Abstract
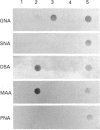
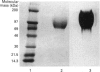
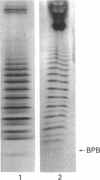
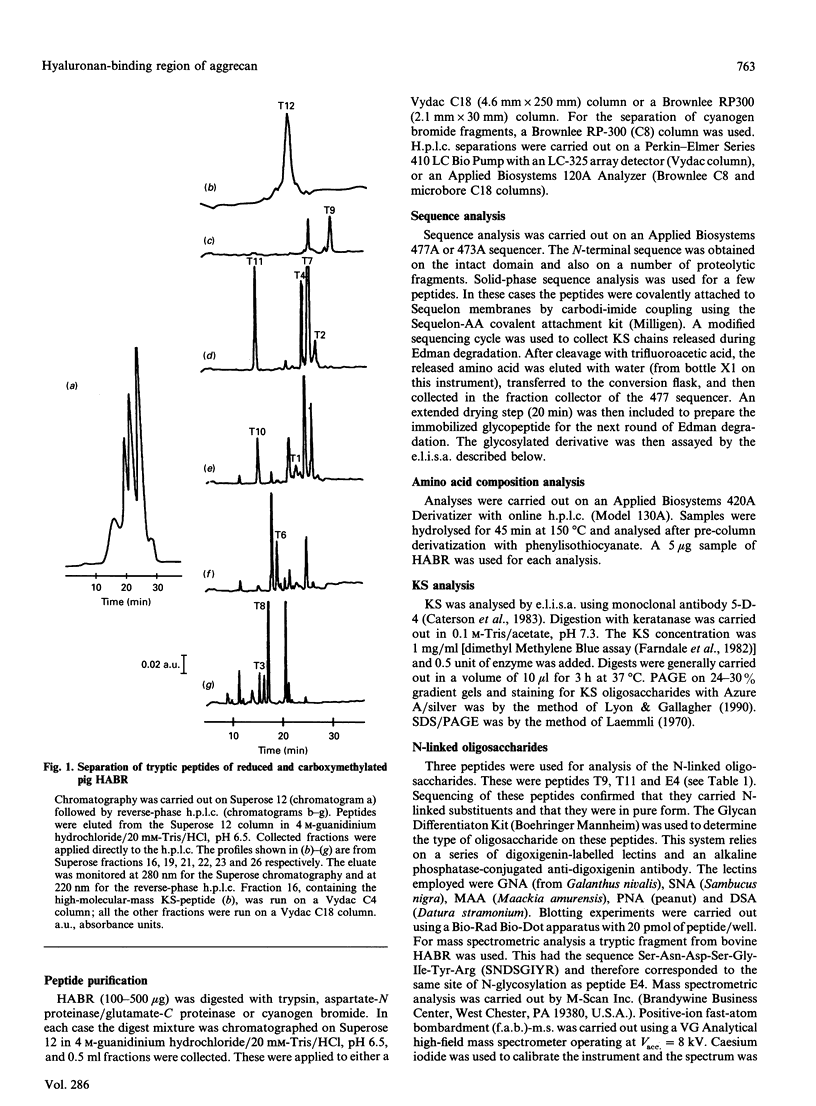
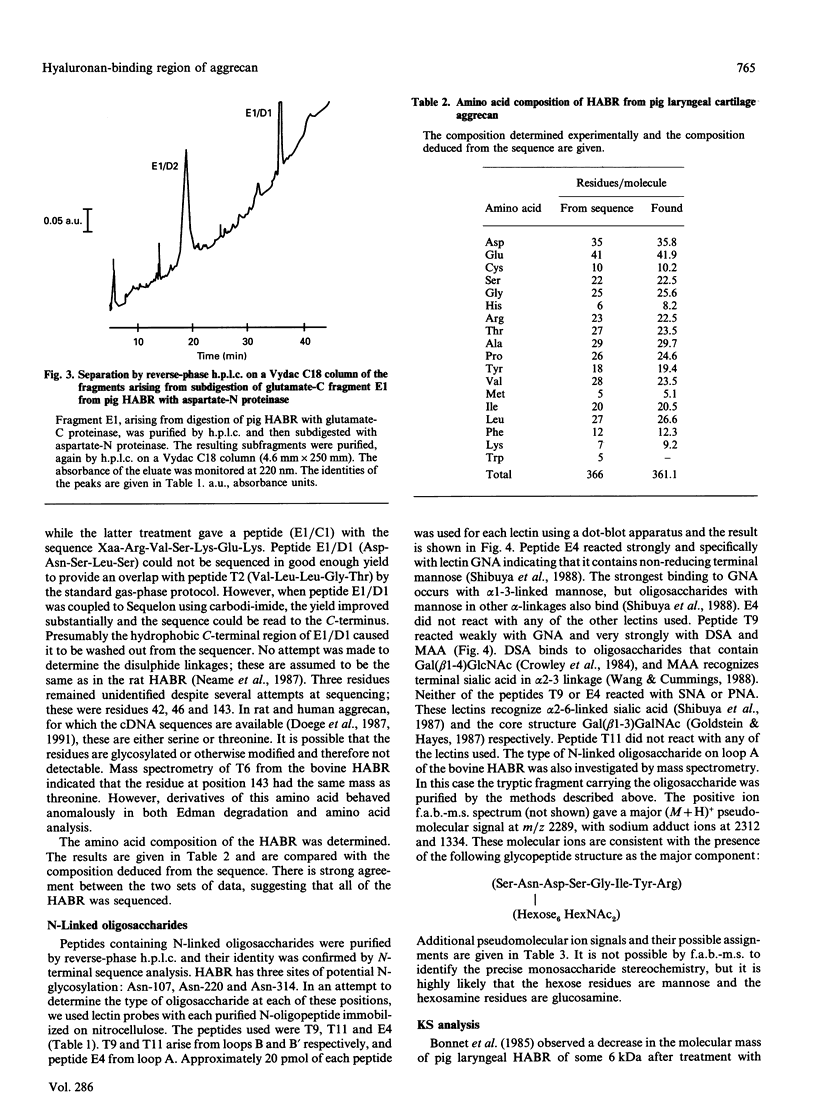
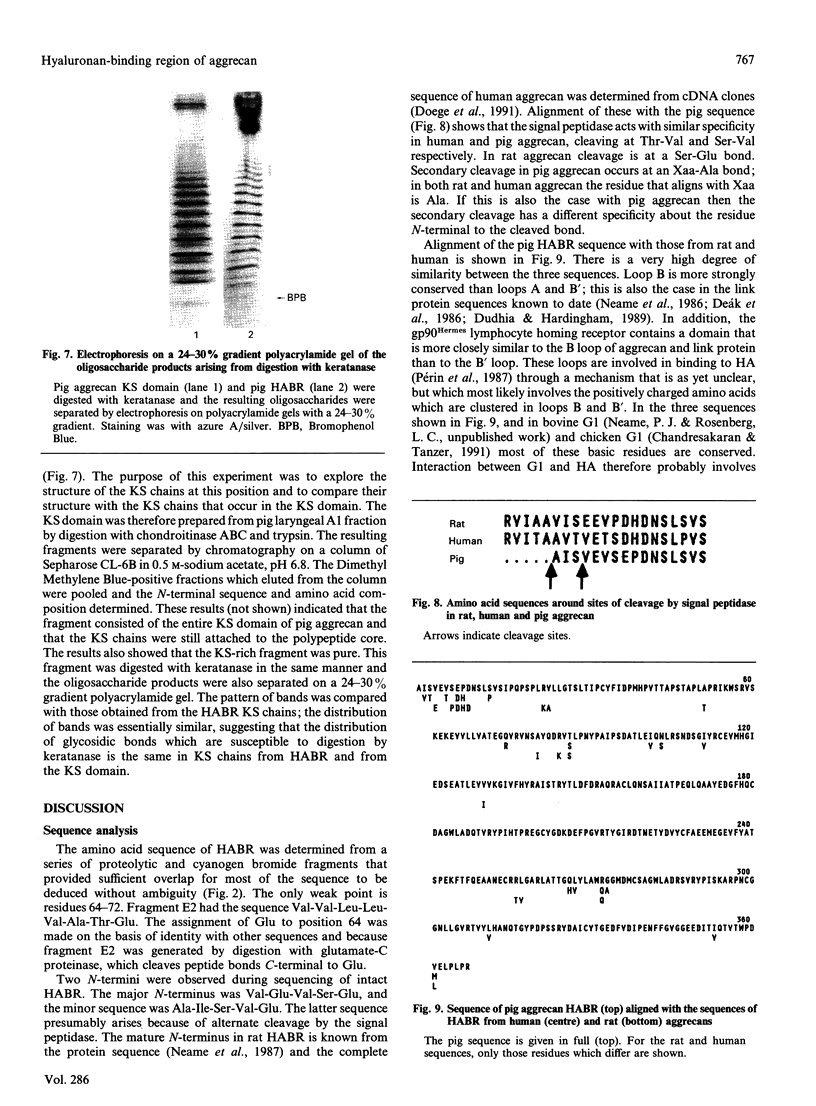
The hyaluronan-binding region (HABR) was prepared from pig laryngeal cartilage aggrecan and the amino acid sequence was determined. The HABR had two N-termini: one N-terminal sequence was Val-Glu-Val-Ser-Glu-Pro (367 amino acids in total), and a second N-terminal sequence (Ala-Ile-Ser-Val-Glu-Val; 370 amino acids in total) was found to arise due to alternate cleavage by the signal peptidase. The N-linked oligosaccharides were analysed by examining their reactivity with a series of lectins. It was found that the N-linked oligosaccharide on loop A was of the mannose type, while that on loop B was of the complex type. No reactivity was detected between the N-linked oligosaccharide on loop B' and any of the lectins. The location of keratan sulphate (KS) in the HABR was determined by Edman degradation of the immobilized KS-containing peptide. The released amino acid derivatives were collected and tested for the presence of epitope to antibody 5-D-4. On the basis of 5-D-4 reactivity and sequencing yields, the KS chains are attached to threonine residues 352 and 357. There is no KS at threonine-355. This site is not in fact in G1, but about 16 amino acid residues into the interglobular domain. Comparison of the structure of the KS chain from the HABR and from the KS domain of pig laryngeal cartilage aggrecan was made by separation on polyacrylamide gels of the oligosaccharides arising from digestion with keratanase. Comparison of the oligosaccharide maps suggests that the KS chains from both parts of the aggrecan molecule have the same structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- Baldwin C. T., Reginato A. M., Prockop D. J. A new epidermal growth factor-like domain in the human core protein for the large cartilage-specific proteoglycan. Evidence for alternative splicing of the domain. J Biol Chem. 1989 Sep 25;264(27):15747–15750. [PubMed] [Google Scholar]
- Bause E., Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981 Jun 1;195(3):639–644. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F., Dunham D. G., Hardingham T. E. Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J. 1985 May 15;228(1):77–85. doi: 10.1042/bj2280077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F., Périn J. P., Lorenzo F., Jollès J., Jollès P. An unexpected sequence homology between link proteins of the proteoglycan complex and immunoglobulin-like proteins. Biochim Biophys Acta. 1986 Sep 5;873(1):152–155. doi: 10.1016/0167-4838(86)90202-5. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Crowley J. F., Goldstein I. J., Arnarp J., Lönngren J. Carbohydrate binding studies on the lectin from Datura stramonium seeds. Arch Biochem Biophys. 1984 Jun;231(2):524–533. doi: 10.1016/0003-9861(84)90417-x. [DOI] [PubMed] [Google Scholar]
- Deák F., Kiss I., Sparks K. J., Argraves W. S., Hampikian G., Goetinck P. F. Complete amino acid sequence of chicken cartilage link protein deduced from cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3766–3770. doi: 10.1073/pnas.83.11.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Fellini S. A., Kimura J. H., Stevens R. L., Hascall V. C. Formation of proteoglycan aggregates in rat chondrosarcoma chondrocyte cultures treated with tunicamycin. J Biol Chem. 1983 Oct 25;258(20):12280–12286. [PubMed] [Google Scholar]
- Lyon M., Gallagher J. T. A general method for the detection and mapping of submicrogram quantities of glycosaminoglycan oligosaccharides on polyacrylamide gels by sequential staining with azure A and ammoniacal silver. Anal Biochem. 1990 Feb 15;185(1):63–70. doi: 10.1016/0003-2697(90)90255-8. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. Cartilage proteoglycan aggregates. The link protein and proteoglycan amino-terminal globular domains have similar structures. J Biol Chem. 1987 Dec 25;262(36):17768–17778. [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Mar 15;261(8):3519–3535. [PubMed] [Google Scholar]
- Nilsson B., De Luca S., Lohmander S., Hascall V. C. Structures of N-linked and O-linked oligosaccharides on proteoglycan monomer isolated from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Sep 25;257(18):10920–10927. [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 1989 Sep;8(9):2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Mörgelin M., Wiedemann H., Beardmore-Gray M., Dunham D., Hardingham T., Heinegård D., Timpl R., Engel J. Extended and globular protein domains in cartilage proteoglycans. Biochem J. 1987 Aug 1;245(3):763–772. doi: 10.1042/bj2450763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dudhia J., Hardingham T. E. Immunoglobulin fold and tandem repeat structures in proteoglycan N-terminal domains and link protein. J Mol Biol. 1989 Apr 20;206(4):737–753. doi: 10.1016/0022-2836(89)90580-9. [DOI] [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Thurieau C., Jollès P. Link protein interactions with hyaluronate and proteoglycans. Characterization of two distinct domains in bovine cartilage link proteins. J Biol Chem. 1987 Sep 25;262(27):13269–13272. [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987 Feb 5;262(4):1596–1601. [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Van Damme E. J., Peumans W. J. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988 Jan 15;263(2):728–734. [PubMed] [Google Scholar]
- Wang W. C., Cummings R. D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988 Apr 5;263(10):4576–4585. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]