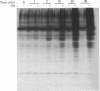
Abstract
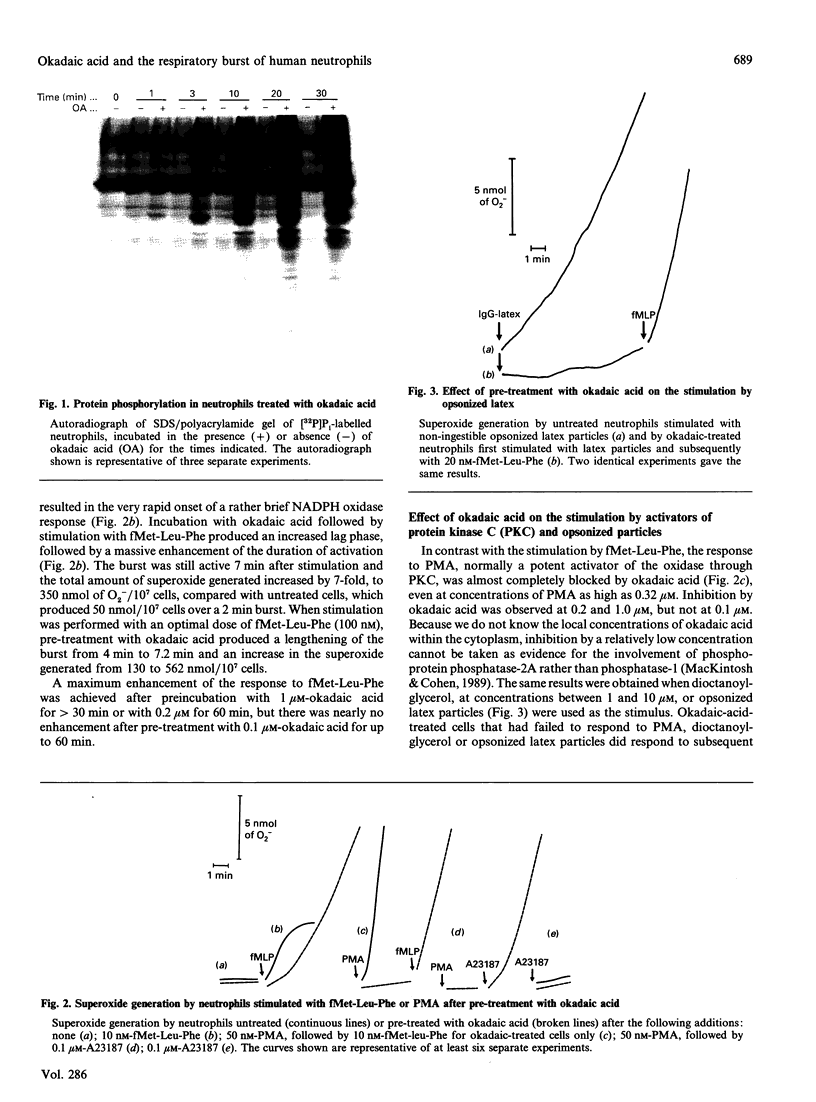
Okadaic acid, a potent inhibitor of protein phosphatases 1 and 2A, profoundly influenced the activity of the NADPH oxidase of human neutrophils. It strongly inhibited stimulation of superoxide generation by phorbol 12-myristate 13-acetate (PMA) and impaired translocation of protein kinase activity and of the two cytosolic components p47-phox and p67-phox to the plasma membrane. The increase in the phosphorylation of the cytochrome b-245 subunits p22-phox and gp91-phox after stimulation was also blocked. Inhibition of activity was associated with a decrease in cytosolic free Ca2+ and was reversed by the Ca2+ ionophore A23187, which also restored protein translocation and phosphorylation of the cytochrome. This effect of A23187 was itself blocked by preincubation with cyclosporin A, suggesting that calcineurin might be involved in the re-activation process. In contrast with PMA, the response to the bacterial peptide fMet-Leu-Phe was greatly prolonged after an initial decrease in the rate of onset of NADPH oxidase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkow R. L., Dodson R. W., Kraft A. S. The effect of a protein kinase C inhibitor, H-7, on human neutrophil oxidative burst and degranulation. J Leukoc Biol. 1987 May;41(5):441–446. doi: 10.1002/jlb.41.5.441. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir C. M., Bu-Ghanim H. N., Rodaway A. R., Bentley D. L., Rowe P., Segal A. W. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2753–2757. doi: 10.1073/pnas.88.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Combadière C., Hakim J., Giroud J. P., Périanin A. Staurosporine, a protein kinase inhibitor, up-regulates the stimulation of human neutrophil respiratory burst by N-formyl peptides and platelet activating factor. Biochem Biophys Res Commun. 1990 Apr 16;168(1):65–70. doi: 10.1016/0006-291x(90)91675-i. [DOI] [PubMed] [Google Scholar]
- Cooke E., Hallett M. B. The role of C-kinase in the physiological activation of the neutrophil oxidase. Evidence from using pharmacological manipulation of C-kinase activity in intact cells. Biochem J. 1985 Dec 1;232(2):323–327. doi: 10.1042/bj2320323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D., Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Garcia R. C., Cross A. R., Segal A. W. The development of cytochrome b-245 in maturing human macrophages. Biochem J. 1986 Nov 1;239(3):647–651. doi: 10.1042/bj2390647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R. C., Segal A. W. Phosphorylation of the subunits of cytochrome b-245 upon triggering of the respiratory burst of human neutrophils and macrophages. Biochem J. 1988 Jun 15;252(3):901–904. doi: 10.1042/bj2520901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C., McPhail L. C., Marfat A., Stimler-Gerard N. P., Bass D. A., McCall C. E. Role of protein kinases in stimulation of human polymorphonuclear leukocyte oxidative metabolism by various agonists. Differential effects of a novel protein kinase inhibitor. J Clin Invest. 1986 Jan;77(1):61–65. doi: 10.1172/JCI112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Receptor-mediated activation of electropermeabilized neutrophils. Evidence for a Ca2+- and protein kinase C-independent signaling pathway. J Biol Chem. 1988 Feb 5;263(4):1779–1783. [PubMed] [Google Scholar]
- Grinstein S., Hill M., Furuya W. Activation of electropermeabilized neutrophils by adenosine 5'-[gamma-thio]triphosphate (ATP[S]). Role of phosphatases in stimulus-response coupling. Biochem J. 1989 Aug 1;261(3):755–759. doi: 10.1042/bj2610755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hardie D. G., Haystead T. A., Sim A. T. Use of okadaic acid to inhibit protein phosphatases in intact cells. Methods Enzymol. 1991;201:469–476. doi: 10.1016/0076-6879(91)01042-z. [DOI] [PubMed] [Google Scholar]
- Harper A. M., Dunne M. J., Segal A. W. Purification of cytochrome b-245 from human neutrophils. Biochem J. 1984 Apr 15;219(2):519–527. doi: 10.1042/bj2190519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Segal A. W. Further evidence for the involvement of a phosphoprotein in the respiratory burst oxidase of human neutrophils. Biochem J. 1986 Nov 1;239(3):723–731. doi: 10.1042/bj2390723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Shrimpton C. F., Segal A. W. Localization of the 47 kDa phosphoprotein involved in the respiratory-burst NADPH oxidase of phagocytic cells. Biochem J. 1989 May 15;260(1):243–248. doi: 10.1042/bj2600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Lomax K. J., Volpp B. D., Nunoi H., Sechler J. M., Nauseef W. M., Clark R. A., Gallin J. I., Malech H. L. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990 May 11;248(4956):727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Henson P. M., Johnston R. B., Jr Respiratory burst enzyme in human neutrophils. Evidence for multiple mechanisms of activation. J Clin Invest. 1981 Mar;67(3):710–716. doi: 10.1172/JCI110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T., Nakamura M., Hiura M., Yoshida K., Okamura N., Ishibashi S. Translocation of the 46 kDa protein(s) in response to activation of NADPH oxidase in guinea pig polymorphonuclear leukocytes. J Biochem. 1990 Aug;108(2):169–174. doi: 10.1093/oxfordjournals.jbchem.a123177. [DOI] [PubMed] [Google Scholar]
- Okamura N., Curnutte J. T., Roberts R. L., Babior B. M. Relationship of protein phosphorylation to the activation of the respiratory burst in human neutrophils. Defects in the phosphorylation of a group of closely related 48-kDa proteins in two forms of chronic granulomatous disease. J Biol Chem. 1988 May 15;263(14):6777–6782. [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Okamura N., Ohashi S., Nagahisa N., Ishibashi S. Changes in protein phosphorylation in guinea pig polymorphonuclear leukocytes by treatment with membrane-perturbing agents which stimulate superoxide anion production. Arch Biochem Biophys. 1984 Jan;228(1):270–277. doi: 10.1016/0003-9861(84)90067-5. [DOI] [PubMed] [Google Scholar]
- Phillips W. A., Fujiki T., Rossi M. W., Korchak H. M., Johnston R. B., Jr Influence of calcium on the subcellular distribution of protein kinase C in human neutrophils. Extraction conditions determine partitioning of histone-phosphorylating activity and immunoreactivity between cytosol and particulate fractions. J Biol Chem. 1989 May 15;264(14):8361–8365. [PubMed] [Google Scholar]
- Rodaway A. R., Teahan C. G., Casimir C. M., Segal A. W., Bentley D. L. Characterization of the 47-kilodalton autosomal chronic granulomatous disease protein: tissue-specific expression and transcriptional control by retinoic acid. Mol Cell Biol. 1990 Oct;10(10):5388–5396. doi: 10.1128/mcb.10.10.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Regional intracellular calcium changes in neutrophils related to morphology and F-actin formation. Trans Assoc Am Physicians. 1986;99:197–205. [PubMed] [Google Scholar]
- Schneider C., Zanetti M., Romeo D. Surface-reactive stimuli selectively increase protein phosphorylation in human neutrophils. FEBS Lett. 1981 May 5;127(1):4–8. doi: 10.1016/0014-5793(81)80327-4. [DOI] [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Dorling J., Coade S. Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J Cell Biol. 1980 Apr;85(1):42–59. doi: 10.1083/jcb.85.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Cohen H. J. Disorders of respiratory burst termination. Hematol Oncol Clin North Am. 1988 Jun;2(2):289–299. [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]
- Wymann M. P., von Tscharner V., Deranleau D. A., Baggiolini M. The onset of the respiratory burst in human neutrophils. Real-time studies of H2O2 formation reveal a rapid agonist-induced transduction process. J Biol Chem. 1987 Sep 5;262(25):12048–12053. [PubMed] [Google Scholar]