Abstract
beta-Very-low-density lipoproteins (beta-VLDL)O were conjugated to the 19F-containing residualizing label, NN-dilactitol-3,5-bis(trifluoromethyl)benzylamine (DLBA), to determine whether the metabolism of this lipoprotein fraction could be characterized in vivo with n.m.r. spectroscopy. Solution state 19F high-resolution n.m.r. spectroscopy of DLBA-beta-VLDL, containing either intact apoproteins or selectively enzymically digested products, demonstrated that the extent of degradation could be distinguished by differences in spin-spin relaxation times (T2 times). DLBA-beta-VLDL was injected intravenously into rabbits, and accumulation of 19F in hepatic tissue was quantified non-invasively by n.m.r. spectroscopy 5 and 30 h after injection. In addition to quantifying the accumulation of DLBA-beta-VLDL in hepatic tissue, a marked decrease (approx. 100 Hz) in the linewidth of 19F resonance from labelled lipoproteins was observed at 30 h compared with the 5 h interval in continuously monitored animals. The change in linewidth was consistent with a decrease in molecular size that occurred during protein degradation, resulting in increased T2 times. To demonstrate that T2 times can be used as an index to quantify apoprotein degradation in vivo, relaxation measurements were performed on livers excised 20 h after injection of DLBA-beta-VLDL into rabbits. Two molecular motional fractions were revealed by relaxation profiles representing either an intact or an extensively degraded form of apoprotein. The amplitudes of each component were compared with results from trichloroacetic acid precipitation of liver homogenates acquired from rabbits 20 h after injection of beta-VLDL labelled with the radioiodinated analogue of DLBA, dilactitol-125I-tyramine. The amount of degraded apoprotein determined by n.m.r. spectroscopy and acid precipitation was 68.6 +/- 7.0% and 58.7 +/- 7.5% (n = 4) respectively. The results of this study demonstrate that 19F n.m.r. spectroscopy can be used to define the temporal characteristics of the hepatic metabolism of lipoproteins in vivo by quantifying both the tissue-specific accumulation and extent of apoprotein degradation. The methodology developed offers promise for the non-invasive, sequential and longitudinal evaluation of lipoprotein metabolism in vivo.
Full text
PDF


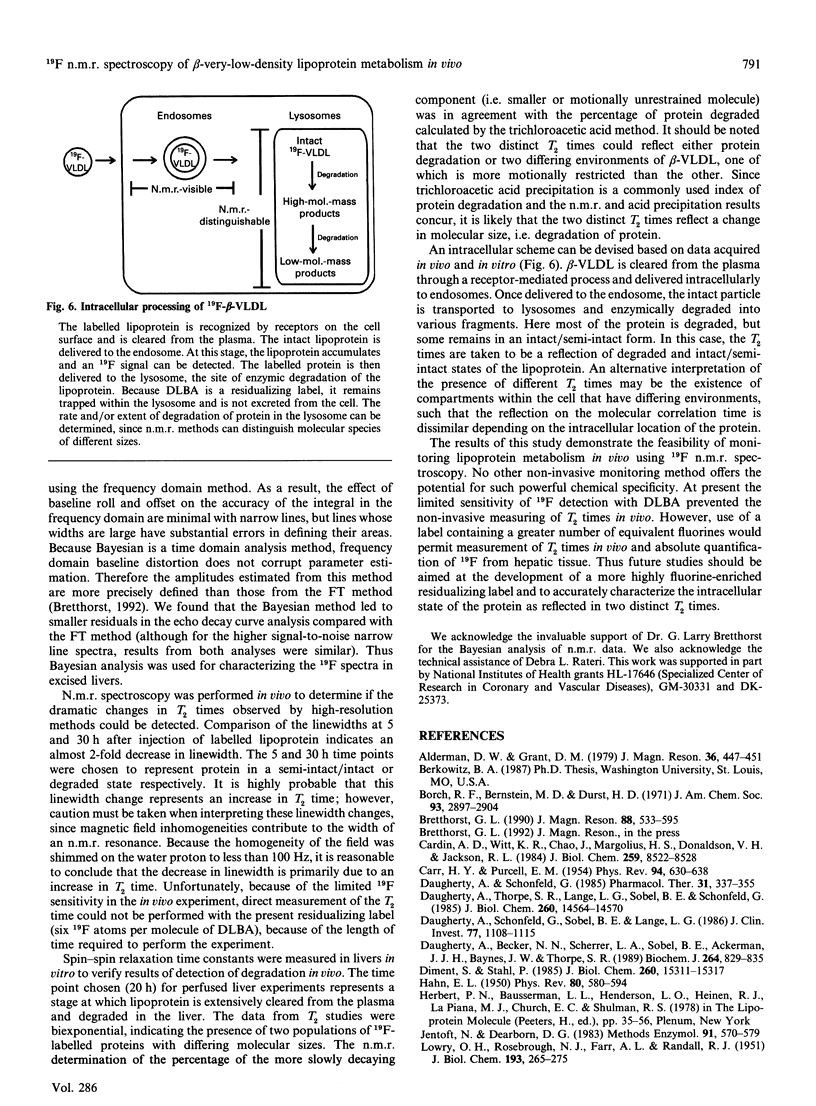

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardin A. D., Witt K. R., Chao J., Margolius H. S., Donaldson V. H., Jackson R. L. Degradation of apolipoprotein B-100 of human plasma low density lipoproteins by tissue and plasma kallikreins. J Biol Chem. 1984 Jul 10;259(13):8522–8528. [PubMed] [Google Scholar]
- Daugherty A., Becker N. N., Scherrer L. A., Sobel B. E., Ackerman J. J., Baynes J. W., Thorpe S. R. Non-invasive detection of protein metabolism in vivo by n.m.r. spectroscopy. Application of a novel 19F-containing residualizing label. Biochem J. 1989 Dec 15;264(3):829–835. doi: 10.1042/bj2640829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Schonfeld G., Sobel B. E., Lange L. G. Metabolism of very low density lipoproteins after cessation of cholesterol feeding in rabbits. A factor potentially contributing to the slow regression of atheromatous plaques. J Clin Invest. 1986 Apr;77(4):1108–1115. doi: 10.1172/JCI112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Thorpe S. R., Lange L. G., Sobel B. E., Schonfeld G. Loci of catabolism of beta-very low density lipoprotein in vivo delineated with a residualizing label, 125I-dilactitol tyramine. J Biol Chem. 1985 Nov 25;260(27):14564–14570. [PubMed] [Google Scholar]
- Diment S., Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985 Dec 5;260(28):15311–15317. [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Protein labeling by reductive alkylation. Methods Enzymol. 1983;91:570–579. doi: 10.1016/s0076-6879(83)91052-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund-Katz S., Ibdah J. A., Letizia J. Y., Thomas M. T., Phillips M. C. A 13C NMR characterization of lysine residues in apolipoprotein B and their role in binding to the low density lipoprotein receptor. J Biol Chem. 1988 Sep 25;263(27):13831–13838. [PubMed] [Google Scholar]
- McFeeters R. F. A manual method for reducing sugar determinations with 2,2'-bicinchoninate reagent. Anal Biochem. 1980 Apr;103(2):302–306. doi: 10.1016/0003-2697(80)90614-4. [DOI] [PubMed] [Google Scholar]
- Moldoveanu Z., Moro I., Radl J., Thorpe S. R., Komiyama K., Mestecky J. Site of catabolism of autologous and heterologous IgA in non-human primates. Scand J Immunol. 1990 Dec;32(6):577–583. doi: 10.1111/j.1365-3083.1990.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N. M., Kintzios J. A. Determination of human serum lipoprotein patterns by agarose gel electrophoresis. Anal Biochem. 1969 Sep;30(3):421–426. doi: 10.1016/0003-2697(69)90136-5. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel J. L., Baynes J. W., Thorpe S. R. 125I-glycoconjugate labels for identifying sites of protein catabolism in vivo: effect of structure and chemistry of coupling to protein on label entrapment in cells after protein degradation. Arch Biochem Biophys. 1985 Aug 1;240(2):635–645. doi: 10.1016/0003-9861(85)90071-2. [DOI] [PubMed] [Google Scholar]
- Strobel J. L., Cady S. G., Borg T. K., Terracio L., Baynes J. W., Thorpe S. R. Identification of fibroblasts as a major site of albumin catabolism in peripheral tissues. J Biol Chem. 1986 Jun 15;261(17):7989–7994. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Yedgar S., Carew T. E., Pittman R. C., Beltz W. F., Steinberg D. Tissue sites of catabolism of albumin in rabbits. Am J Physiol. 1983 Jan;244(1):E101–E107. doi: 10.1152/ajpendo.1983.244.1.E101. [DOI] [PubMed] [Google Scholar]