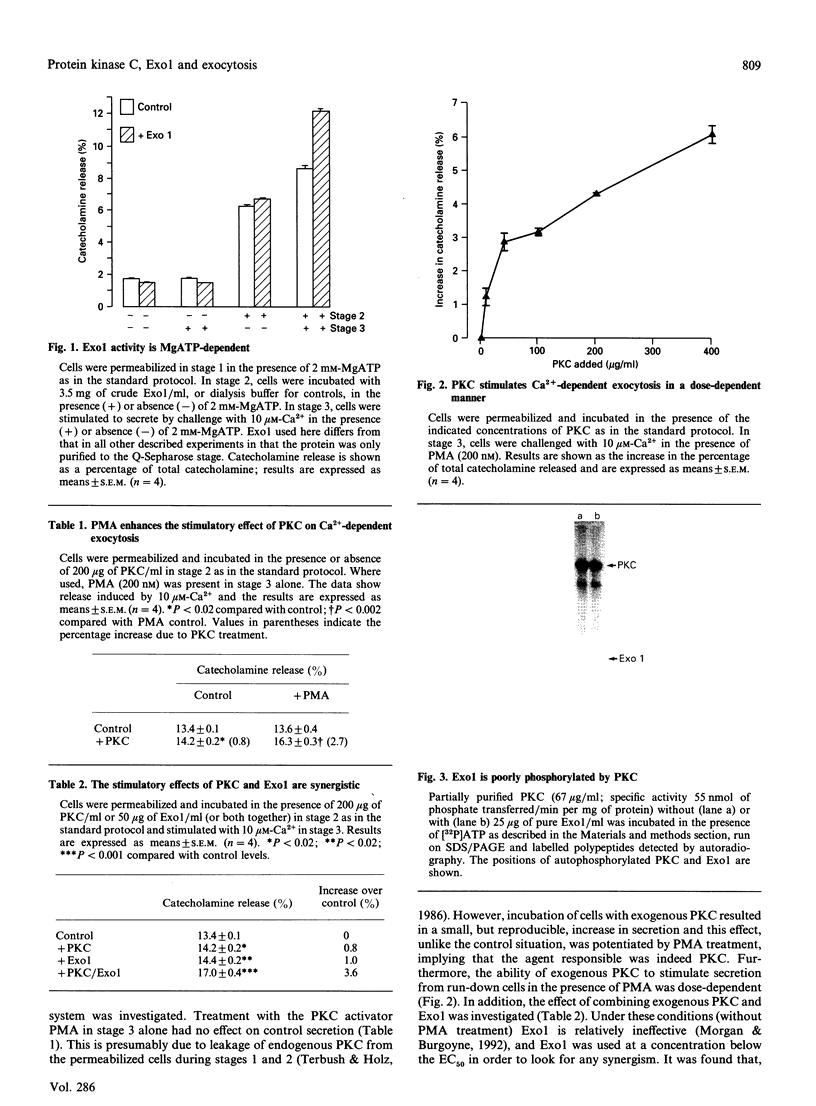
Abstract
The roles of protein kinase C (PKC) and Exo1 in exocytosis from digitonin-permeabilized adrenal chromaffin cells were explored by using exogenous purified proteins in a run-down/reconstitution system. The stimulatory action of Exo1 on exocytosis from run-down cells was found to be completely dependent on the continuous presence of exogenous MgATP, suggesting that it acts on the slow phase of exocytosis [Holz, Bittner, Peppers, Senter & Eberhard (1989), J. Biol. Chem. 264, 5412-5419]. Partially purified rat brain PKC was found to be able to stimulate Ca(2+)-dependent exocytosis from run-down cells in a dose-dependent manner. This effect was indeed due to PKC and not a contaminant in the PKC fraction, since the PKC activator phorbol 12-myristate 13-acetate (PMA), under conditions in which control secretion was not affected, potentiated the effect of the exogenous PKC in stimulating secretion. Furthermore, although either PKC or Exo1 alone could stimulate exocytosis from run-down cells, the effect of combining the fractions was synergistic, as had previously been observed using PMA treatment combined with Exo1 incubation [Morgan & Burgoyne (1992) Nature (London) 355, 833-836]. The observed synergy between PKC and Exo1 was not due to PKC-mediated phosphorylation of Exo1, and Exo1 was found not to affect PKC activity in enzyme assays. We conclude that PKC and Exo1 act synergistically in the slow phase of Ca(2+)-dependent exocytosis from adrenal chromaffin cells. Furthermore, PKC does not directly affect Exo1, but rather enhances the activity of Exo1 by a putative phosphorylation of another, unidentified, component of the exocytotic machinery which facilitates the action of Exo1 in exocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Ellis C. A., Harris A., Sellers L. A., Toker A. Kinase and neurotransmitters. Nature. 1990 Apr 12;344(6267):594–594. doi: 10.1038/344594a0. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Burgoyne R. D. The stimulatory effect of calpactin (annexin II) on calcium-dependent exocytosis in chromaffin cells: requirement for both the N-terminal and core domains of p36 and ATP. Cell Signal. 1990;2(3):265–276. doi: 10.1016/0898-6568(90)90054-e. [DOI] [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Aunis D., Bader M. F. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988 Sep;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):83–103. doi: 10.1098/rstb.1981.0174. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo H., Sigmund O., Stabel S., Reiss N., Naor Z. Preferential release of catecholamine from permeabilized PC12 cells by alpha- and beta-type protein kinase C subspecies. Biochem J. 1991 Nov 15;280(Pt 1):65–69. doi: 10.1042/bj2800065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Evidence for a role of calpactin in calcium-dependent exocytosis. Biochem Soc Trans. 1990 Dec;18(6):1101–1104. doi: 10.1042/bst0181101. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. The control of cytoskeletal actin and exocytosis in intact and permeabilized adrenal chromaffin cells: role of calcium and protein kinase C. Cell Signal. 1989;1(4):323–334. doi: 10.1016/0898-6568(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Burgoyne R. D. Cyclic AMP inhibits both nicotine-induced actin disassembly and catecholamine secretion from bovine adrenal chromaffin cells. J Biol Chem. 1987 Aug 25;262(24):11663–11666. [PubMed] [Google Scholar]
- Greenberg A., Zinder O. Alpha- and beta-receptor control of catecholamine secretion from isolated adrenal medulla cells. Cell Tissue Res. 1982;226(3):655–665. doi: 10.1007/BF00214792. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Bittner M. A., Peppers S. C., Senter R. A., Eberhard D. A. MgATP-independent and MgATP-dependent exocytosis. Evidence that MgATP primes adrenal chromaffin cells to undergo exocytosis. J Biol Chem. 1989 Apr 5;264(10):5412–5419. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Sugden D., Baker P. F. Evidence implicating protein kinase C in exocytosis from electropermeabilized bovine chromaffin cells. J Membr Biol. 1988 Aug;104(1):21–34. doi: 10.1007/BF01871899. [DOI] [PubMed] [Google Scholar]
- Lee S. A., Holz R. W. Protein phosphorylation and secretion in digitonin-permeabilized adrenal chromaffin cells. Effects of micromolar Ca2+, phorbol esters, and diacylglycerol. J Biol Chem. 1986 Dec 25;261(36):17089–17098. [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Naor Z., Dan-Cohen H., Hermon J., Limor R. Induction of exocytosis in permeabilized pituitary cells by alpha- and beta-type protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4501–4504. doi: 10.1073/pnas.86.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocotte S. L., Frye R. A., Senter R. A., TerBush D. R., Lee S. A., Holz R. W. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Natl Acad Sci U S A. 1985 Feb;82(3):930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., Bader M. F. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J Biol Chem. 1987 Dec 5;262(34):16671–16676. [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Effects of phorbol esters, diglyceride, and cholinergic agonists on the subcellular distribution of protein kinase C in intact or digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Dec 25;261(36):17099–17106. [PubMed] [Google Scholar]
- Toker A., Ellis C. A., Sellers L. A., Aitken A. Protein kinase C inhibitor proteins. Purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Eur J Biochem. 1990 Jul 31;191(2):421–429. doi: 10.1111/j.1432-1033.1990.tb19138.x. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]



