Abstract
Massive small-bowel resection results in a marked adaptive response in the residual terminal ileum. Increased polyamine synthesis is a necessary component of this response. The ileal L-cell-derived peptides enteroglucagon and peptide tyrosine tyrosine (PYY) have been implicated as humoral mediators of this response. We have previously reported a rapid and sustained increase in glucagon mRNA concentrations after massive small-bowel resection. In this study using an inhibitor of the rate-limiting enzyme in polyamine biosynthesis, ornithine decarboxylase, we have demonstrated that the response of the glucagon and PYY genes to massive small-bowel resection is dependent on polyamine biosynthesis. In addition, we have examined the response of both the ornithine decarboxylase and c-jun genes in this model of intestinal adaptation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Savage A. P., Fuessl H. S., Wolfe K., Besterman H. S., Bloom S. R. Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery. 1987 Jun;101(6):715–719. [PubMed] [Google Scholar]
- Al-Mukhtar M. Y., Sagor G. R., Ghatei M. A., Bloom S. R., Wright N. A. The role of pancreatico-biliary secretions in intestinal adaptation after resection, and its relationship to plasma enteroglucagon. Br J Surg. 1983 Jul;70(7):398–400. doi: 10.1002/bjs.1800700703. [DOI] [PubMed] [Google Scholar]
- Alarcon P., Lebenthal E., Lee P. C. Effect of difluoromethyl ornithine (DFMO) on small intestine of adult and weanling rats. Dig Dis Sci. 1987 Aug;32(8):883–888. doi: 10.1007/BF01296713. [DOI] [PubMed] [Google Scholar]
- Ali-Rachedi A., Varndell I. M., Adrian T. E., Gapp D. A., Van Noorden S., Bloom S. R., Polak J. M. Peptide YY (PYY) immunoreactivity is co-stored with glucagon-related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry. 1984;80(5):487–491. doi: 10.1007/BF00495439. [DOI] [PubMed] [Google Scholar]
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Erdman S. H., Park J. H., Thompson J. S., Grandjean C. J., Hart M. H., Vanderhoof J. A. Suppression of diamine oxidase activity enhances postresection ileal proliferation in the rat. Gastroenterology. 1989 Jun;96(6):1533–1538. doi: 10.1016/0016-5085(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Marlowe M., Pekala P. H., Seidel E. R. Multiple pathways for the regulation of ornithine decarboxylase in intestinal epithelial cells. Am J Physiol. 1990 Mar;258(3 Pt 1):G454–G460. doi: 10.1152/ajpgi.1990.258.3.G454. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Osborne D. L., Seidel E. R. Putrescine stimulates DNA synthesis in intestinal epithelial cells. Am J Physiol. 1989 Jul;257(1 Pt 1):G145–G150. doi: 10.1152/ajpgi.1989.257.1.G145. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Ghatei M. A., Domin J., Bloom S. R., Gregory H., Wright N. A. Plasma enteroglucagon, peptide YY and gastrin in rats deprived of luminal nutrition, and after urogastrone-EGF administration. A proliferative role for PYY in the intestinal epithelium? Experientia. 1989 Feb 15;45(2):168–169. doi: 10.1007/BF01954862. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Ghatei M. A., Domin J., Bloom S. R., Wright N. A. Is peptide YY trophic to the intestinal epithelium of parenterally fed rats? Digestion. 1990;46 (Suppl 2):177–181. doi: 10.1159/000200383. [DOI] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Hanson W. R., Rijke R. P., Plaisier H. M., Van Ewijk W., Osborne J. W. The effect of intestinal resection on Thiry-Vella fistulae of jejunal and ileal origin in the rat: evidence for a systemic control mechanism of cell renewal. Cell Tissue Kinet. 1977 Nov;10(6):543–555. doi: 10.1111/j.1365-2184.1977.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Hosomi M., Lirussi F., Stace N. H., Vaja S., Murphy G. M., Dowling R. H. Mucosal polyamine profile in normal and adapting (hypo and hyperplastic) intestine: effects of DFMO treatment. Gut. 1987;28 (Suppl):103–107. doi: 10.1136/gut.28.suppl.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Kashiwagi K., Watanabe S., Kameji T., Hayashi S., Igarashi K. Influence of the 5'-untranslated region of ornithine decarboxylase mRNA and spermidine on ornithine decarboxylase synthesis. J Biol Chem. 1990 Aug 5;265(22):13036–13041. [PubMed] [Google Scholar]
- Iwami K., Wang J. Y., Jain R., McCormack S., Johnson L. R. Intestinal ornithine decarboxylase: half-life and regulation by putrescine. Am J Physiol. 1990 Feb;258(2 Pt 1):G308–G315. doi: 10.1152/ajpgi.1990.258.2.G308. [DOI] [PubMed] [Google Scholar]
- Kingsnorth A. N., Abu-Khalaf M., LaMuraglia G. M., McCann P. P., Diekema K. A., Ross J. S., Malt R. A. Inhibition of ileal and colonic ornithine decarboxylase activity by alpha-difluoromethylornithine in rats: transient atrophic changes and loss of postresectional adaptive growth. Surgery. 1986 Jun;99(6):721–727. [PubMed] [Google Scholar]
- Kitabayashi I., Saka F., Gachelin G., Yokoyama K. Nucleotide sequence of rat c-jun protooncogene. Nucleic Acids Res. 1990 Jun 11;18(11):3400–3400. doi: 10.1093/nar/18.11.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter A. B., Toder A., Wolfe H. J., Taylor I. L., Cooperman S., Mandel G., Goodman R. H. Peptide YY. Structure of the precursor and expression in exocrine pancreas. J Biol Chem. 1987 Sep 25;262(27):12984–12988. [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Inhibition of intestinal epithelial DNA synthesis and adaptive hyperplasia after jejunectomy in the rat by suppression of polyamine biosynthesis. J Clin Invest. 1984 Sep;74(3):698–704. doi: 10.1172/JCI111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Polyamines and intestinal growth--increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983 Nov;245(5 Pt 1):G656–G660. doi: 10.1152/ajpgi.1983.245.5.G656. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Yang P. Distribution of polyamines and their biosynthetic enzymes in intestinal adaptation. Am J Physiol. 1988 Feb;254(2 Pt 1):G194–G200. doi: 10.1152/ajpgi.1988.254.2.G194. [DOI] [PubMed] [Google Scholar]
- Lund P. K., Ulshen M. H., Rountree D. B., Selub S. E., Buchan A. M. Molecular biology of gastrointestinal peptides and growth factors: relevance to intestinal adaptation. Digestion. 1990;46 (Suppl 2):66–73. doi: 10.1159/000200369. [DOI] [PubMed] [Google Scholar]
- Manzella J. M., Blackshear P. J. Regulation of rat ornithine decarboxylase mRNA translation by its 5'-untranslated region. J Biol Chem. 1990 Jul 15;265(20):11817–11822. [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Sagor G. R., Al-Mukhtar M. Y., Ghatei M. A., Wright N. A., Bloom S. R. The effect of altered luminal nutrition on cellular proliferation and plasma concentrations of enteroglucagon and gastrin after small bowel resection in the rat. Br J Surg. 1982 Jan;69(1):14–18. doi: 10.1002/bjs.1800690106. [DOI] [PubMed] [Google Scholar]
- Sakai M., Okuda A., Hatayama I., Sato K., Nishi S., Muramatsu M. Structure and expression of the rat c-jun messenger RNA: tissue distribution and increase during chemical hepatocarcinogenesis. Cancer Res. 1989 Oct 15;49(20):5633–5637. [PubMed] [Google Scholar]
- Savage A. P., Gornacz G. E., Adrian T. E., Ghatei M. A., Goodlad R. A., Wright N. A., Bloom S. R. Is raised plasma peptide YY after intestinal resection in the rat responsible for the trophic response? Gut. 1985 Dec;26(12):1353–1358. doi: 10.1136/gut.26.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrino G., Lorenzini E. C., Ferioli M. E. Polyamines and mammalian hormones. Part I: Biosynthesis, interconversion and hormone effects. Mol Cell Endocrinol. 1991 May;77(1-3):1–35. doi: 10.1016/0303-7207(91)90056-x. [DOI] [PubMed] [Google Scholar]
- Seidel E. R., Haddox M. K., Johnson L. R. Ileal mucosal growth during intraluminal infusion of ethylamine or putrescine. Am J Physiol. 1985 Oct;249(4 Pt 1):G434–G438. doi: 10.1152/ajpgi.1985.249.4.G434. [DOI] [PubMed] [Google Scholar]
- Spiller R. C., Trotman I. F., Adrian T. E., Bloom S. R., Misiewicz J. J., Silk D. B. Further characterisation of the 'ileal brake' reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988 Aug;29(8):1042–1051. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
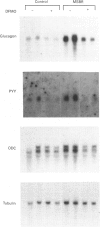
- Taylor R. G., Verity K., Fuller P. J. Ileal glucagon gene expression: ontogeny and response to massive small bowel resection. Gastroenterology. 1990 Sep;99(3):724–729. doi: 10.1016/0016-5085(90)90961-y. [DOI] [PubMed] [Google Scholar]
- Van Steeg H., Van Oostrom C. T., Hodemaekers H. M., Peters L., Thomas A. A. The translation in vitro of rat ornithine decarboxylase mRNA is blocked by its 5' untranslated region in a polyamine-independent way. Biochem J. 1991 Mar 1;274(Pt 2):521–526. doi: 10.1042/bj2740521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Blackshear P. J. Rat ornithine decarboxylase gene. Nucleotide sequence, potential regulatory elements, and comparison to the mouse gene. J Biol Chem. 1989 May 25;264(15):9016–9021. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L. Evidence for an enterotropic hormone: compensatory hyperplasia in defunctioned bowel. Br J Surg. 1978 Oct;65(10):736–739. doi: 10.1002/bjs.1800651018. [DOI] [PubMed] [Google Scholar]
- van Daalen Wetters T., Macrae M., Brabant M., Sittler A., Coffino P. Polyamine-mediated regulation of mouse ornithine decarboxylase is posttranslational. Mol Cell Biol. 1989 Dec;9(12):5484–5490. doi: 10.1128/mcb.9.12.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kranen H. J., van de Zande L., van Kreijl C. F., Bisschop A., Wieringa B. Cloning and nucleotide sequence of rat ornithine decarboxylase cDNA. Gene. 1987;60(2-3):145–155. doi: 10.1016/0378-1119(87)90222-8. [DOI] [PubMed] [Google Scholar]