Abstract
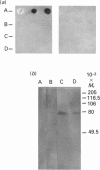
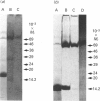
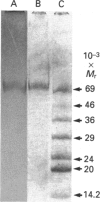
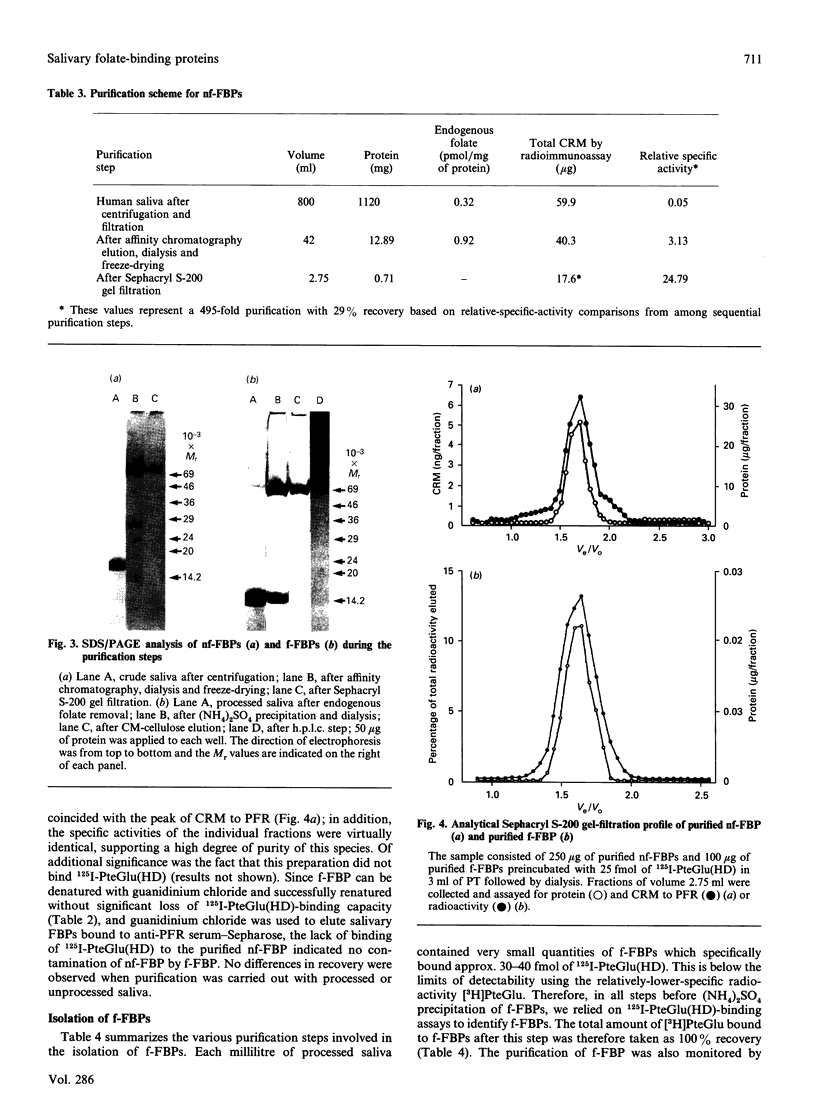
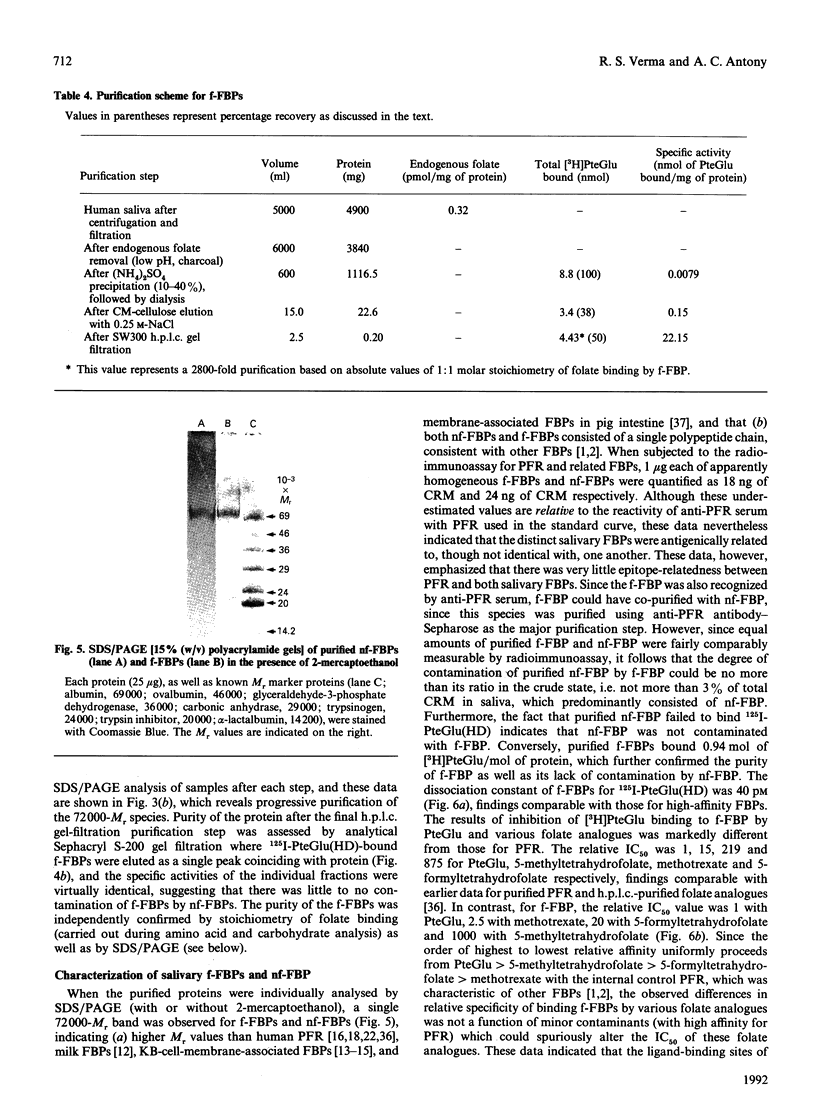
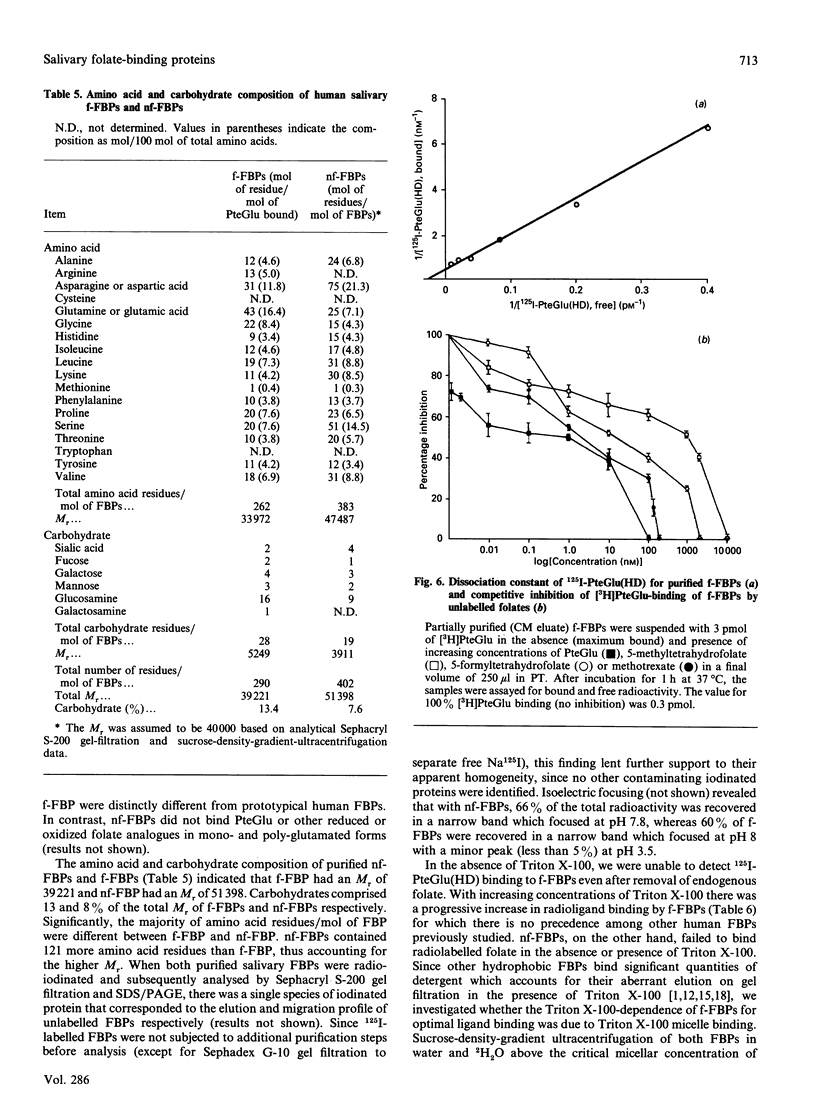
Human saliva contains a single 72,000-M(r) species which specifically reacted with rabbit anti-[human placental folate receptor (PFR)] serum on SDS/PAGE and Western blots. Although a specific radioimmunoassay for human PFR and related folate-binding proteins (FBPs) identified 55 ng of cross-reacting material (CRM) per mg of crude salivary proteins, only a minor fraction (1.6 ng) specifically bound radiolabelled folate. The major fraction of CRM did not contain bound endogenous folate and did not bind radiolabelled folates. On the basis of folate binding, salivary CRM species to PFR were designated as either functional (f-FBP) or non-functional (nf-FBP) species respectively. nf-FBPs and f-FBPs were isolated by different purification schemes. Both purified f-FBPs and nf-FBPs migrated as a single apparent 72,000-M(r) species on SDS/PAGE, but on Sephacryl S-200 gel filtration and sucrose-density-gradient ultracentrifugation they were eluted/sedimented with 40,000-M(r) markers. Each microgram of purified f-FBP and nf-FBP was measured in the radioimmunoassay for PFR as being equivalent to 18 ng and 24 ng of CRM respectively, indicating low epitope-relatedness to PFR. The Kd of f-FBPs was 50 pM and 0.94 mol of folate was bound/mol of protein. f-FBPs exhibited an unusual dependence on Triton X-100 for optimal ligand binding, despite the fact that Triton X-100 micelle binding was not demonstrated. The relative order of affinity of f-FBPs for pteroylglutamate greater than methotrexate greater than 5-formyltetrahydrofolate greater than 5-methyltetrahydrofolate was also distinct from that of purified PFR. Whereas amino acid and carbohydrate analysis revealed that nf-FBP (M(r) 51,400) and f-FBP (M(r) 39,200) were distinct glycoproteins with 8 and 13% carbohydrate respectively, isoelectric focusing and immunological studies suggested some structural identity. The presence of f-FBP and nf-FBP in normal saliva raises new questions about their possible role in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Briddell R. A., Brandt J. E., Straneva J. E., Verma R. S., Miller M. E., Kalasinski L. A., Hoffman R. Megaloblastic hematopoiesis in vitro. Interaction of anti-folate receptor antibodies with hematopoietic progenitor cells leads to a proliferative response independent of megaloblastic changes. J Clin Invest. 1991 Jan;87(1):313–325. doi: 10.1172/JCI114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Bruno E., Briddell R. A., Brandt J. E., Verma R. S., Hoffman R. Effect of perturbation of specific folate receptors during in vitro erythropoiesis. J Clin Invest. 1987 Dec;80(6):1618–1623. doi: 10.1172/JCI113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Kincade R. S., Verma R. S., Krishnan S. R. Identification of high affinity folate binding proteins in human erythrocyte membranes. J Clin Invest. 1987 Sep;80(3):711–723. doi: 10.1172/JCI113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C. The biological chemistry of folate receptors. Blood. 1992 Jun 1;79(11):2807–2820. [PubMed] [Google Scholar]
- Antony A. C., Utley C. S., Marcell P. D., Kolhouse J. F. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem. 1982 Sep 10;257(17):10081–10089. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Antony A. C., Verma R. S. Hydrophobic erythrocyte folate binding proteins are converted to hydrophilic forms by trypsin in vitro. Biochim Biophys Acta. 1989 Feb 13;979(1):62–68. doi: 10.1016/0005-2736(89)90523-3. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Verma R. S., Kincade R. S. Development of a specific radioimmunoassay for the placental folate receptor and related high-affinity folate binding proteins in human tissues. Anal Biochem. 1987 Apr;162(1):224–235. doi: 10.1016/0003-2697(87)90031-5. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Verma R. S., Unune A. R., LaRosa J. A. Identification of a Mg2+-dependent protease in human placenta which cleaves hydrophobic folate-binding proteins to hydrophilic forms. J Biol Chem. 1989 Feb 5;264(4):1911–1914. [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Colman N., Hettiarachchy N., Herbert V. Detection of a milk factor that facilitates folate uptake by intestinal cells. Science. 1981 Mar 27;211(4489):1427–1429. doi: 10.1126/science.6781067. [DOI] [PubMed] [Google Scholar]
- Deutsch J. C., Elwood P. C., Portillo R. M., Macey M. G., Kolhouse J. F. Role of the membrane-associated folate binding protein (folate receptor) in methotrexate transport by human KB cells. Arch Biochem Biophys. 1989 Nov 1;274(2):327–337. doi: 10.1016/0003-9861(89)90446-3. [DOI] [PubMed] [Google Scholar]
- Elwood P. C., Deutsch J. C., Kolhouse J. F. The conversion of the human membrane-associated folate binding protein (folate receptor) to the soluble folate binding protein by a membrane-associated metalloprotease. J Biol Chem. 1991 Feb 5;266(4):2346–2353. [PubMed] [Google Scholar]
- Elwood P. C., Kane M. A., Portillo R. M., Kolhouse J. F. The isolation, characterization, and comparison of the membrane-associated and soluble folate-binding proteins from human KB cells. J Biol Chem. 1986 Nov 25;261(33):15416–15423. [PubMed] [Google Scholar]
- Elwood P. C. Molecular cloning and characterization of the human folate-binding protein cDNA from placenta and malignant tissue culture (KB) cells. J Biol Chem. 1989 Sep 5;264(25):14893–14901. [PubMed] [Google Scholar]
- Ford J. E. Some observations on the possible nutritional significance of vitamin B12-and folate-binding proteins in milk. Br J Nutr. 1974 Mar;31(2):243–257. doi: 10.1079/bjn19740030. [DOI] [PubMed] [Google Scholar]
- Hansen S. I., Holm J., Lyngbye J. Detergent activation of the binding protein in the folate radioassay. Clin Chem. 1982 Jan;28(1):117–118. [PubMed] [Google Scholar]
- Henderson G. B. Folate-binding proteins. Annu Rev Nutr. 1990;10:319–335. doi: 10.1146/annurev.nu.10.070190.001535. [DOI] [PubMed] [Google Scholar]
- Jansen G., Westerhof G. R., Jarmuszewski M. J., Kathmann I., Rijksen G., Schornagel J. H. Methotrexate transport in variant human CCRF-CEM leukemia cells with elevated levels of the reduced folate carrier. Selective effect on carrier-mediated transport of physiological concentrations of reduced folates. J Biol Chem. 1990 Oct 25;265(30):18272–18277. [PubMed] [Google Scholar]
- Jansen G., Westerhof G. R., Kathmann I., Rademaker B. C., Rijksen G., Schornagel J. H. Identification of a membrane-associated folate-binding protein in human leukemic CCRF-CEM cells with transport-related methotrexate resistance. Cancer Res. 1989 May 1;49(9):2455–2459. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. A., Portillo R. M., Elwood P. C., Antony A. C., Kolhouse J. F. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986 Jan 5;261(1):44–49. [PubMed] [Google Scholar]
- Lacey S. W., Sanders J. M., Rothberg K. G., Anderson R. G., Kamen B. A. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989 Aug;84(2):715–720. doi: 10.1172/JCI114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs C. A., Pitiranggon P., da Costa M., Rothenberg S. P., Slomiany B. L., Brink L., Tous G. I., Stein S. Purified membrane and soluble folate binding proteins from cultured KB cells have similar amino acid compositions and molecular weights but differ in fatty acid acylation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6546–6549. doi: 10.1073/pnas.84.18.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs C. A., Sadasivan E., da Costa M., Rothenberg S. P. The isolation and properties of multiple forms of folate binding protein in cultured KB cells. Arch Biochem Biophys. 1986 Oct;250(1):94–105. doi: 10.1016/0003-9861(86)90705-8. [DOI] [PubMed] [Google Scholar]
- Luhrs C. A., Slomiany B. L. A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail. J Biol Chem. 1989 Dec 25;264(36):21446–21449. [PubMed] [Google Scholar]
- Ratnam M., Marquardt H., Duhring J. L., Freisheim J. H. Homologous membrane folate binding proteins in human placenta: cloning and sequence of a cDNA. Biochemistry. 1989 Oct 3;28(20):8249–8254. doi: 10.1021/bi00446a042. [DOI] [PubMed] [Google Scholar]
- Reisenauer A. M. Affinity labelling of the folate-binding protein in pig intestine. Biochem J. 1990 Apr 1;267(1):249–252. doi: 10.1042/bj2670249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan E., Rothenberg S. P. The complete amino acid sequence of a human folate binding protein from KB cells determined from the cDNA. J Biol Chem. 1989 Apr 5;264(10):5806–5811. [PubMed] [Google Scholar]
- Sadasivan E., Rothenberg S. P., da Costa M., Brink L. Characterization of multiple forms of folate-binding protein from human leukemia cells. Biochim Biophys Acta. 1986 Jul 16;882(3):311–321. doi: 10.1016/0304-4165(86)90253-9. [DOI] [PubMed] [Google Scholar]
- Said H. M., Horne D. W., Wagner C. Effect of human milk folate binding protein on folate intestinal transport. Arch Biochem Biophys. 1986 Nov 15;251(1):114–120. doi: 10.1016/0003-9861(86)90057-3. [DOI] [PubMed] [Google Scholar]
- Tani M., Iwai K. Some nutritional effects of folate-binding protein in bovine milk on the bioavailability of folate to rats. J Nutr. 1984 Apr;114(4):778–785. doi: 10.1093/jn/114.4.778. [DOI] [PubMed] [Google Scholar]
- Verma R. S., Antony A. C. Kinetic analysis, isolation, and characterization of hydrophilic folate-binding proteins released from chorionic villi cultured under serum-free conditions. J Biol Chem. 1991 Jul 5;266(19):12522–12535. [PubMed] [Google Scholar]
- Verma R. S., Gullapalli S., Antony A. C. Evidence that the hydrophobicity of isolated, in situ, and de novo-synthesized native human placental folate receptors is a function of glycosyl-phosphatidylinositol anchoring to membranes. J Biol Chem. 1992 Feb 25;267(6):4119–4127. [PubMed] [Google Scholar]
- Zheng D. B., Lim H. M., Pène J. J., White H. B., 3rd Chicken riboflavin-binding protein. cDNA sequence and homology with milk folate-binding protein. J Biol Chem. 1988 Aug 15;263(23):11126–11129. [PubMed] [Google Scholar]