Abstract
Background
Consistent evidence shows that magnesium (Mg) intake is associated with lower blood pressure (BP), and that lower BP is associated with improved cerebral health. However, recent findings indicate that the positive effect of dietary Mg intake on cerebral health is not mediated by a decrease in BP. As Mg’s anti-inflammatory action is a plausible alternative mechanism, the objective of this study was to investigate the associations between Mg intake and inflammation to determine whether it mediates any neuroprotective effect.
Methods
Participants from the UK Biobank (n = 5775, aged 40–73 years, 54.7% female) were assessed for dietary magnesium using an online food questionnaire, brain and white matter lesion (WML) volumes were segmented with FreeSurfer software, and inflammation markers including high-sensitivity C-reactive protein (hs-CRP), leukocyte, erythrocyte count, and Glycoprotein acetylation (GlycA) were measured using specific laboratory techniques such as immunoturbidimetry, automated cell counting, and nuclear magnetic resonance. Hierarchical linear regression models were performed to investigate the association between dietary Mg, and inflammatory markers and between dietary Mg, brain and WMLs volumes. Mediation analysis was performed to test a possible mediation role of inflammation on the association between dietary Mg and brain and WMLs volumes.
Results
Higher dietary Mg intake was associated with lower inflammation: hs-CRP level (− 0.0497%; 95% confidence interval [CI] − 0.0497%, − 0.0199%) leukocytes count (− 0.0015%; 95%CI − 0.00151%, − 0.0011%), and GlycA (− 0.0519%; 95%CI − 0.1298%, − 0.0129%). Moreover, higher dietary Mg intake was associated with larger grey matter volume (0.010%; 95%CI 0.004%, 0.017%), white matter volume (0.012%; 95%CI 0.003, 0.022) and right hippocampal volume (0.002%; 95%CI 0.0007, –0.0025%). Lower hs-CRP levels mediated the positive association between higher dietary Mg intake and larger grey matter volume.
Conclusions
The anti-inflammatory effects of dietary Mg intake in the general population, appears to mediate its neuroprotective effect.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-024-03383-1.
Keywords: Magnesium, Inflammation, Brain volumes, White matter lesions, UK biobank
Introduction
Ageing of the population is projected to lead to an increase in the proportion of individuals aged 65 years and older, with estimates suggesting it will increase from 10% today to 16% of the world’s population by 2050 [1]. This trend has important healthcare and economic implications for our society [2]. In particular, older age is associated with an increase prevalence of dementia and other neurodegenerative disease. Therefore, there is a pressing need for the development of effective strategies to promote healthy brain ageing and mitigate the risk of neurodegenerative diseases and dementia, both for the benefit of individuals and for the sustainability of healthcare systems.
Nutrition is an important modifiable risk factor that influences cerebral health and that is highly amenable to interventions that are scalable and cost-effective [3]. Dietary magnesium (Mg), in particular, is associated with better cognitive function [4] and may reduce the risk and delay the onset of dementia [5]. However, the underlying biological mechanisms responsible for the neuroprotective effect of Mg are not well understood. This is a crucial question to address, as optimising Mg intake through diet may contribute to reducing the risk of dementia in the general population.
Mg plays an important role in neuronal health. It is essential for nerve transmission and neuromuscular conduction [6]. Mg deficiency has been linked to the development of several neurological pathologies related to ageing [7]. A recent study that investigated the link between Mg and brain volumes in a large population (n = 6000; mean age 40–70 years) found that higher dietary Mg intake was associated with larger brain volumes, particularly in the hippocampus [8]. Another study (n = 1406; mean age = 62.5 years) with an eight-year follow-up found that higher dietary Mg intake (≥ 434 mg; recommended minimum intake 350-400 mg) was also linked to a lower risk of progressing from normal cognition to mild cognitive impairment (MCI; hazard ratios [HR] = 0.07, 95% confidence interval [CI] 0.01, 0.56) [9]. In a recent study with 1565 participants (mean age 71.7 years) from an urban Shanghai community, it was found that high Mg intake (> 267.5 mg/day) at baseline was linked to an elevated risk of dementia within a 5-year follow-up period [10]. It is important to note that the study had a relatively short follow-up duration, and despite dementia cases were excluded, participants with MCI were not excluded in this study [10]. Moreover, a recent systematic review demonstrated that individuals with Alzheimer’s disease (AD) have significantly lower plasma Mg levels (standardized mean difference [SMD] = − 0.89; 95%CI − 1.36, − 0.43) than healthy controls [11]. Contrarily, a study involving 102,648 individuals showed no association between plasma Mg concentrations and AD, suggesting a reverse causation of explanation [12]. It is also possible that these contradictory results are due to the fact that Mg is primarily stored intracellular, with only around 1% found in the bloodstream. As a result, serum Mg levels may not accurately represent total Mg levels in the body, underscoring the importance of dietary intake in assessing Mg levels [13]. In combination, these findings suggest that dietary Mg intake contributes to the modulation of neurodegenerative processes, and further research is needed to better understand this relationship.
However, the precise mechanisms underlying Mg’s neuroprotective effects remain unclear. A plausible mechanism is the known anti-hypertensive effect of Mg, as numerous studies have indicated that Mg supplementation lowers blood pressure (BP) and helps in the management of hypertension [14, 15], because Mg acts as a calcium antagonist on the smooth muscles, leading to vasorelaxation [16]. Since elevated BP is closely linked to neurodegeneration [17–20], cognitive decline [21], and dementia [22], we recently conducted research in a large population to investigate the link between dietary Mg intake and BP and examined whether BP mediates any neuroprotective effects. Contrary to expectations, our findings did not support a blood-lowering effect as the main mechanism mediating the relationship between Mg and cerebral health. This suggests that other mechanisms may be involved [8].
A potential alternative mechanism is the anti-inflammatory effect of Mg. Animal studies have shown that low Mg intake is associated with microglia activation and the production of pro-inflammatory cytokines, including interleukin 1 beta (IL-1), IL-6, and tumour necrosis factor alpha (TNF-α) [23]. Mg supplementation, in contrast, has an anti-inflammatory impact by reducing the production of pro-inflammatory cytokines, including IL-1, IL-6, and TNF-α [24]. Moreover, a recent systematic review including 11 randomized controlled trials (RCTs) found that Mg supplementation significantly decreased C-reactive protein (CRP) serum levels (SMD = − 0.356; 95%CI − 0.659,−0.054) [25]. CRP is produced by the liver in response to the acute inflammatory phase following infections or trauma, and its production is regulated by IL-1 and IL-6 [26]. Moreover, it is worth noting that there is evidence suggesting a link between higher inflammation markers including IL-6 and CRP levels and an increased risk of neurodegeneration [27], cognitive impairment [28], and dementia [29, 30]. However, conflicting evidence suggests no discernible differences in CRP levels between AD patients and controls [31].
Taken together, the current evidence suggests that dietary Mg intake has a positive impact on reducing inflammation and protecting cerebral health. However, it is unknown whether its anti-inflammatory action is responsible for its neuroprotective effect. Consequently, the aim of this study was to investigate the association between dietary Mg and high-sensitivity CRP (hs-CRP), an indicator of inflammation. Due to the unavailability of Interleukins such as, IL-1 and IL-6 in the UK Biobank database, the study also aimed to explore the associations between Mg intake and other available inflammatory markers, including leukocyte and erythrocyte count, and Glycoprotein acetylation (GlycA) levels. The leukocyte count, in particular, is important as it is a key component of the innate immune system’s defence mechanism and is responsible for expressing and secreting Interleukins [32, 33] Recent studies also suggest that erythrocytes are vulnerable to oxidative stress and upregulation of cytokines which are associated with inflammatory diseases [34]. Furthermore, the GlycA biomarker has been used to assess pro-inflammatory cytokines and predicts the development of cardiovascular disease [35–38]. Finally, the second aim of this study was to examine whether any effect of Mg on inflammation mediates the associations between Mg and brain and WMLs volumes, as measures of cerebral health.
Methods
Study design and participants
Participants recruited into the UK biobank study, which has previously been described [39], were considered for inclusion in this study. Briefly, the UK Biobank is a prospective cohort study of 502,655 participants aged 37 to 73 years at baseline who were evaluated at 22 assessment centres across the United Kingdom between 2006 and 2023. Participants who had both baseline diastolic BP (DBP) and systolic BP (SBP) measurements (n = 456,990) at baseline in 2006–2009, completed a structural magnetic resonance imaging (MRI) scan at the second assessment in 2014 (n = 36,260), for whom dietary Mg intake and inflammatory markers at baseline in 2006–2009 (n = 30,484), and who did not have any neurological disordered (n = 3,275) were excluded. This resulted in a final sample of participants (n = 5776 leukocytes and erythrocytes, n = 5641 hs-CRP, and n = 1457 GlycA; Supplementary material; Fig. S1).
The North-West Multi-Centre Research Ethics Committee approved the UK Biobank Study (#06/MRE08/65). All participants provided informed consent. This study follows to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [40].
Measurement of mg intake Dietary
Dietary intake was measured using the Oxford WebQ, a computerised 24-hour recall questionnaire administered online [41, 42]. The WebQ was designed to be completed multiple times to minimize measurement error that might occur with a single 24-hour recall assessment. It includes 200 items in various quantity sizes. The overall dietary Mg was computed using McCance and Widdowson’s “The Composition of Food and its Supplements” [42]. Specific details regarding the calculation of Mg intake are described elsewhere [41, 42].The WebQ has been validated against a 24-hour recall assessment performed by an interviewer, with only minor discrepancies in nutrient intake reported using both procedures [42].
MRI acquisition
MRI images were collected at one of three imaging locations using the same scanner (3T Siemens Skyra, running VD13A SP4 using a 32-channel head coil). Detailed imaging protocol are available online (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1977) [39]. Briefly, T1-weighted brain MRI scans were acquired in sagittal orientation using a 3D magnetization-prepared rapid acquisition gradient echo sequence (resolution = 1 × 1 × 1 mm; matrix size = 208 × 256 × 256; T1/TR = 880/2000 ms).
Segmentation and image analysis
MRI data was segmented and analysed using FreeSurfer (version6.0.5) [43]. The FreeSurfer pipeline has been detailed elsewhere [44], but in brief, it includes motion correction, transformation to Talairach image space, inhomogeneity normalisation, non-brain tissue removal using hybrid watershed, volumetric segmentation [45, 46], and cortical surface reconstruction and parcellation [47]. The region of interest (ROI) was selected based on previous investigation showing a relationship between dietary Mg and brain ageing [8]. They included total grey matter volume (GM), total white matter volume (WM), left and right hippocampus volume (LHC, RHC) and white matter lesions (WMLs).
Inflammatory markers
Inflammatory markers included: hs-CRP level (mg/L) serum was assessed using immunoturbidimetric high-sensitivity analysis on a Beckman Coulter AU5800 [31–33]. leukocytes count (109 cells/Litter) and erythrocyte count (1012 cells/Litter) were measured as an absolute number per unit volume on fresh samples using an automated, clinically validated Coulter LH 750 (Beckman Coulter). Calibration and quality control were carried out in accordance with the manufacturer’s instructions. GlycA (mmol/Litter) was measured using an NMR metabolomics platform (Nightingale Health, Helsinki, Finland). More information can be found on the UK Biobank website (http://www.ukbiobank.ac.uk).
Covariates
The covariates included age, sex, body mass index (BMI), serum high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), education level, diabetes mellitus diagnosed by a doctor, self-reported smoking status (i.e., never, previous, or current), diagnosed with hypertension (participants with SBP/DBP of ≥ 140/90 or who reported taking BP medication), alcohol intake (drinks/week), and physical activity (metabolic equivalent [METs]/week) [48].
Statistical analyses
Statistical analyses were computed using the R statistical package (Version 1.2.5019) under Rstudio (Version 1.2.5019) [49]. Descriptive analyses were conducted using Chi-square tests for categorical data and t-tests to compare groups on continuous variables. The skewed distribution of the WMLs was transformed using log transformations. All dependent and independent variables remained unstandardized. Mg intake was centered on 350 mg (recommended daily intake ~ 310–420) to facilitate interpretation [50].
Hierarchical linear regression models were performed to investigate the association between (1) baseline Mg intake and inflammatory markers (hs-CRP, leukocytes, erythrocytes, and GlycA); and (2) baseline Mg intake and brain volumes (GM, WM, LHC, RHC, WMLs). The main three models were fit as follows: Model 1 was controlled for age, sex and education. Model 2 additionally controlled for the cardiovascular risk factors including diabetes mellitus, hypertension, BP medication, HDL, TC, alcohol intake, physical activity, smoking status, BMI and the time difference between baseline and follow-up, owing to significant variability in the time span with average 8 years. Model 3 additionally tested the two-way interactions between baseline Mg intake and the cardiovascular risk factors. Sensitivity analyses were conducted to evaluate the influence of calcium (Ca), considering the biological link between Mg and Ca [51]. Additionally, adjustments for energy intake were performed to address confounding variables related to high energy associated with high food intake. Since a prior study has highlighted sex differences in Mg intake analysis [52], a sensitivity analysis was performed to investigate sex differences in the association between Mg and brain volumes and Mg and inflammation by stratifying the sample into men and women. All brain analyses were controlled for the intra-cranial volume (ICV), to correct for head size differences. The unstandardized beta coefficient, standard error, and p-values for outcomes measures are reported. The significance threshold was set at p < 0.05 and corrected for multiple comparisons (Bonferroni).
Possible mediation of dietary Mg intake on the brain volume and WMLs through inflammation was investigated using Baron and Kenny’s method [53, 54]. The bootstrapping of indirect effect was set to n = 1000. The three main steps were tested. Step 1 tested the effect of dietary Mg intake and other covariates on the brain volumes and WMLs. Step 2 tested the effects of dietary Mg intake and other covariates on the inflammatory markers meeting criteria. Step 3 tested the total effects of inflammatory markers, dietary Mg, and other covariates on brain volumes and WMLs. Step 4 tested the causal mediation analysis of the indirect effect of inflammation on brain volumes and WMLs through dietary Mg.
Results
Participants characteristics
Participant characteristics are presented in Table 1. While the average Mg intake (mean = 361.9, SD = 125.11) was above the recommended 350 mg mg/day, this was the case for men (mean = 383.83, SD = 133.03), but not women (mean = 342.16, SD = 113.91) even though it was within the normal range for both sexes. Moreover, men were slightly older (~ 1year) and had slightly higher BMI (1.2 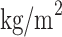 ), SBP (6.5mmHg), DBP (3.84mmHg) and had a higher prevalence of BP medication (1.8%) and diabetes mellitus (1.6%), than women.
), SBP (6.5mmHg), DBP (3.84mmHg) and had a higher prevalence of BP medication (1.8%) and diabetes mellitus (1.6%), than women.
Table 1.
Participants demographic characteristic
| Measures | Whole Sample | Males | Females | (P value) |
|---|---|---|---|---|
| (n = 5766) | (n = 2745) | (n = 3021) | ||
| Age, year (SD) | 55.37 (7.45) | 56.05 (7.51) | 54.75 (7.35) | (< 0.001) |
| Magnesium intake, mg | 361.99 (125.11) | 383.83 (133.03) | 342.16 (113.91) | (< 0.001) |
| GM volume, mm3 (SD) | 668753.30 (59323.58) | 702489.76 (53567.20) | 638115.21 (46234.46) | (< 0.001) |
| WM volume, mm3 (SD) | 479969.55 (57361.26) | 510020.62 (54142.82) | 452678.38 (45297.32) | (< 0.001) |
| Left HC volume, mm3 (SD) | 3696.18 (395.93) | 3829.82 (401.13) | 3574.81 (349.42) | (< 0.001) |
| Right HC volume, mm3 (SD) | 3811.71 (402.65) | 3951.46 (409.86) | 3684.79 (350.71) | (< 0.001) |
| WMLs volume, mm3(SD) | 7.38 (0.66) | 7.50 (0.65) | 7.26 (0.64) | (< 0.001) |
| ICV volume, mm3 (SD) | 1554471.18 (150902.35) | 1643379.11 (134101.86) | 1473728.56 (115865.44) | (< 0.001) |
| Leukocyte count, 109 cells/L (SD) | 6.59 (1.78) | 6.60 (1.60) | 6.58 (1.93) | (0.645) |
| Erythrocyte count, 1012 cells/Litre (SD) | 4.55 (0.40) | 4.78 (0.35) | 4.34 (0.32) | (< 0.001) |
| hs-CRP Level, mg/L | 2.01 (3.32) | 1.85 (2.95) | 2.15 (3.61) | (< 0.001) |
| Glycoprotein Acetyls, mmol/L | 0.77 (0.11) | 0.76 (0.11) | 0.77 (0.11) | (0.634) |
| SBP, mmHg (SD) | 134.77 (17.83) | 138.20 (16.60) | 131.66 (18.32) | (< 0.001) |
| DBP, mmHg (SD) | 81.15 (9.95) | 83.17 (9.91) | 79.31 (9.63) | (< 0.001) |
| BMI, kg/m2 (SD) | 26.51 (4.14) | 27.08 (3.77) | 26.00 (4.39) | (< 0.001) |
| Cholesterol, mmol/L, (SD) | 5.74 (1.07) | 5.62 (1.08) | 5.86 (1.06) | (< 0.001) |
| HDL mmol/L, (SD) | 1.49 (0.36) | 1.32 (0.29) | 1.64 (0.36) | (< 0.001) |
| Hypertension, n (%) | 2352 (40.65%) | 1312 (47.81%) | 1030 (34.08%) | (< 0.001) |
| BP medication, n (%) | 435 (7.53%) | 231 (8.40%) | 204 (6.74%) | (0.019) |
| Diabetes, n (%) | 163 (2.82%) | 97 (3.53%) | 66 (2.18%) | (0.003) |
| Higher Education, n (%) | 2845 (49.26%) | 1427 (51.91%) | 1418 (46.85%) | (< 0.001) |
Significance: p < 0.05. Abbreviation; SBP: systolic blood pressure; DBP: diastolic blood pressure; GM: grey matter; WM: white matter; HC: hippocampus; WMLs: white matter lesions; ICV: intracranial volume; BMI: body mass index; HDL: high-density lipoprotein; hs-CRP: high-sensitivity c-reactive protein. Note. The statistical test reported based on the group comparison of men relative to women
Dietary mg intake and inflammation
Associations between Mg, and inflammation levels are presented in Table 2. Higher dietary Mg intake was significantly associated with lower inflammation levels, with some variation across inflammatory markers. Every additional 1 mg in Mg intake above 350 mg/day was associated with a – 0.049% lower hs-CRP, − 0.0015% lower leukocytes, and − 0.0519% lower GlycA. However, this association did not reach the significance for erythrocyte levels (Table S1-S5 and Fig. S2-S6).
Table 2.
Association between dietary Mg intake and inflammatory markers
| Leukocytes (109 cells/L) | ESR (1012 cells/L) | hs-CRP Level (mg/L) | GlycA (mmol/L) | |
|---|---|---|---|---|
| Beta (CI) | Beta (CI) | Beta (CI) | Beta (CI) | |
| Mg |
-0.0001* (-0.0001, -0.00007) p = 0.01925 |
-0.00001 (-0.0001, 0.0001) p = 0.21675 |
-0.001*** (-0.001, -0.0004) p = 0.00000 |
-0.0004*** (-0.001, -0.0001) p = 0.00075 |
| BP medication |
0.010 (-0.014, 0.035) p = 0.403 |
0.006 (-0.028, 0.039) p = 0.737 |
0.457*** (0.157, 0.757) p = 0.003 |
0.012 (-0.009, 0.034) p = 0.264 |
| Hypertension |
0.013* (-0.0002, 0.027) p = 0.054 |
0.073*** (0.054, 0.092) p = 0.00 |
-0.087 (-0.244, 0.071) p = 0.281 |
0.006 (-0.005, 0.017) p = 0.306 |
| Smoking status |
0.044*** (0.034, 0.054) p = 0.00 |
-0.043*** (-0.057, -0.029) p = 0.00 |
0.079*** (0.038, 0.119) p = 0.0002 |
0.010** (0.001, 0.019) p = 0.356 |
| Cholesterol |
0.001 (-0.005, 0.007) p = 0.707 |
0.041*** (0.033, 0.050) p = 0.000 |
0.070*** (0.046, 0.094) p = 0.00 |
0.032*** (0.027, 0.037) p = 0.00 |
| Mg x Smoking status |
0.0001** (0.00001, 0.0002) p = 0.033 |
— | — | — |
| Mg x BP medication | — | — |
-0.001*** (-0.002, -0.0003) p = 0.033 |
— |
| Mg x Hypertension | — | — |
0.0004** (0.00004, 0.001) p = 0.032 |
— |
| Mg x Cholesterol | — | — | — |
0.0001*** (0.00002, 0.0001) p = 0.005 |
| Constant |
-4.292*** (-4.368, -4.217) p = 0.000 |
4.536*** (4.432, 4.640) p = 0.000 |
-2.826*** (-3.139, -2.513) p = 0.000 |
0.503*** (0.441, 0.566) p = 0.000 |
| Observations | 5,766 | 5,766 | 5,468 | 1,417 |
Significance. * p < 0.05; ** p < 0.01; *** p < 0.001. Abbreviations: CI-confidence interval - standard error; Mg - magnesium; hs-CRP - high-sensitivity C-reactive protein; GlycA - Glycoprotein acetylation. Note: The hierarchical analysis presents results on the association between dietary Mg intake and inflammatory markers, including leukocytes, erythrocytes, hs-CRP, and GlycA, using data from the UK Biobank study. In Model 3, adjustments were made for covariates including as age, sex, education, BP medication, high-density lipoprotein (HDL), cholesterol, diabetes mellitus, smoking status, higher education, physical activity, alcohol intake and body mass index (BMI). We also tested two-way interactions between Mg and smoking status, Mg and hypertension, Mg and BP medication, and Mg and cholesterol levels. The data represents unstandardized beta coefficients with 95% Confidence Interval (CI). Beta values correspond to a 1 mg unit increment in Mg intake variables
Effects of cardiovascular risk factors
Two-way interactions between Mg intake and cardiovascular risk factors as predictors of the inflammatory markers are presented in Table 2 and Fig. S7. Cardiovascular risk factors were found to significantly modulate the association between Mg intake and inflammation with some notable differences between markers.
The negative association between Mg intake and hs-CRP was weaker (0.029% lower leukocyte for every 1 mg Mg above 350 mg/day) in hypertensive. However, the negative association between Mg intake and hs-CRP was stronger (–0.059% lower hs-CRP for every 1 mg Mg above 350 mg/day) in those who were treated with BP medication.
Moreover, the negative association between Mg intake and leukocytes was weaker (0.001% lower leukocyte for every 1 mg Mg above 350 mg/day) in those who smoked. Also, association between Mg and GlycA was weaker (0.013% lower GlycA for every 1 mg Mg above 350 mg/day) with higher cholesterol levels.
Sensitivity analysis
Sensitivity analyses were performed to account for Ca and energy intake when assessing the relationship between dietary Mg intake and inflammatory markers. The significant association between dietary Mg intake and inflammatory markers remained unchanged after adjusting for Ca, suggesting that this association is independent of Ca (Table S12). Furthermore, the association between dietary Mg intake and inflammatory markers persisted even after controlling for energy intake, except for the association between dietary Mg and ESR levels, which became stronger (-0.002%) (Table S12).
Additional analyses stratified by sex were conducted to better characterise sex-specific associations between Mg intake and inflammation levels, (Table S13). Higher dietary Mg intake was significantly associated with lower leucocyte levels in women, with a weaker association in men. Furthermore, higher dietary Mg intake was significantly associated with lower GlycA in men, and this association was weaker in women.
Dietary mg intake and brain volumes and WMLs
Associations between baseline Mg, and brain and WMLs volumes are presented in Table 3. Higher dietary Mg intake was significantly associated with larger brain volumes. Every 1 mg higher in Mg intake above 350 mg/day was associated with a 0.0105% larger GM, 0.0122% larger WM, and 0.002% larger RHC. The association did not reach significance for LHC, and WMLs (Table S6-S11 and Fig. S8-S13).
Table 3.
Association between dietary Mg intake and brain volumes and WMLs.
| Gray matter volume (mm3) | White matter volume (mm3) | Left hippocampal volume (mm3) | Right hippocampal volume (mm3) | White matter lesions volume (mm3) | |
|---|---|---|---|---|---|
| Beta (CI) | Beta (CI) | Beta (CI) | Beta (CI) | Beta (CI) | |
| Mg |
70.321*** (26.028, 114.615) p = 0.0004 |
58.751** (13.766, 103.735) p = 0.002 |
0.021 (-0.043, 0.086) p = 0.103 |
0.063* (0.029,0.095) p = 0.012 |
0.00003 (-0.0001, 0.0001) p = 0.123 |
| Age |
-1,574.842*** (-1,679.220, -1,470.465) p = 0.000 |
-1,100.555*** (-1,206.560, -994.549) p = 0.000 |
-16.550*** (-17.667, -15.434) p = 0.000 |
-15.325*** (-16.455, -14.194) p = 0.000 |
0.041*** (0.039, 0.043) p = 0.000 |
| Mg x Age |
-1.151*** (-1.946, -0.356) p = 0.005 |
-1.024** (-1.832, -0.217) p = 0.013 |
|||
| Constant |
300,081.900*** (285,775.300, 314,388.400) p = 0.000 |
85,425.380*** (70,895.690, 99,955.070) p = 0.000 |
2,699.007*** (2,545.463, 2,852.551) p = 0.000 |
2,583.895*** (2,428.393, 2,739.397) p = 0.000 |
2.601*** (2.333, 2.869) p = 0.000 |
| Observations | 5,766 | 5,766 | 5,766 | 5,766 | 5,766 |
Significance. * p < 0.05; ** p < 0.01; *** p < 0.001. Abbreviations: CI - confidence interval; Mg - magnesium; GM - grey matter; WM - white matter; LHC - left hippocampal; RHC - right hippocampal; WMLs - white matter lesions. Note: This section presents the hierarchical analysis results of the association between Mg intake and brain volumes, including GM, WM, LHC, RHC, and WMLs. In Model 3, adjustments were made for covariates including age, sex, education, ICV (intracranial volume), BP medication, high-density lipoprotein (HDL), cholesterol, diabetes mellitus, smoking status, higher education, physical activity, alcohol intake, body mass index (BMI), and time differences between measurements. Additionally, we tested the two-way interaction between Mg and age. The data represents unstandardized beta coefficients with 95% Confidence Interval (CI). Beta values correspond to a 1 mg unit increment in Mg intake variables
Effects of age
Two-way interactions between Mg and age as predictors of the brain volumes are presented in Table 3 and Fig. S14. Age was found to significantly modulate the association between Mg intake and brain volumes. Therefore, a further analysis stratified by age group (age ≤ 45; age ≥ 55; age ≥ 65) were conducted. The positive association between Mg intake and GM and WM volumes was weaker with advancing age. Compared to individuals ≤ 45 years, every additional 1 mg in Mg intake above 350 mg/day was associated with − 0.0001% lower GM volume and − 0.0002% lower WM volume in individuals at age 55 years.
Sensitivity analysis
Sensitivity analysis was performed to control for Ca and energy intake in assessing the relationship between dietary Mg intake and brain volumes. The significant association between dietary Mg intake and brain volumes remained unchanged after accounting for Ca, indicating that this relationship is independent of Ca levels (Table S12). Furthermore, the association between dietary Mg intake and brain volumes persisted after controlling for energy intake, suggesting that energy intake does not impact this association (Table S12).
Additional analyses stratified by sex were conducted to better characterise sex-specific associations between Mg intake and brain regions (Table S14). Higher dietary Mg intake was significantly associated with larger GM volume, with a stronger association observed in men compared to women.
Mediation effects of inflammation
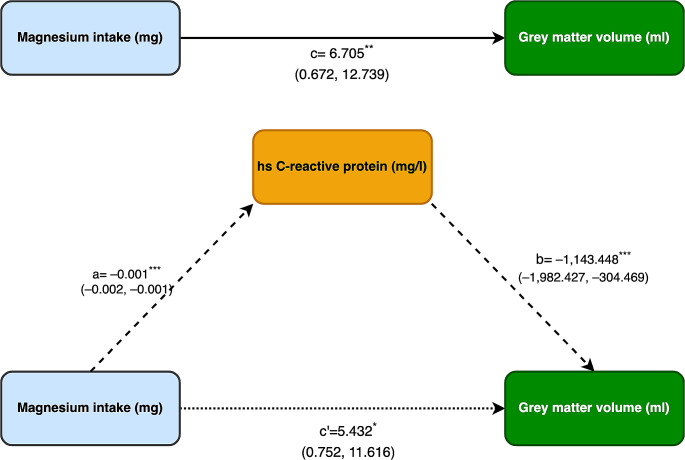
The possible mediating role of inflammatory markers on the relationship between dietary Mg intake and brain volumes and WMLs was investigated. Significant mediation was only detected for GM for which the effect of dietary Mg intake was partially-mediated by hs-CRP levels (direct effect, B = 5.432, indirect effect; B = 1.1434) (Fig. 1).
Fig. 1.
Mediation analysis testing whether the dietary magnesium (Mg) intake on grey matter volume (GM) is mediated through high-sensitivity c-reactive protein (hs-CRP).
a = the effect of dietary Mg intake on hs-CRP, b = the effect of hs-CRP on the GM, c = the effect of dietary Mg intake on GM, c’= is the direct effect of dietary Mg intake on GM. The indirect effect was calculated with bootstrapping method (stimulation number = 1000)
Discussion
This study produced several important findings. Firstly, dietary Mg intake was found to be associated with reduced inflammation. Secondly, the neuroprotective effect of dietary Mg intake was confirmed. Finally, Mg’s neuroprotective effect was found to be in part mediated by inflammation.
A key finding was that low dietary Mg intake is related to higher levels of inflammation. The findings demonstrate that individuals who consume ~ 15% (50 mg) below the daily recommended intake of Mg (350 mg) have on average a 2.48% higher hs-CRP level, a 0.075% higher leukocyte count, and a 2.59% higher GlycA level. The increase in hs-CRP levels observed in individuals with lower Mg intake is likely due to the elevated expression and secretion of interleukins. This is consistent with findings from a meta-analysis of randomized control trials that found a significant inverse relationship between Mg supplementation and lower CRP and IL-6 levels [25]. Additionally, this interpretation is also supported by our findings that low Mg intake was associated with higher leukocyte counts. Indeed, leucocytes proliferation is up-regulated in the early stages of the immune response and in turn leads to an increased production of interleukins and particularly IL-1 and IL-6, which are known to be implicated in neurodegenerative processes [32, 55]. Moreover, similar inverse association between serum Mg levels and leukocyte count was demonstrated in COVID-19 patients [56]. Therefore, these results are consistent with a plausible mechanistic cascade linking Mg intake to a pro-inflammatory immune response reflected by higher hs-CRP levels.
Furthermore, our study is to our knowledge the first to show that dietary Mg intake is associated with lower GlycA levels. This is important because GlycA is a recently discovered biomarker that has been shown to be associated with atherosclerosis [57–59], cardiovascular events [35–38, 60], and heart failure [26]. Higher GlycA levels have been linked to higher levels of hs-CRP and pro-inflammatory cytokines including IL-6, as well as TNF-α, and fibrinogen [55]. The present findings suggest that the relationship between Mg intake and BP levels may be mediated by the inflammatory biomarker GlycA. This may also partially explain the neuroprotective effect of GlycA in vascular diseases. Further research is needed to investigate the specific mechanisms by which Mg intake affects BP levels possibly via GlycA mediation. Together, the present findings and evidence from the broader literature present a consistent picture of Mg being associated with a lower inflammatory response at different stages of the immunological process.
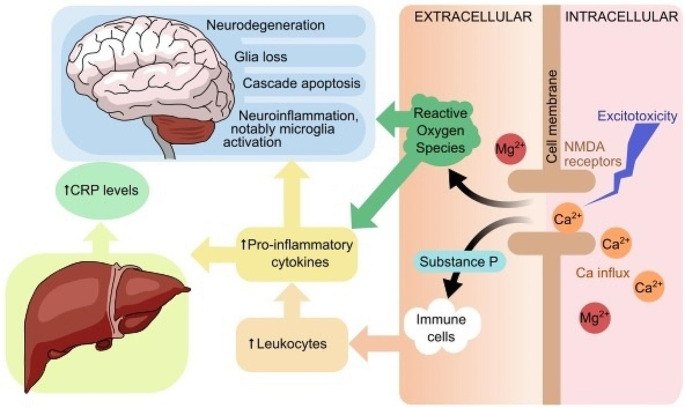
The novel and the most significant finding of this study is that the neuroprotective effect associated with dietary Mg intake is mediated by lower inflammation. Specifically, lower hs-CRP levels significantly mediated the positive association between higher dietary Mg intake and larger GM volume. Our findings are in line with evidence from animal studies, which have demonstrated a relationship between lower Mg intake and higher neuroinflammation. Research has suggested that a reduction in Mg intake can trigger microglia activation, which may result in an increase in proinflammatory cytokines including IL-6, TNF-α, and nitric oxide [23]. Treatment with Mg has been shown to decrease microglia activation and inhibit TNF-α production in rats [24]. Although the exact mechanisms linking dietary Mg, inflammation, and cerebral health are not yet fully understood, some evidence suggests that a decrease in extracellular Mg ion concentrations could activate macrophages and increase the influx of calcium ions into various types of cells, such as adipocytes, neurons, and peritoneal cells. This, in turn, could lead to hyperexcitability of cells due to overstimulation of N-methyl-D-aspartate (NMDA) receptors [61]. Additionally, the reduction in Mg ions may increase the release of neuromediators such as substance P and neuroinflammatory tachykinins, as well as stimulate pro-inflammatory cytokines such as IL-6, TNF-α, and nitric oxide. The latter can act as signalling molecules and increase the release of CRP from the liver as part of the acute phase response, and may prolong the inflammatory response [62]. On the other hand, higher TNF-α levels further upregulate the production of pro-inflammatory cytokines (e.g. IL-1, IL-6), which has been linked to activation of the apoptosis cascade, glial cell loss, and GM atrophy both in rats experiments and human observational studies [63–65] (Fig. 2). These findings suggest that maintaining a sufficient, and possibly somewhat raised, dietary Mg intake may contribute to decreasing neurodegeneration and therefore protect cerebral health through its anti-inflammatory action. While the effects observed in this study were small, the fact that mediation was detected in an epidemiological context with many uncontrolled factors suggests that the findings are noteworthy and may have been under-estimated. However, it is important to state that further research is needed to confirm these results and to better understand the underlying mechanisms.
Fig. 2.
The figure illustrates the cellular mechanisms that connect higher levels of magnesium to a reduction in inflammatory response and improved cerebral health
Importantly, sensitivity analyses revealed that the association between Mg and GM volume are stronger in men. This is consistent with findings from previous studies which have shown sex related differences in the association between Mg intake and brain volumes [52]. This sex difference may be attributed to hormonal effects, including variations in estrogen and testosterone levels, aligning with Mg’s known role in modulating hormonal pathways and regulating levels in the body [66–68]. Another possible cause may relate to the fact that exposure to lifestyle factors, which is known to differ between men and women, such as physical activity which can differentially impact cardiovascular health [69], and contribute to the observed sex-specific effects. However, it should also be acknowledged that lacking sufficient statistical power for stratified analyses may increase the risk of identifying significant associations within a specific group, and thus may lead to contradictory results between sex interactions in combined models and stratified sex effects. This may lead to confusing and potentially misleading conclusions [9–11]. Therefore, future studies should aim to clarify sex differences in the association between Mg and neurodegeneration.
Nonetheless, it should be noted we did not find any evidence of inflammation playing a mediating role in the association between dietary Mg intake and other brain regions. This may be due to the decreased capacity to detect diffuse effects in smaller brain areas, or attributable to other mechanisms. For example, Mg has been shown to help prevent synaptic loss by blocking the cytotoxic effects of NMDA and therefore, increase neurogenesis and decrease neurodegeneration [70, 71].
Finally, it is noteworthy that the anti-inflammatory benefits of dietary Mg intake appear to be weakened in the presence of cardiovascular risk factors such as smoking, high cholesterol levels, and hypertension. Indeed, in those who presented with these risk factors the association between higher Mg and lower inflammation was weaker than in those who did not. In contrast, these associations were stronger in individuals taking BP medication. The reasons for these interactions are unclear and need further investigation. It is possible that smoking in particular may interfere with the absorption and utilization of Mg in the body [72], leading to a weaker anti-inflammatory response. It is also possible that the strong pro-inflammatory and/or neurodegenerative effects of cardiovascular risk factors may have obscured or dampened an Mg effect. In contrast, BP medication may work synergistically with dietary Mg intake in reducing inflammation [73]. It is important to highlight that the exact mechanisms behind these observations are not clear and further research is needed to identify them.
Limitation and strength
This study has a number of limitations but also significant strengths. Mg intake was assessed indirectly, using food frequency questionnaires [41, 42]. This method is known to be reliable [74], although it is also linked to more measurement error, which may have reduced our ability to detect some relationships. Nonetheless, nutrition was assessed multiple times during the follow-up period, which is likely to have reduced any recall bias [74]. Another possible conceptual limitation is the use of a single nutrient investigation, which may oversimplify the complex interplay of nutrients within the human diet [75–77]. The health benefits associated with magnesium-rich foods, such as, green vegetables, nuts, seeds, and unrefined grains, include anti-inflammatory, antioxidant, as well as other effects that are only partly attributable to its Mg content [78] and which therefore cannot be estimated in single nutrient analyses. On the other hand, this approach provides more specific estimates of dose-effects which can contribute to systematic review and may be useful in informing clinical practice and population health interventions [9]. Another limitation is the lack of availability of more detailed inflammatory measures, in particular IL-1 and IL-6 which have been consistently implicated in neurodegenerative processes [27, 79], as well as the biological pathways linking Mg to inflammatory processes [80]. lastly, due to the observational nature of our study it is difficult to establish a conclusive cause-and-effect relationship.
A particular strength is that this study examined a very large number of participants with enough statistical power to test hypothesised associations and mediation while adjusting for a large number of cardiovascular factors. To our knowledge, it was also the first to use neuroimaging and structural brain measures in humans to examine the mediation effect of dietary Mg intake on cerebral health through the inflammatory process.
Conclusion
In summary, the current findings provide convergent evidence suggesting that the anti-inflammatory effects of dietary Mg in middle-to early old-age adults may have a neuroprotective effect. Thus, these findings are a promising step towards understanding the potential benefits of dietary Mg intake, and in gauging the value Mg supplementation and dietary advice may contribute to interventions aimed at reducing inflammation and promoting cerebral health in the general population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge and thank all UK Biobank participants and the UK Biobank team for their work (application number 47813), and also thank the Deanship of scientific research in King Saud University for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
Author contributions
Alateeq contributed to the conceptualization of the study and the development of the methodological approach, conducted the formal analyses and managed data curation, and produced the original draft and graphics; Walsh contributed to the conceptualization of the study and the development of the methodological approach and the writing and editing of the manuscript; Ambikairajah the optimization of the study data and the writing and editing of the manuscript; and Cherbuin contributed to the conceptualization of the study and the development of the methodological approach, the writing and editing of the manuscript, and the supervision and administration of the project.
Funding
There is no financial support for the research, authorship and/or publication of this article.
Open Access funding enabled and organized by CAUL and its Member Institutions
Data availability
UK Biobank is an open access resource accessible to confirmed researchers upon request (ukbiobank.ac.uk/).
Declarations
Conflict of interest
The author has no financial interests or ethical conflicts of interest regarding this submission.
References
- 1.Department of Economic and Social, Affairs PD (2022) World Population Prospects 2022
- 2.Velandia PP, Miller-Petrie MK, Chen C et al (2022) Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: an economic modelling exercise. 10.1016/j.eclinm.2022.101337. eClinicalMedicine 45: [DOI] [PMC free article] [PubMed]
- 3.Scarmeas N, Anastasiou CA, Yannakoulia M (2018) Nutrition and prevention of cognitive impairment. Lancet Neurol 17:1006–1015 10.1016/S1474-4422(18)30338-7 [DOI] [PubMed] [Google Scholar]
- 4.Tao M, Liu J, Cervantes D (2022) Association between magnesium intake and cognition in US older adults: National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Alzheimer’s Dement Transl Res Clin Interv 8. 10.1002/trc2.12250 [DOI] [PMC free article] [PubMed]
- 5.Ozawa M, Ninomiya T, Ohara T et al (2012) Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the hisayama study. J Am Geriatr Soc 60:1515–1520. 10.1111/j.1532-5415.2012.04061.x 10.1111/j.1532-5415.2012.04061.x [DOI] [PubMed] [Google Scholar]
- 6.Kirkland AE, Sarlo GL, Holton KF (2018) The role of Magnesium in Neurological disorders. Nutrients 10. 10.3390/nu10060730 [DOI] [PMC free article] [PubMed]
- 7.Cherbuin N (2016) Dietary Mineral Intake (Magnesium, Calcium, and Potassium) and the biological processes of aging. Elsevier Inc
- 8.Alateeq K, Walsh EI, Cherbuin N (2023) Dietary magnesium intake is related to larger brain volumes and lower white matter lesions with notable sex differences. Eur J Nutr. 10.1007/s00394-023-03123-x 10.1007/s00394-023-03123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherbuin N, Kumar R, Sachdev PS, Anstey KJ (2014) Dietary mineral intake and risk of mild cognitive impairment: the PATH through life project. Front Aging Neurosci 6:1–8. 10.3389/fnagi.2014.00004 10.3389/fnagi.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J, Zhang C, Zhao Q et al (2022) Dietary calcium and magnesium intake and risk for incident dementia: the Shanghai Aging Study. Alzheimer’s Dement Transl Res Clin Interv 8. 10.1002/trc2.12362 [DOI] [PMC free article] [PubMed]
- 11.Du K, Zheng X, Ma Z-T et al (2022) Association of Circulating Magnesium Levels in patients with Alzheimer’s Disease from 1991 to 2021: a systematic review and Meta-analysis. Front Aging Neurosci 13:799824. 10.3389/fnagi.2021.799824 10.3389/fnagi.2021.799824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomassen JQ, Tolstrup JS, Nordestgaard BG et al (2021) Plasma concentrations of Magnesium and Risk of Dementia: a General Population Study of 102 648 individuals. Clin Chem 67:899–911. 10.1093/clinchem/hvab041 10.1093/clinchem/hvab041 [DOI] [PubMed] [Google Scholar]
- 13.Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J (2018) Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients 10:1–21. 10.3390/nu10020168 10.3390/nu10020168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibaba DT, Xun P, Song Y et al (2017) The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am J Clin Nutr 106:921–929. 10.3945/ajcn.117.155291 10.3945/ajcn.117.155291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kass L, Weekes J, Carpenter L (2012) Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr 66:411–418. 10.1038/ejcn.2012.4 10.1038/ejcn.2012.4 [DOI] [PubMed] [Google Scholar]
- 16.Bo S, Pisu E (2008) Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol 19:50–56. 10.1097/MOL.0b013e3282f33ccc 10.1097/MOL.0b013e3282f33ccc [DOI] [PubMed] [Google Scholar]
- 17.Alateeq K, Walsh EI, Abhayaratna WP, Cherbuin N (2022) Effects of higher normal blood pressure on Brain are detectable before Middle-Age and Differ by Sex. J Clin Med 11:3127. 10.3390/jcm11113127 10.3390/jcm11113127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alateeq K, Walsh EI, Cherbuin N (2021) Higher blood pressure is associated with greater white matter lesions and brain atrophy: a systematic review with meta-analysis. J Clin Med 10:1–22 10.3390/jcm10040637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherbuin N, Mortby ME, Janke AL et al (2015) Blood pressure, brain structure, and cognition: opposite associations in men and women. Am J Hypertens 28:225–231. 10.1093/ajh/hpu120 10.1093/ajh/hpu120 [DOI] [PubMed] [Google Scholar]
- 20.Cherbuin N, Walsh EI, Shaw M et al (2021) Optimal blood pressure keeps our brains younger. Front Aging Neurosci 13:529. 10.3389/fnagi.2021.694982 10.3389/fnagi.2021.694982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forte G, De Pascalis V, Favieri F, Casagrande M (2019) Effects of blood pressure on cognitive performance: a systematic review. J Clin Med 9:34. 10.3390/jcm9010034 10.3390/jcm9010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CJ, Lee JY, Han K et al (2022) Blood pressure levels and risks of dementia: a nationwide study of 4.5 million people. Hypertension 79:218–229. 10.1161/HYPERTENSIONAHA.121.17283 10.1161/HYPERTENSIONAHA.121.17283 [DOI] [PubMed] [Google Scholar]
- 23.Mazur A, Maier JAM, Rock E et al (2007) Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys 458:48–56. 10.1016/j.abb.2006.03.031 10.1016/j.abb.2006.03.031 [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Li TY, van Dam RM et al (2007) Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr 85:1068–1074. 10.1093/ajcn/85.4.1068 10.1093/ajcn/85.4.1068 [DOI] [PubMed] [Google Scholar]
- 25.Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M et al (2017) Effect of Magnesium supplementation on plasma C-reactive protein concentrations: a systematic review and Meta-analysis of Randomized controlled trials. Curr Pharm Des 23. 10.2174/1381612823666170525153605 [DOI] [PubMed]
- 26.Jang S, Ogunmoroti O, Ndumele CE et al (2020) Association of the novel inflammatory marker GlycA and Incident Heart failure and its subtypes of preserved and reduced ejection fraction: the multi-ethnic study of atherosclerosis. Circ Hear Fail 13:E007067. 10.1161/CIRCHEARTFAILURE.120.007067 10.1161/CIRCHEARTFAILURE.120.007067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherbuin N, Walsh EI, Leach L et al (2022) Systemic inflammation predicts Alzheimer Pathology in Community samples without dementia. Biomedicines 10:1240. 10.3390/biomedicines10061240 10.3390/biomedicines10061240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo HK, Yen CJ, Chang CH et al (2005) Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol 4:371–380 10.1016/S1474-4422(05)70099-5 [DOI] [PubMed] [Google Scholar]
- 29.Cooper J, Pastorello Y, Slevin M (2023) A meta-analysis investigating the relationship between inflammation in autoimmune disease, elevated CRP, and the risk of dementia. Front Immunol 14:1087571. 10.3389/fimmu.2023.1087571 10.3389/fimmu.2023.1087571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long S, Chen Y, Meng Y et al (2023) Peripheral high levels of CRP predict progression from normal cognition to dementia: a systematic review and meta-analysis. J Clin Neurosci 107:54–63 10.1016/j.jocn.2022.11.016 [DOI] [PubMed] [Google Scholar]
- 31.Ng A, Tam WW, Zhang MW et al (2018) IL-1β, IL-6, TNF- α and CRP in Elderly patients with Depression or Alzheimer’s disease: systematic review and Meta-analysis. Sci Rep 8. 10.1038/s41598-018-30487-6 [DOI] [PMC free article] [PubMed]
- 32.Justiz Vaillant AA, Qurie A (2022) Interleukin. StatPearls Publishing [PubMed]
- 33.Brocker C, Thompson D, Matsumoto A et al (2010) Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics 5:30–55. 10.1186/1479-7364-5-1-30 10.1186/1479-7364-5-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchla A, Kriebardis AG, Georgatzakou HT et al (2022) Red blood cell abnormalities as the Mirror of SARS-CoV-2 Disease Severity: a pilot study. Front Physiol 12:2487. 10.3389/fphys.2021.825055 10.3389/fphys.2021.825055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruppen EG, Riphagen IJ, Connelly MA et al (2015) GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with C-reactive protein and renal function. PLoS ONE 10. 10.1371/journal.pone.0139057 [DOI] [PMC free article] [PubMed]
- 36.Akinkuolie AO, Glynn RJ, Padmanabhan L et al (2016) Circulating N-Linked glycoprotein side-chain biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: an analysis from the JUPITER Trial. J Am Heart Assoc 5. 10.1161/JAHA.116.003822 [DOI] [PMC free article] [PubMed]
- 37.Akinkuolie AO, Buring JE, Ridker PM, Mora S (2014) A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 3. 10.1161/JAHA.114.001221 [DOI] [PMC free article] [PubMed]
- 38.Duprez DA, Otvos J, Sanchez OA et al (2016) Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem 62:1020–1031. 10.1373/clinchem.2016.255828 10.1373/clinchem.2016.255828 [DOI] [PubMed] [Google Scholar]
- 39.Miller KL, Alfaro-Almagro F, Bangerter NK et al (2016) Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19:1523–1536. 10.1038/nn.4393 10.1038/nn.4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Elm E, Altman DG, Egger M et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. 10.1136/bmj.39335.541782.AD 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galante J, Adamska L, Young A et al (2016) The acceptability of repeat internet-based hybrid diet assessment of previous 24-h dietary intake: administration of the Oxford WebQ in UK Biobank. Br J Nutr 115:681–686. 10.1017/S0007114515004821 10.1017/S0007114515004821 [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Young H, Crowe FL et al (2011) Development and evaluation of the oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr 14:1998–2005. 10.1017/S1368980011000942 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 43.Reuter M, Fischl B (2011) Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage 57:19–21. 10.1016/j.neuroimage.2011.02.076 10.1016/j.neuroimage.2011.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majrashi NA, Ahearn TS, Williams JHG, Waiter GD (2020) Sex differences in the association of photoperiod with hippocampal subfield volumes in older adults: a cross-sectional study in the UK Biobank cohort. Brain Behav 10:01593–01603. 10.1002/brb3.1593 10.1002/brb3.1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ségonne F, Dale AM, Busa E et al (2004) A hybrid approach to the skull stripping problem in MRI. NeuroImage 22:1060–1075. 10.1016/j.neuroimage.2004.03.032 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- 46.Fischl B (2012) FreeSurfer. Neuroimage [DOI] [PMC free article] [PubMed]
- 47.Alfaro-Almagro F, Jenkinson M, Bangerter NK et al (2018) Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 166:400–424. 10.1016/j.neuroimage.2017.10.034 10.1016/j.neuroimage.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ainsworth BE, Haskell WL, Whitt MC et al (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498–S504. 10.1001/jama.1916.02590120017006 10.1001/jama.1916.02590120017006 [DOI] [PubMed] [Google Scholar]
- 49.R Development Core Team (2018) R: a language and environment for statistical computing. Vienna, Austria [Google Scholar]
- 50.Office of Dietary Supplements (ODS) (2022) https://ods.od.nih.gov/. Accessed 10
- 51.Cormick G, Ciapponi A, Cafferata ML et al (2022) Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst. Rev. 2022 [DOI] [PMC free article] [PubMed]
- 52.Alateeq K, Walsh EI, Cherbuin N (2023) Dietary magnesium intake is related to larger brain volumes and lower white matter lesions with notable sex differences. Eur J Nutr 1–13. 10.1007/s00394-023-03123-x [DOI] [PMC free article] [PubMed]
- 53.Hayes AF (2009) Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 76:408–420. 10.1080/03637750903310360 10.1080/03637750903310360 [DOI] [Google Scholar]
- 54.MacKinnon DP, Fairchild AJ, Fritz MS (2007) Mediation analysis. Annu Rev Psychol 58:593–614. 10.1146/annurev.psych.58.110405.085542 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connelly MA, Otvos JD, Shalaurova I et al (2017) GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med 15 [DOI] [PMC free article] [PubMed]
- 56.Anuk AT, Polat N, Akdas S et al (2021) The relation between Trace element Status (Zinc, Copper, Magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol Trace Elem Res 199:3608–3617. 10.1007/s12011-020-02496-y 10.1007/s12011-020-02496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ezeigwe A, Fashanu OE, Zhao D et al (2019) The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: the multi-ethnic study of atherosclerosis. Atherosclerosis 282:91–99. 10.1016/j.atherosclerosis.2019.01.011 10.1016/j.atherosclerosis.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tibuakuu M, Fashanu OE, Zhao D et al (2019) GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS 33:547–557. 10.1097/QAD.0000000000002079 10.1097/QAD.0000000000002079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshi AA, Lerman JB, Aberra TM et al (2016) GlycA is a novel biomarker of inflammation and subclinical Cardiovascular Disease in Psoriasis. Circ Res 119:1242–1253. 10.1161/CIRCRESAHA.116.309637 10.1161/CIRCRESAHA.116.309637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muhlestein JB, May HT, Galenko O et al (2018) GlycA and hsCRP are independent and additive predictors of future cardiovascular events among patients undergoing angiography: the intermountain heart collaborative study. Am Heart J 202:27–32. 10.1016/j.ahj.2018.04.003 10.1016/j.ahj.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 61.Nielsen FH (2010) Magnesium, inflammation, and obesity in chronic disease. Nutr Rev 68:333–340 10.1111/j.1753-4887.2010.00293.x [DOI] [PubMed] [Google Scholar]
- 62.Liu S, Chacko SA (2013) Dietary mg intake and biomarkers of inflammation and endothelial dysfunction. Magnesium in Human Health and Disease. Humana Press Inc., pp 35–50
- 63.Baune BT, Ponath G, Rothermundt M et al (2009) Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol 22:23–34. 10.1177/0891988708328216 10.1177/0891988708328216 [DOI] [PubMed] [Google Scholar]
- 64.Rao JS, Kellom M, Kim HW et al (2012) Neuroinflammation and synaptic loss. Neurochem Res 37:903–910 10.1007/s11064-012-0708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bettcher BM, Wilheim R, Rigby T et al (2012) C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 26:103–108. 10.1016/j.bbi.2011.07.240 10.1016/j.bbi.2011.07.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haul D (2001) Nutritional influences on Estrogen Metabolism. Appl Nutr Sci Rep 1–8
- 67.Cinar V, Polat Y, Baltaci AK, Mogulkoc R (2011) Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol Trace Elem Res 140:18–23. 10.1007/s12011-010-8676-3 10.1007/s12011-010-8676-3 [DOI] [PubMed] [Google Scholar]
- 68.Maggio M, De Vita F, Lauretani F et al (2014) The interplay between magnesium and testosterone in modulating physical function in men. Int. J. Endocrinol. 2014 [DOI] [PMC free article] [PubMed]
- 69.Walker KA, Power MC, Gottesman RF (2017) Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep 19:24 10.1007/s11906-017-0724-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parsons CG, Danysz W, Quack G (1998) Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect 11:523. 10.1358/dnp.1998.11.9.863689 10.1358/dnp.1998.11.9.863689 [DOI] [PubMed] [Google Scholar]
- 71.Lo K, Liu Q, Madsen T et al (2019) Relations of magnesium intake to cognitive impairment and dementia among participants in the women’s Health Initiative Memory Study: a prospective cohort study. BMJ Open 9:030052. 10.1136/bmjopen-2019-030052 10.1136/bmjopen-2019-030052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuchardt JP, Hahn A (2017) Intestinal absorption and factors influencing bioavailability of Magnesium- An Update. Curr Nutr Food Sci 13:260. 10.2174/1573401313666170427162740 10.2174/1573401313666170427162740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Houston M (2011) The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens 13:843–847 10.1111/j.1751-7176.2011.00538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lowenstein FW, Stanton MF (1986) Serum magnesium levels in the United States, 1971–1974. J Am Coll Nutr 5:399–414. 10.1080/07315724.1986.10720143 10.1080/07315724.1986.10720143 [DOI] [PubMed] [Google Scholar]
- 75.Wang S, Meckling KA, Marcone MF et al (2011) Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J Agric Food Chem 59:960–968. 10.1021/jf1040977 10.1021/jf1040977 [DOI] [PubMed] [Google Scholar]
- 76.Jeejeebhoy KN (2015) Nutritional Assessment. Nutritional Care of the patient with gastrointestinal disease. CRC, pp 1–14
- 77.Tapsell LC, Neale EP, Satija A, Hu FB (2016) Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr 7:445–454 10.3945/an.115.011718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martínez-Lapiscina EH, Clavero P, Toledo E et al (2013) Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 84:1318–1325. 10.1136/jnnp-2012-304792 10.1136/jnnp-2012-304792 [DOI] [PubMed] [Google Scholar]
- 79.Williamson LL, Bilbo SD (2013) Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun 30:186–194. 10.1016/j.bbi.2013.01.077 10.1016/j.bbi.2013.01.077 [DOI] [PubMed] [Google Scholar]
- 80.Maier JA, Castiglioni S, Locatelli L et al (2021) Magnesium and inflammation: advances and perspectives. Semin Cell Dev Biol 115:37–44. 10.1016/j.semcdb.2020.11.002 10.1016/j.semcdb.2020.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UK Biobank is an open access resource accessible to confirmed researchers upon request (ukbiobank.ac.uk/).