6.25) | |
| Suicide risk score (8Q score), n (%) | < 0.0001* | ||
| No suicide risk (score = 0) | 0 (0.00) | 59 (61.46) | |
| Low suicide risk (score = 1–16) | 22 (51.16) | 34 (35.42) | |
| High suicide risk (score ≥ 17) | 21 (48.84) | 3 (3.12) | |
| WBC count (× 109 cells/L) | 7.90 ± 1.78 | 7.92 ± 2.30 | 0.959 |
| Neutrophil (%) | 61.67 ± 5.96 | 60.06 ± 6.06 | 0.148 |
| Neutrophil count (× 109 cells/L) | 4.89 ± 1.32 | 4.79 ± 1.63 | 0.727 |
| Lymphocyte (%) | 29.95 ± 5.36 | 33.04 ± 5.36 | 0.002* |
| Lymphocyte count (× 109 cells/L) | 2.35 ± 0.63 | 2.58 ± 0.75 | 0.082 |
| Monocyte (%) | 5.98 ± 3.37 | 5.04 ± 2.36 | 0.063 |
| Monocyte count (× 109 cells/L) | 0.46 ± 0.25 | 0.39 ± 0.21 | 0.106 |
| Platelet count (× 109 cells/L) | 282.6 ± 58.12 | 293.7 ± 71.05 | 0.368 |
| NLR | 2.15 ± 0.55 | 1.92 ± 0.78 | 0.090 |
| MLR | 0.21 ± 0.13 | 0.16 ± 0.08 | 0.005* |
| PLR | 126.29 ± 36.17 | 121.21 ± 40.59 | 0.482 |
| SII (109 cells/L) | 603.05 ± 192.85 | 560.49 ± 232.89 | 0.297 |
| SIRI (109 cells/L) | 0.97 ± 0.59 | 0.74 ± 0.45 | 0.012* |
Data are shown as mean ± SD, unless otherwise noted. *P < 0.05.
MDD major depressive disorder, SA had made at least one suicide attempts during the month prior to assessment, n number of patients, BMI body mass index, 8Q score depression scale from the 8Q Scale, WBC white blood cell, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Baseline blood immune cell markers as indicators of MDD
Compared to HC, MDD patients showed significantly higher baseline white blood cell (WBC) count, neutrophil percentage, neutrophil count, and platelet count, along with lower lymphocyte percentage (p-values = 0.002, < 0.0001, < 0.0001, = 0.001, and < 0.0001, respectively). In comparison to the HC group, NLR, PLR, SII, and SIRI were significantly higher in MDD patients (all p-values < 0.0001) (Table 1).
The area under the ROC curves of baseline inflammatory markers for the prediction of MDD diagnosis was determined to test their diagnostic performance. NLR and SII had AUC greater than 0.7. The ROC characteristics of the inflammatory markers were as follows: NLR (AUC: = 0.73, p-value < 0.0001; cut-off = 1.645, sensitivity = 71.2%, specificity = 67.2%) and SII (AUC = 0.74, p-value < 0.0001; cut-off = 428.67, sensitivity = 75.5%, specificity = 62.5%) (Table 3 and Fig. 1).
Table 3.
Area under the receiver operating characteristic (ROC) curve and cut-off points of baseline inflammatory markers for the prediction of MDD and of suicide attempts in patients with MDD.
| Parameters | AUC | 95% CI of AUC | Cut-off point | P-value | % sensitivity | % specificity |
|---|---|---|---|---|---|---|
| For the prediction of MDD | ||||||
| NLR | 0.73 | 0.66–0.81 | 1.645 | < 0.000* | 71.2 | 67.2 |
| MLR | 0.52 | 0.44–0.60 | – | 0.603 | – | – |
| PLR | 0.57 | 0.48–0.65 | – | 0.137 | – | – |
| SII | 0.74 | 0.67–0.82 | 428.67 | < 0.000* | 75.5 | 62.5 |
| SIRI | 0.62 | 0.54–0.70 | 0.592 | 0.007* | 61.9 | 50.0 |
| For the prediction of suicide attempts | ||||||
| NLR | 0.71 | 0.62–0.80 | 1.82 | < 0.0001* | 72.1 | 65.6 |
| MLR | 0.60 | 0.50–0.71 | 0.165 | 0.039* | 60.5 | 56.2 |
| PLR | 0.58 | 0.49–0.68 | 112.8 | 0.094 | 60.5 | 55.6 |
| SII | 0.67 | 0.59–0.76 | 478.02 | 0.001* | 72.1 | 55.6 |
| SIRI | 0.66 | 0.56–0.76 | 0.69 | 0.001* | 69.8 | 61.3 |
*P < 0.05.
AUC area under the ROC curve, 95% CI 95% confidence intervals, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Figure 1.
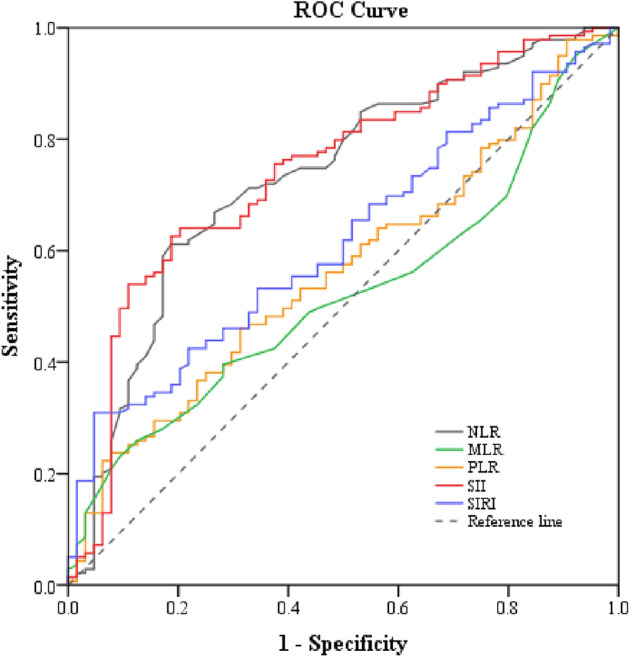
The receiver operating characteristic (ROC) curve of baseline inflammatory markers for the prediction of MDD. NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Comparison of MDD patients with and without suicide attempts
Within the MDD group, further exploration focused on suicide attempts. Characteristics, baseline complete blood parameters, and inflammatory markers among the MDD patients by SA history are detailed in Table 2. Among the 139 cases of MDD patients, 43 (30.94%) had made at least one suicide attempt during the month prior to assessment. While there were no significant differences in sex, age, BMI, or state of depressive episode between MDD patients with and without suicide attempts, the lymphocyte percentage was significantly lower in MDD patients with suicide attempts (p-value = 0.002). There was a tendency for neutrophil percentage, monocyte percentage, and monocyte count to increase in MDD with suicide attempts, but these findings did not reach statistical significance (Table 2).
When compared to the MDD without SA group, MLR and SIRI were significantly higher in MDD patients with SA (p-values = 0.005 and 0.012, respectively). Although NLR displayed a tendency to increase, this difference did not reach statistical significance (p-value = 0.090) (Table 2).
Table 3 and Fig. 2 provide information on the area under the ROC curves and cut-off points of baseline inflammatory markers for predicting suicide attempts in our sample with MDD. NLR outperformed MLR, PLR, SII, and SIRI in predicting suicide attempts. Only NLR had AUC greater than 0.7, with the ROC characteristics as follows: (AUC: area under the curve = 0.71, p-value < 0.0001; cut-off = 1.82, sensitivity = 72.1%, specificity = 65.6%) (Table 3).
Figure 2.
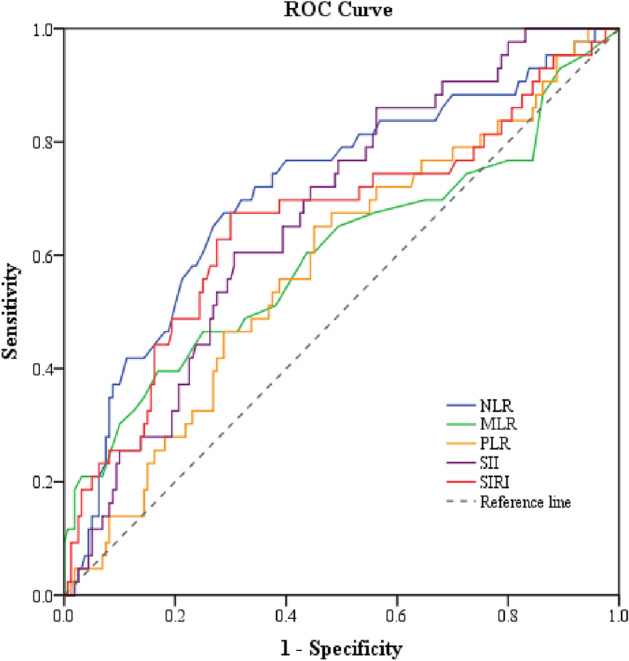
The receiver operating characteristic (ROC) curve of baseline inflammatory markers for the prediction of suicide attempts in patients with MDD. NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Comparison of MDD patients who responded and did not respond to SSRI treatment
As reported in our previous publication17, CBC parameters at baseline did not differ between responders (n = 25) and nonresponders (n = 20) to SSRI treatment, with the exception of significantly lower platelet counts (p-value = 0.016) among the responders. We have expanded upon these findings to include new analyses for SII and SIRI in the present work. The responders and non-responders did not vary in their levels of SII (responders (× 109 cells/L): 552.89 ± 208.48; nonresponders (× 109 cells/L): 629.88 ± 190.75, p-value = 0.208) or SIRI (responders (× 109 cells/L): 0.856 ± 0.46; nonresponders (× 109 cells/L): 0.972 ± 0.66, p-value = 0.494). Both PLR and platelet count demonstrated an AUC exceeding 0.7, accompanied by high sensitivity and specificity. In terms of AUC, sensitivity, and specificity, platelet count had greater performance compared to PLR as an inflammatory marker in predicting non-response to SSRI treatment in young adults with MDD. The area under the ROC curves and the corresponding cut-off points of baseline PLR and platelet count as well as NLR, MLR, SII and SIRI in predicting non-response to SSRI therapy are presented in Table 4 and Fig. 3.
Table 4.
Area under the receiver operating characteristic (ROC) curve and cut-off points of baseline inflammatory markers for the prediction of having no response to SSRI treatment in patients with MDD.
| AUC | 95% CI of AUC | Cut-off point | P-value | % sensitivity | % specificity | |
|---|---|---|---|---|---|---|
| Platelet count (× 109 cells/L) | 0.79 | 0.64–0.95 | 317.5 | 0.011* | 75.7 | 75.0 |
| NLR | 0.42 | 0.20–0.63 | 1.85 | 0.458 | 48.6 | 37.5 |
| MLR | 0.47 | 0.28–0.66 | 0.18 | 0.789 | 56.8 | 62.5 |
| PLR | 0.74 | 0.56–0.92 | 135.77 | 0.033* | 83.8 | 62.5 |
| SII (109 cells/L) | 0.64 | 0.45–0.83 | 676.98 | 0.224 | 78.4 | 37.5 |
| SIRI (109 cells/L) | 0.46 | 0.25–0.67 | 0.748 | 0.722 | 45.9 | 37.5 |
*P < 0.05.
AUC area under the ROC curve, 95% CI 95% confidence intervals, NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Figure 3.
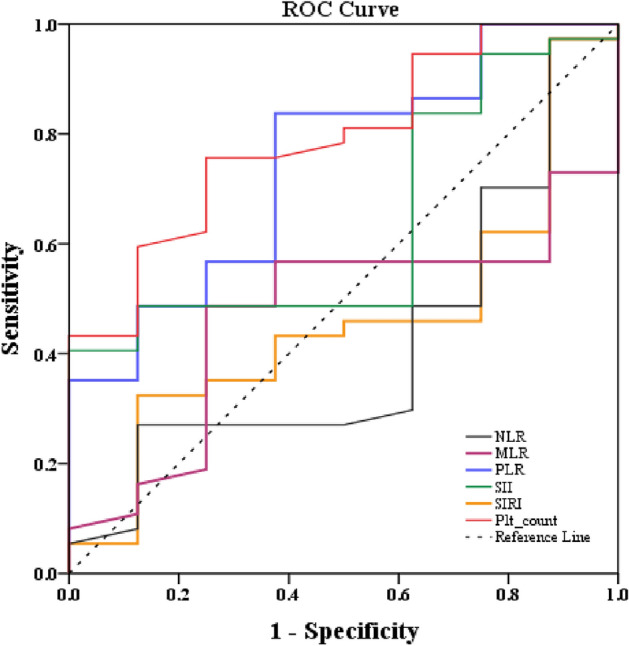
The receiver operating characteristic (ROC) curve of baseline platelet count and inflammatory markers for the prediction of having no response to SSRI treatment in patients with MDD. NLR neutrophil-to-lymphocyte ratio, MLR monocyte-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammatory index, SIRI systemic inflammation response index.
Discussion
Several simple biological markers, such as NLR, MLR, and PLR, have been suggested as potential diagnostic and prognostic tools for depression and its severity. We hypothesized that the novel systemic inflammatory markers SII and/or SIRI might offer a better diagnostic value for depression and poor clinical outcomes of the disease, including suicide attempts and nonresponse to SSRI treatment, compared to NLR, MLR, PLR, and platelet count. In this study, we report that, in comparison to healthy controls, MDD patients showed a significantly higher baseline white blood cell (WBC) count, neutrophil percentage, neutrophil count, and platelet count, along with a lower lymphocyte percentage. As a result, the NLR, PLR, SII, and SIRI were significantly elevated in individuals with MDD. Moreover, we employed ROC curve analysis to investigate the diagnostic values of the inflammatory markers for MDD. The result revealed that NLR and SII were better than MLR, PLR, and SIRI as inflammatory markers for distinguishing between MDD and healthy controls. Both baseline NLR and SII had AUC values greater than 0.7, indicating their potential utility in distinguishing MDD samples from healthy samples in young adults. Similarly, MDD patients with SA were characterized by reduced baseline lymphocytes and increased MLR and SIRI in comparison to the levels among MDD patients without SA. However, ROC curve analysis demonstrated NLR as a superior diagnostic marker for SA among MDD sample over MLR, SIRI, SII, and PLR. Furthermore, platelet count outperformed PLR and other markers as an inflammatory marker for predicting non-response to SSRI treatment. To our knowledge, this is the first study to evaluate and compare the diagnostic values of baseline SII and SIRI versus NLR, MLR, PLR, and platelet counts in distinguishing MDD, and suicide attempts, as well as evaluating prognostic value of these biomarkers in assessing SSRI treatment response among patients with MDD.
These notable differences in various hematological parameters between young adults with MDD and HC indicate immune cell alterations that could lead to an inflammatory condition associated with MDD and possible cellular origins of cytokine changes linked to depression. Additionally, our data showed a marked increase in the two inflammatory indexes, SII and SIRI, in patients with MDD. These changes are apparently driven by an increase in neutrophil and platelet counts and a decrease in lymphocyte count. This observation aligns with NHANES study by Li et al.28, which found that both SII and SIRI are associated with an increased risk of depression. This supports the findings of meta-analysis by Foley et al.10, which indicated that multiple arms of the immune system are involved in the pathophysiology of MDD.
Systematic reviews and meta-analyses have highlighted the association between certain inflammatory ratios, including NLR, MLR, and PLR, and depression and suicidal behaviors4,18,19,22. These systematic reviews and meta-analyses suggested that NLR, PLR, and MLR may serve as potential indicators or predictors of depression and suicide risk, although their significance may vary in different patient populations and regions. Mazza et al.18 reviewed 11 studies focusing on subjects with bipolar disorder (BD) and found that NLR and PLR were higher in BD subjects compared to healthy controls. Cheng et al.19 analyzed 18 studies involving 2264 depressed patients and 2415 controls. They observed that patients with depression had notably higher NLR and PLR values compared to controls, with MLR showing a slight elevation in depressed individuals. In a subgroup analysis of Chinese participants and matched age and gender groups, NLR, PLR, and MLR were all elevated in depressed patients. Additionally, individuals with post-stroke depression had higher NLR and MLR values compared to those without depression. Su et al.4 conducted a study with 2580 cases and 2664 controls, revealing that depressed individuals had significantly higher NLR levels than healthy controls, although no significant differences were observed in PLR and MLR values between the two groups. Velasco et al.22 analyzed 11 studies involving 1701 depressed patients. They found that NLR was higher in depressed patients with a history of suicide attempts compared to those without suicidal behavior. Indeed, several studies have reported higher PLR in suicidal behavior among patients with MDD compared to those with MDD alone11,14,22,32.
There have been several studies that have explored the relationship between NLR, MLR, PLR, SII, and SIRI within the context of depression, with a specific focus on BD. In Dadouli et al.'s study33, they found that NLR, MLR, and SII indices were significantly higher in patients with BD, both during manic episodes as well as depressive episodes, than in HC. Notably, neutrophils and NLR had the highest area under the curve in the ROC curve. In the study by Dionisie et al.34, patients in manic episodes demonstrated heightened NLR, MLR, PLR, and SII index compared to those with unipolar depression, as well as increased NLR and SII index when compared to bipolar depression. Additionally, the NLR and SII index were higher in individuals with bipolar depression compared to those with unipolar depression. However, to our knowledge, there is no study comparing the prognostic values of these indices with clinical outcomes, including suicide attempts and the response to treatment within the context of depression or among patients with MDD.
Baseline NLR outperformed SII, SIRI, MLR, and PLR in distinguishing suicide attempts. Predicting suicide attempts is crucial for depressed individuals. We further examined and compared the predictive performance of baseline SII and SIRI against baseline MLR, NLR, PLR, and platelet counts in distinguishing suicide attempts among young adult MDD patients. We found that MLR and SIRI were significantly higher in MDD patients with SA compared to those without suicide attempts, while NLR displayed a tendency to increase. However, it is noteworthy that the AUC for NLR in distinguishing suicide attempts was superior to MLR, PLR, SII, and SIRI. Additionally, NLR demonstrated an AUC greater than 0.7, indicating its potential as an indicator for suicide attempts. These suggest that NLR was superior to MLR, PLR, SII, and SIRI as inflammatory markers for predicting suicide attempts, underscoring its potential as an indicator of suicide attempts in young adult MDD. Nevertheless, its sensitivity and specificity were slightly below conventional thresholds (as shown in Table 4 and Fig. 2). However, in the context of predicting suicide attempts, where achieving early detection is of critical importance, NLR's performance could be considered acceptable.
Baseline platelet count and PLR were the only inflammatory indicators related to nonresponse to SSRI. Platelet count predicted SSRI non-response better than PLR. This study showed that NLR, PLR, SII, SIRI, and platelet count had a tendency to decrease following SSRI treatment; however, SSRI treatment significantly diminished only MLR and SIRI. In our previous study17, we proposed the potential utility of baseline platelet count as an indicator for predicting MDD individuals unlikely to respond to SSRI treatment. This study expanded its original scope by incorporating an investigation of composite measures of inflammatory reaction, i.e., SII and SIRI, as potential predictors for individuals with MDD who do not respond to SSRI therapy. This study showed that the only inflammatory biomarkers associated with the incidence of nonresponse to SSRI treatment were a higher baseline platelet count and PLR. Both PLR and platelet count demonstrated AUC values higher than 0.7, suggesting the potential utility of platelet count and PLR as indicators for non-response to SSRI treatment (as shown in Table 4). Importantly, in terms of AUC, sensitivity, and specificity, baseline platelet count was better than baseline PLR as a more reliable inflammatory marker for non-response to SSRI treatment in these patients. This observation implies that baseline platelet count and PLR could serve as early indicators of non-response to SSRI therapy.
Against the backdrop of these important findings, cutoffs for abnormal levels of these novel hematological marker indicating risk for specific conditions are yet to be established. As an important first step, population level data from a Dutch sample (n = 8711) showed that mean values of NLR, PLR and SII lie around 1.76, 120 and 459, respectively35. Interestingly, the optimal cutoffs identified in our analysis approximated these values, indicating that translation of these cutoffs into risk evaluations will require further investigation.
In summary, the findings of this study shed light on the significance of specific baseline inflammatory markers and platelet count as potential indicators for predicting MDD outcomes in young adult populations. Baseline SII and NLR exhibited strong diagnostic potential for MDD and suicide attempts in young adults. Baseline SII outperformed SIRI, NLR, MLR, and PLR in distinguishing MDD. In terms of AUC, sensitivity, and specificity, NLR was better than SII, SIRI, MLR, and PLR in predicting suicide attempts. The only inflammatory biomarkers associated with the incidence of nonresponse to SSRI treatment were a higher baseline platelet count and PLR. Platelet count was superior to PLR as an indicator of non-response to SSRI treatment in these patients. These findings demonstrate the potential benefit of integrating both novel and classical hematological parameters to enhance the predictive accuracy for depression, suicide attempts, and treatment response. Howerver, for an inflammatory marker to be clinically useful, it must not only demonstrate a strong association with MDD but also possess high sensitivity and specificity for the disorder, distinguishing it from other conditions with similar symptoms or inflammatory profiles. This necessitates extensive validation studies to ascertain the clinical relevance and utility of these markers.
Strengths and limitations
The study's strength lies in its carefully planned cohort design, which excluded confounding variables or comorbidities that could influence inflammatory changes. We also adjusted for education level, age, and sex. The eligibility criteria were stringent in a narrow age range in a relatively young population. Thus, these factors should not have an impact on the outcomes. Despite these strengths, the study is constrained by its relatively small sample size, particularly the samples who completed the 12-week treatment protocol, potentially affecting the robustness of the conclusions drawn in this section. Additionally, based on the limitations of the retrospective nature of the study, which relies on existing records that could be prone to recall bias or incomplete or inconsistent data, it is fortunately that all participants in this study had their diagnosis and treatment made by only one licensed psychiatrist, thereby minimizing the possibility of inconsistent documentation.
Implications
Prediction models are sorely needed to identify suicide risk in depression. The findings from this study highlight the potential utility of integrated immune inflammatory signatures, particularly baseline NLR, SII, or platelet count, as indicators for predicting outcomes in MDD, including the prediction of suicide attempts and the identification of individuals unlikely to respond to SSRI treatment. These markers offer potential advantages in terms of early detection and personalized treatment approaches. Depression patients with a high baseline NLR, SII, or platelet count should be closely monitored, since this might be a viable treatment strategy for limiting suicide attempts. Additionally, identifying individuals who are unlikely to respond to treatment early in the course of their illness is essential for therapeutic decisions and minimizing the risk of prolonged suffering. These indices are easy to calculate and based on routinely performed clinical tests of blood cell counts, making them feasible to translate into practice. Future studies should investigate the utility of composite indicators incorporating both cellular and protein biomarkers. Additionally, research should focus on validating these markers with a larger sample size and proposing optimal cutoffs. Futue studies should also examine the utility of these markers in heterogeneous patient groups, including different age ranges, genders, ethnic backgrounds, and clinical settings. Furthermore, research should encompass major affective disorders, including not only MDD but also various states of BD.
Acknowledgements
We would like to thank the staff from the Central Laboratory of Walailak University Hospital for their assistance with blood sample collection.
Author contributions
PN, PK, SPN, PP, and PK contributed to the conception and design of the study. PN, PK, and PP contributed to the funding acquisition. PN, PP, HJ, and PC collected blood samples and data. PN performed the statistical analysis and wrote the main manuscript text. SPN substantively revised the manuscript. All authors reviewed and approved the manuscript.
Funding
This research was financially supported by Walailak University, Thailand (Grant No. WU-IRG-63–042) and the BDMS Health Research Center, Bangkok Dusit Medical Services PLC, Thailand.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
PK and PK are employed by the Bangkok Dusit Medical Services PLC, Thailand. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Haruthai Jongkrijak, Potiga Chotipong and Pakin Kaewpijit.
References
- 1.Berk, M. et al. So depression is an inflammatory disease, but where does the inflammation come from?. BMC Med.11, 200. 10.1186/1741-7015-11-200 (2013). 10.1186/1741-7015-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felger, J. C. Role of inflammation in depression and treatment implications. in Antidepressants. (ed. Macaluso, M. & Preskorn, S.) 255–286 (Springer, 2019) 10.1007/164_2018_166 [DOI] [PubMed]
- 3.Osimo, E. F., Baxter, L. J., Lewis, G., Jones, P. B. & Khandaker, G. M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med.49(12), 1958–1970. 10.1017/S0033291719001454 (2019). 10.1017/S0033291719001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su, M., Ouyang, X. & Song, Y. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and monocyte to lymphocyte ratio in depression: A meta-analysis. J. Affect. Disord.308, 375–383. 10.1016/j.jad.2022.04.038 (2022). 10.1016/j.jad.2022.04.038 [DOI] [PubMed] [Google Scholar]
- 5.Köhler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand.135(5), 373–387. 10.1111/acps.12698 (2017). 10.1111/acps.12698 [DOI] [PubMed] [Google Scholar]
- 6.Smith, K. J., Au, B., Ollis, L. & Schmitz, N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp. Gerontol.102, 109–132. 10.1016/j.exger.2017.12.005 (2018). 10.1016/j.exger.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 7.D’Acunto, G., Nageye, F., Zhang, J., Masi, G. & Cortese, S. Inflammatory cytokines in children and adolescents with depressive disorders: A systematic review and meta-analysis. J. Child Adolesc. Psychopharmacol.29(5), 362–369. 10.1089/cap.2019.0015 (2019). 10.1089/cap.2019.0015 [DOI] [PubMed] [Google Scholar]
- 8.Mac Giollabhui, N., Ng, T. H., Ellman, L. M. & Alloy, L. B. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Mol. Psychiatry.26(7), 3302–3314. 10.1038/s41380-020-00867-4 (2021). 10.1038/s41380-020-00867-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam, M. R. et al. Evaluation of inflammatory cytokines in drug-naïve major depressive disorder: A systematic review and meta-analysis. Int. J. Immunopathol. Pharmacol.37, 3946320231198828. 10.1177/03946320231198828 (2023). 10.1177/03946320231198828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley, É. M., Parkinson, J. T., Mitchell, R. E., Turner, L. & Khandaker, G. M. Peripheral blood cellular immunophenotype in depression: A systematic review and meta-analysis. Mol. Psychiatry.28(3), 1004–1019. 10.1038/s41380-022-01919-7 (2023). 10.1038/s41380-022-01919-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekinci, O. & Ekinci, A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: A specific relationship with the neutrophil-to-lymphocyte ratio. Nord. J. Psychiatry.71(8), 574–580. 10.1080/08039488.2017.1363285 (2017). 10.1080/08039488.2017.1363285 [DOI] [PubMed] [Google Scholar]
- 12.Kayhan, F., Gündüz, Ş, Ersoy, S. A., Kandeğer, A. & Annagür, B. B. Relationships of neutrophil-lymphocyte and platelet-lymphocyte ratios with the severity of major depression. Psychiatry Res.247, 332–335. 10.1016/j.psychres.2016.11.016 (2017). 10.1016/j.psychres.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 13.Velasco, Á. et al. Neutrophil-to-lymphocyte ratio: A potential new peripheral biomarker of suicidal behavior. Eur. Psychiatry.63(1), e14. 10.1192/j.eurpsy.2019.20 (2020). 10.1192/j.eurpsy.2019.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amitai, M. et al. Neutrophil to-lymphocyte and platelet-to-lymphocyte ratios as biomarkers for suicidal behavior in children and adolescents with depression or anxiety treated with selective serotonin reuptake inhibitors. Brain Behav. Immun.104, 31–38. 10.1016/j.bbi.2022.04.018 (2022). 10.1016/j.bbi.2022.04.018 [DOI] [PubMed] [Google Scholar]
- 15.Puangsri, P. & Ninla-aesong, P. Potential usefulness of complete blood count parameters and inflammatory ratios as simple biomarkers of depression and suicide risk in drug-naive, adolescents with major depressive disorder. Psychiatry Res.305, 114216. 10.1016/j.psychres.2021.114216 (2021). 10.1016/j.psychres.2021.114216 [DOI] [PubMed] [Google Scholar]
- 16.Ninla-aesong, P., Puangsri, P., Kietdumrongwong, P., Jongkrijak, H. & Noipha, K. Being overweight and obese increases suicide risk, the severity of depression, and the inflammatory response in adolescents with major depressive disorders. Front. Immunol.14, 1197775. 10.3389/fimmu.2023.1197775 (2023). 10.3389/fimmu.2023.1197775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puangsri, P., Jinanarong, V. & Ninla-aesong, P. Impact of antidepressant treatment on complete blood count parameters and inflammatory ratios in adolescents with major depressive disorder. J. Psychiatr. Res.157, 26–35. 10.1016/j.jpsychires.2022.11.017 (2023). 10.1016/j.jpsychires.2022.11.017 [DOI] [PubMed] [Google Scholar]
- 18.Mazza, M. G. et al. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry.84(Pt A), 229–236. 10.1016/j.pnpbp.2018.03.012 (2018). 10.1016/j.pnpbp.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Cheng, Y. et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in depression: An updated systematic review and meta-analysis. Front. Psychiatry.13, 893097. 10.3389/fpsyt.2022.893097 (2022). 10.3389/fpsyt.2022.893097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarejloo, S. et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in poststroke depression: A systematic review and meta-analysis. Dis. Markers.10.1155/2022/5911408 (2022). 10.1155/2022/5911408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neupane, S. P. et al. Immune-related biomarkers and suicidal behaviors: A meta-analysis. Eur. Neuropsychopharmacol.75, 15–30. 10.1016/j.euroneuro.2023.05.009 (2023). 10.1016/j.euroneuro.2023.05.009 [DOI] [PubMed] [Google Scholar]
- 22.Velasco, A. et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in depressed patients with suicidal behavior: A systematic review. Eur. Psychiatry.16, 1–25. 10.1192/j.eurpsy.2023.18 (2023). 10.1192/j.eurpsy.2023.18 [DOI] [PubMed] [Google Scholar]
- 23.Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res.20(23), 6212–6222. 10.1158/1078-0432.CCR-14-0442 (2014). 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 24.Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer.122(14), 2158–2167. 10.1002/cncr.30057 (2016). 10.1002/cncr.30057 [DOI] [PubMed] [Google Scholar]
- 25.Wang, J., Zhou, D., Dai, Z. & Li, X. Association between systemic immune-inflammation index and diabetic depression. Clin. Interv. Aging.16, 97–105. 10.2147/CIA.S285000 (2021). 10.2147/CIA.S285000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu, J. et al. The association between systemic inflammatory markers and post-stroke depression: A Prospective Stroke Cohort. Clin. Interv. Aging.16, 1231–1239. 10.2147/CIA.S314131 (2021). 10.2147/CIA.S314131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, X. et al. Association between depression or anxiety symptoms and immune-inflammatory characteristics in in-patients with tuberculosis: A cross-sectional study. Front. Psychiatry.13, 985823. 10.3389/fpsyt.2022.985823 (2022). 10.3389/fpsyt.2022.985823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X., Huan, J., Lin, L. & Hu, Y. Association of systemic inflammatory biomarkers with depression risk: Results from National Health and Nutrition Examination Survey 2005–2018 analyses. Front. Psychiatry.14, 1097196. 10.3389/fpsyt.2023.1097196 (2023). 10.3389/fpsyt.2023.1097196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kittirattanapaiboon, P. & Khamwongpin, M. The Validity of the Mini International Neuropsychiatric Interview (M.I.N.I.)-Thai Version. J. Ment. Health Thai.13(3), 125–135 (2005). [Google Scholar]
- 30.Coley, R. Y. et al. Defining success in measurement-based care for depression: A comparison of common metrics. Psychiatr. Serv.71(4), 312–318. 10.1176/appi.ps.201900295 (2020). 10.1176/appi.ps.201900295 [DOI] [PubMed] [Google Scholar]
- 31.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA.310(20), 2191–2194. 10.1001/jama.2013.281053 (2013). 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Botía, P. et al. Sex-dependent grades of haematopoietic modulation in patients with major depressive episodes are associated with suicide attempts. Eur. Neuropsychopharmacol.40, 17–30. 10.1016/j.euroneuro.2020.06.006 (2020). 10.1016/j.euroneuro.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Dadouli, K. et al. Neutrophil-to-lymphocyte, monocyte-to-lymphocyte, platelet-to-lymphocyte ratio and systemic immune-inflammatory index in different states of bipolar disorder. Brain Sci.12(8), 1034. 10.3390/brainsci12081034 (2022). 10.3390/brainsci12081034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dionisie, V. et al. Neutrophil-to-lymphocyte ratio, a novel inflammatory marker, as a predictor of bipolar type in depressed patients: A quest for biological markers. J. Clin. Med.10(9), 2021. 10.3390/jcm10091924 (1924). 10.3390/jcm10091924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fest, J. et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep.8(1), 10566. 10.1038/s41598-018-28646-w (2018). 10.1038/s41598-018-28646-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


