Abstract
The dose dependence of the effect of enzyme inducers and the effect of the combined administration of two inducers that exert their effect via the same induction pathway (pregnane X receptor) have not been well studied. Using oral midazolam microdoses (30 μg), we have investigated CYP3A4 induction by St. John's wort (SJW) in 11 healthy volunteers using low (300 mg/day containing 7.48 mg hyperforin), therapeutic (900 mg/day), and supratherapeutic doses of SJW (1800 mg/day) for 14 days. SJW was then co‐administered with rifampin (600 mg/day) for a further 7 days to evaluate the effect of the combined administration of two inducers. In addition, intravenous midazolam microdoses (10 μg) were administered before SJW, at SJW 1800 mg/day, and during administration of the two inducers to assess the hepatic contribution to total induction (semi‐simultaneous administration). Administration of SJW increased oral midazolam clearance 1.96‐fold (300 mg/day), 3.86‐fold (900 mg/day), and 5.62‐fold (1800 mg/day), and 17.5‐fold after the addition of rifampin. Concurrently, the clearance of intravenous midazolam increased 2.05‐fold (1800 mg/day) and 2.93‐fold (SJW + rifampin). These results show that rifampin significantly enhances the induction of the highest SJW doses both hepatically and overall and suggest that these metabolic effects occur predominantly in the gut. These findings also suggest that in drug interactions involving strong and moderate enzyme inducers, the perpetrator effects of the strong inducer are decisive for the interaction.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
An increasing number of drugs that can activate the pregnane X receptor (PXR) are on the market. These drugs have the potential to increase the expression of important pharmacokinetic targets such as cytochrome P450 (CYP) isozymes and drug transporters, thereby substantially altering the clearance of co‐administered substrates. Limited evidence in humans suggests that PXR induction is dose‐dependent and that the extent of induction can vary depending on the inducer. Little is known about the co‐administration of two inducers acting through the same pathway.
WHAT QUESTION DID THIS STUDY ADDRESS?
Our aim was to characterize the dose‐dependent effect of St. John's wort (SJW) on oral clearance of microdosed midazolam as a marker for CYP3A4 activity and to investigate the effect of adding a strong inducer (rifampin) to the highest SJW dose. In addition, we assessed the contribution of hepatic clearance to total midazolam clearance at the highest SJW dose and during its combination with rifampin.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This is the first trial thoroughly characterizing the dose dependence of CYP3A4 induction by SJW in humans. It revealed that the clearance increases occur dose‐dependently, predominantly extrahepatically, and likely at the level of the gut. The trial also revealed that the co‐administration of rifampin further increased midazolam clearance to values known from trials that analyzed rifampin induction without SJW.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The findings of this trial indicate that if a moderate inducer (SJW) and a strong inducer (rifampin) acting via the same PXR pathway are combined, the perpetrator effects of the stronger inducer dominate.
INTRODUCTION
Phenotyping the in vivo activity of drug‐metabolizing enzymes such as cytochrome P450 (CYP) isozymes is an important technique that enables (a) the screening and quantitative assessment of the potential of a drug to induce pharmacokinetic drug interactions during drug development, (b) the investigation of the effects of medical conditions on the drug elimination capacity of the human body, and (c) the development of patient‐specific strategies for precision dosing of drugs with a narrow therapeutic index whose clearance (CL) is CYP‐dependent. 1 Hence, the development and refinement of techniques that facilitate phenotyping, such as limited‐sampling strategies (LSS), use of endogenous biomarkers, phenotyping cocktails, and microdosing, are important as they allow to phenotype more vulnerable populations more often and in a more economical way. 1 , 2 , 3
Midazolam is the paradigm marker substrate recommended by the European Medicines Agency and the US Food and Drug Administration (FDA) for measuring the activity of the CYP3A isozyme. 4 , 5 Microdoses as low as 10 ng can be used to characterize pharmacokinetic drug interactions. 6 , 7 Midazolam microdoses can be administered orally to measure apparent oral CL (CL/F) or intravenously (i.v.) to measure systemic CL (CLsys). 6 , 7 We have used these techniques in the past for repetitive phenotyping to assess the effect of time on complex drug interactions 7 , 8 , 9 or to phenotype critically ill patients suffering from hematological tumors. 10 Up to now, these trials investigated the effect of CYP inhibition. We have recently characterized the time course of induction by rifampin with a continuous infusion of microdosed midazolam. 7 However, with the more common oral or i.v. bolus administration of midazolam microdoses, the effects of CYP3A induction on the pharmacokinetics of midazolam are still unclear.
Depending on the preparation, St. John's wort (SJW) is a strong inducer of CYP3A activity, which can lead to severe drug–drug interactions, 11 for which its active ingredient hyperforin is mainly responsible. 12 Similarly, also rifampin is a strong inducer, and both hyperforin and rifampin act by binding to the intracellular pregnane X receptor (PXR). 13 Mechanistically, the induction of CYP3A4 is the result of the binding of a small molecule to the ligand‐binding pocket of PXR, which in turn leads to the translocation of PXR from the cytoplasm to the nucleus where two PXR form a heterotetramer with two retinoic X receptors (RXR). Binding of the PXR/RXR heterotetramer to a DNA‐binding domain leads to the recruitment cascade of co‐factors that enhances the transcriptional activity and results in increased CYP3A4 gene expression. 14 Although both hyperforin (molecular weight: 536.8 Da) 15 and the larger rifampin (822.9 Da) 16 bind to PXR, their binding properties differ and the binding of the larger rifampin can displace the walls of the PXR ligand pocket. 13 , 17 However, rifampin's binding to the PXR binding pocket is more than 20‐fold weaker (EC50 0.71 μM) than the binding of hyperforin (0.032 μM), 18 suggesting that rifampin may not further enhance induction in the presence of hyperforin.
In this clinical trial in healthy volunteers, we used both oral and i.v. midazolam microdoses in a semi‐simultaneous fashion 19 , 20 to evaluate the dose‐dependent effect of SJW on CYP3A activity and its modification by a second paradigm inducer rifampin.
MATERIALS AND METHODS
Regulatory
The study protocol was approved by the Competent Authority in Germany (BfArM, clinical trial registration: EudraCT No: 2013‐004374‐10 registered on Oct. 10, 2013) and the responsible Ethics Committee of the Medical Faculty of Heidelberg University. It was conducted at the DIN EN ISO9001‐certified Early Clinical Trial Unit (KliPS) of Internal Medicine IX, Department of Clinical Pharmacology and Pharmacoepidemiology in accordance with the standards of Good Clinical Practice (as defined in the ICH E6 Guideline for Good Clinical Practice) and in agreement with the Declaration of Helsinki and the specific legal requirements in Germany.
Study participants
All study participants gave their written informed consent for the study and for the storage of DNA in a biobank for the genotyping of metabolic enzymes before any study measures were carried out. They were physically and mentally healthy as confirmed by the medical history, a physical examination, a 12‐lead electrocardiogram, and routine laboratory analyses including kidney and liver function tests, hematology, glucose, urinalysis, urinary screening for illicit drugs, and pregnancy testing (women of childbearing potential). Female participants had to agree to use two highly effective contraceptive methods. After the conclusion of the trial, the participants were genotyped for CYP3A5 as previously described 6 ; one carried *1/*3 alleles (active enzyme), nine were poor metabolizers (*3/*3), and of two participants no DNA was available.
Study conduct
The study was carried out as an open clinical trial with a fixed‐sequence design without washout phases (Figure 1). The sequence consisted of a baseline assessment of CYP3A activity followed by CYP3A induction with 300 mg SJW (Jarsin®300, Casella‐med, Cologne, Germany; average hyperforin content 7.48 mg/dose unit 21 ) once daily for 14 days (SJW 300). The SJW dose was then increased to 3 × 300 mg daily for 14 days (SJW 900), followed by a further increase to 3 × 600 mg daily for 14 days (SJW 1800). This dose was then maintained for a further 7 days together with 600 mg rifampin once daily (Eremfat®, Riemser Arzneimittel AG, Greifswald, Germany). To assess total and hepatic CYP3A activity, a fixed‐sequence approach with “semi‐simultaneous” oral and subsequent i.v. administration of midazolam was carried out as described earlier, 20 but with microdoses instead of pharmacologically active doses. On three separate study days (baseline, SJW 1800, SJW 1800 + rifampin 600), participants received 30 μg of midazolam solution orally and 6 h later 10 μg of i.v. midazolam over 5 min (Dormicum®V 5 mg/5 mL solution for injection, Roche, Grenzach‐Wyhlen, Germany). Oral medication was self‐administered by the participants, except on days with pharmacokinetics, when all doses were administered on‐site. Blood samples were taken before drug administration and at 15, 30, 45 min and 1, 1.5, 2, 2.5, 3, 4, 5, and 6 h after oral administration of midazolam and at 15, 30, 40, 50 min and 1, 1.25, 1.5, 2, 3, 4, 6, and 8 h after the start of the i.v. administration of midazolam. On the study days of SJW 300 and SJW 900, only a 30‐μg oral dose was administered and a LSS was used taking five blood samples (pre‐dose, 2, 2.5, 3, and 4 h). 22 The blood samples were centrifuged at 2500g at 4°C, the samples were stored at −20°C until analysis.
FIGURE 1.
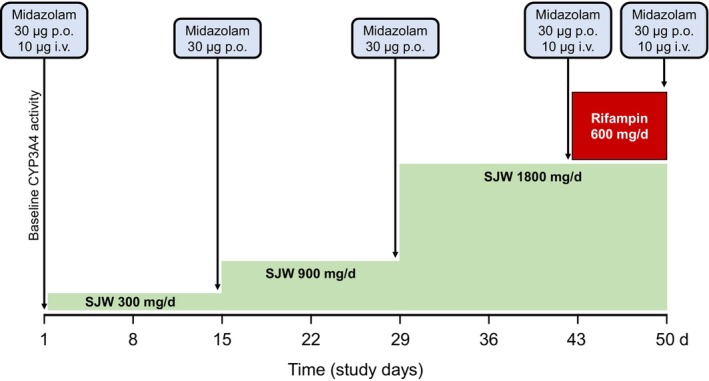
Study design with four consecutive induction phases using 300, 900, and 1800 mg St. John's wort (SJW, green) daily each for 14 days, and combining 1800 mg SJW daily with 600 mg rifampin (red) daily for 7 days. CYP3A activity was determined on five study days with the respective midazolam doses indicated.
Midazolam quantification
Midazolam concentrations in plasma were determined by a validated UPLC‐MS/MS method as previously described. 23 The assay's lower limit of quantification was 0.37 pg/mL and the accuracy/precision values were 96%–99%/≤12.0%.
Pharmacokinetic calculations
Standard non‐compartmental pharmacokinetic parameters of midazolam were calculated using PKanalix (Lixoft Inc, Antony, France) 24 : On days with full pharmacokinetic profiles, the maximum plasma concentration of midazolam (C max) and the time to reach C max (t max) were taken directly from the measurements. The terminal elimination half‐life (t 1/2) was calculated from the plasma concentration–time data up to 8 h after i.v. administration by determining ln 2/λ, where λ is the terminal slope calculated by linear regression of the time versus log concentration data. The area under the plasma concentration–time curve (AUC∞) after oral administration was calculated using the linear‐up log‐down trapezoidal rule and the λ value of the i.v. administration was used to extrapolate the area from 6 h to infinity. The AUC∞ after i.v. administration was calculated as AUC from the start of i.v. administration minus the extrapolated AUC, attributable to the oral dose (AUC6‐∞ po). Similarly, for the definition of C max after i.v. administration, the extrapolated concentration originating from the preceding oral administration was subtracted from the measured C max value. CL/F and CLsys were calculated using the appropriate AUCs. Bioavailability (F) was calculated on the basis of the AUC∞ of oral midazolam and the AUC∞ of i.v. midazolam minus AUC6–∞ po. For the evaluation of the total CYP3A activity on all 5 study days, the midazolam metabolic CL (eCLmet) was estimated by the previously described LSS methodology using the AUC from 2 to 4 h (AUC2‐4) after oral administration. 22 To estimate the relative contribution of extrahepatic midazolam metabolism to total CL (eCLmet), we subtracted CLsys, which is thought to primarily reflect hepatic CL, and expressed the geometric mean values of the resulting estimated intestinal CL as a percentage of eCLmet.
Statistics
Data are presented as geometric mean with 95% confidence intervals (95% CI) unless stated otherwise. Corrected for multiple comparisons using the Sidak test, a repeated measures ANOVA on log‐transformed data was applied to test for differences between inducing substances and baseline. The relationship between SJW dose and CYP3A induction was analyzed using the classical dose–response equation with variable slope and the minimum (E min) fixed to baseline CYP3A activity (Hill equation). The relationship between AUC2‐4 and AUC∞ of midazolam was analyzed using linear regression analysis. All statistical calculations were performed with Prism 9.4 (GraphPad Software, La Jolla, CA, USA), and a p‐value <0.05 was considered significant.
RESULTS
Study participants
Twelve participants (six females) were included but one female participant dropped out during treatment with 1800 mg SJW due to adverse effects (allodynia, erythrodermia), an observation which was reported earlier. 25 Therefore, 11 participants (age: 26 ± 4 years; weight: 71 ± 19 kg; body mass index: 23.8 ± 5.3 kg m2) completed the study.
Midazolam pharmacokinetics
Repeated administration of 1800 mg SJW substantially decreased midazolam exposure after oral and i.v. administration (Figure 2; Table 1) and enhanced midazolam CL 2.04‐fold after i.v. and 4.62‐fold after oral administration. Concurrently, also t 1/2 was reduced and the absolute F of midazolam decreased from 35.1% at baseline to 15.6%.
FIGURE 2.
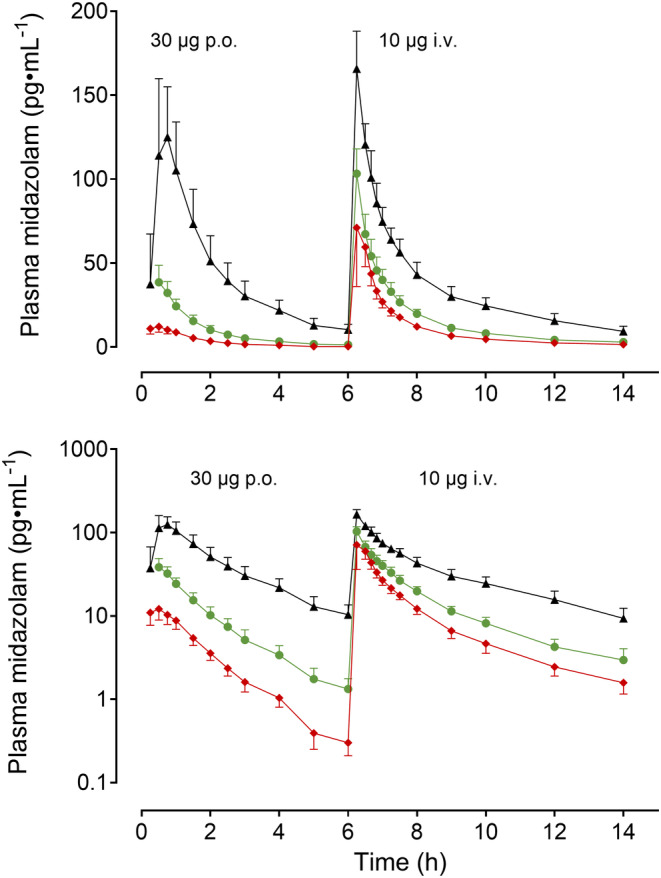
Geometric mean (95% confidence interval) midazolam plasma concentrations (upper panel: Linear concentrations; lower panel: Log concentrations) after 30 μg oral midazolam and 10 μg intravenous midazolam (administered 6 h after oral midazolam) at baseline (black triangles), during 1800 mg St. John's wort (green dots), and during 1800 mg St. John's wort plus 600 mg rifampin (red diamonds) in 11 healthy volunteers.
TABLE 1.
Midazolam pharmacokinetic parameters after 30 μg oral midazolam and 10 μg intravenous midazolam (6 h after oral midazolam) at baseline, at 1800 mg St. John's wort, and 1800 mg St. John's wort plus 600 mg rifampin in 11 healthy volunteers.
| Baseline | SJW 1800 | SJW 1800 + RIFAMPIN 600 | |||
|---|---|---|---|---|---|
| GM (95% CI) | GM (95% CI) | GMR (90% CI) | GM (95% CI) | GMR (90% CI) | |
| 30 μg midazolam orally | |||||
| C max (pg mL−1) | 138.6 (109–177) | 41.7* (31.7–54.9) | 0.3008 (0.2448–0.3696) | 14.0*,# (10.9–17.9) | 0.1006 (0.0846–0.1196) |
| AUC0–6 (h pg mL−1) | 257.6 (205–324) | 60.7* (48.7–75.5) | 0.2354 (0.1980–0.2798) | 20.4*,# (16.3–25.4) | 0.0790 (0.0689–0.0905) |
| AUC∞ (h pg mL−1) | 303.1 (237.0–387.7) | 65.63* (52.55–81.97) | 0.2165 (0.1814–0.2584) | 21.45# (17.08–26.93) | 0.0707 (0.0614–0.0815) |
| CL/F (mL min−1) | 1649 (1290–2109) | 7619* (6100–9515) | 4.620 (3.871–5.514) | 23,312*,# (18,564–29,275) | 14.14 (12.28–16.28) |
| V z/F (L) | 422 (351–507) | 1612* (1195–2173) | 3.819 (3.111–4.688) | 4767*,# (3848–5905) | 11.30 (9.433–13.53) |
| F (%) | 35.1 (30.0–41.2) | 15.6* (12.7–19.2) | 0.4437 (0.3735–0.5271) | 7.28*,# (5.70–9.29) | 0.2070 (0.1704–0.2516) |
| 10 μg midazolam i.v. | |||||
| C max (pg mL−1) | 155.3 (137–177) | 101.9* (89.2–117) | 0.6562 (0.6013–0.7161) | 91.3* (72.6–115) | 0.5880 (0.5082–0.6804) |
| AUC∞ (h pg mL−1) | 334 (283–394) | 145* (128–166) | 0.4348 (0.3839–0.4926) | 99.4*,# (83.6–118) | 0.2974 (0.2569–0.3443) |
| Corr AUC∞ (h pg mL−1) | 287 (249–332) | 140* (124–159) | 0.4880 (0.4336–0.5492) | 98.2*,# (82.6–117) | 0.3417 (0.2956–0.3949) |
| t 1/2 (h) | 2.97 (2.61–3.37) | 2.45+ (2.03–2.95) | 0.8248 (0.7276–0.9350) | 2.35* (2.10–2.62) | 0.7908 (0.7093–0.8816) |
| CLsys (mL min−1) | 580 (503–669) | 1188* (1046–1348) | 2.049 (1.821–2.305) | 1697# (1426–2019) | 2.927 (2.533–3.382) |
| V z (L) | 148 (132–167) | 251* (214–296) | 1.694 (1.533–1.872) | 347*,# (297–405) | 2.340 (2.036–2.688) |
Abbreviations: AUC0–6, area under the concentration–time curve from zero to 6 h; AUC∞, area under the concentration–time curve extrapolated to infinity; Corr AUC∞, AUC after intravenous administration corrected for the AUC contribution from the preceding oral administration; CI, confidence interval; CLsys, systemic clearance; CL/F, apparent oral clearance; C max, peak plasma concentration; F, absolute bioavailability; GM, geometric mean; GMR, geometric mean ratio; SJW, St. John's Wort; t 1/2, terminal elimination half‐life; V z , volume of distribution; V z/F, apparent volume of distribution.
*p < 0.01 versus baseline; + p < 0.05 versus baseline; # p < 0.01 versus SJW 1800.
The addition of 600 mg rifampin to high SJW doses for 7 days further increased midazolam CL 1.43‐fold i.v. (2.93‐fold compared with baseline) and 3.06‐fold orally (14.1‐fold compared with baseline) (Table 1). The effect of the two inducers was several times more pronounced after oral administration of midazolam than after i.v. administration, with CL values being 1.43‐fold higher after i.v. and 3.06‐fold higher after oral administration.
The contribution of extrahepatic metabolism to midazolam CL was estimated by subtracting CLsys from eCLmet. In the absence of inducers, the estimated intestinal contribution to total midazolam CL (eCLmet) was 29.8%, increased to 73.5% with 1800 mg SJW, and reached 88.0% with the combined rifampin and SJW treatment.
Midazolam LSS
To evaluate the validity of the LSS in a state of CYP3A induction, we analyzed the relationship between AUC2‐4 and AUC∞ during the treatment with 1800 mg SJW and 1800 mg SJW plus 600 mg rifampin using a log–log regression analysis. A slope of 1.11 (95% CI: 1.08–1.21) (R 2 = 0.975) confirms the applicability of the abbreviated AUC methodology (Figure 3).
FIGURE 3.
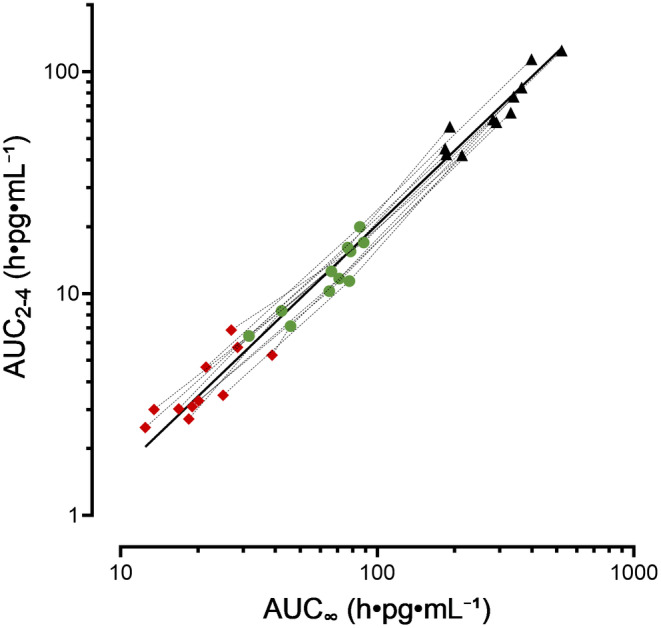
Relationship between AUC2‐4 and AUC∞ of oral midazolam at baseline (black triangles), during treatment with 1800 mg SJW (green dots), during combined administration of 1800 mg SJW plus 600 mg rifampin (red diamonds) analyzed using a log–log linear regression analysis (R 2 = 0.9753).
Using LSS data, the effect of ascending doses of SJW on total CYP3A activity (CLmet of midazolam) was evaluated. All SJW doses reduced midazolam exposure and increased its partial metabolic CL (Table 2, individual changes are shown in Figure S1). The addition of rifampin to the highest SJW dose further increased midazolam CL 3.11‐fold (Figure 4; Table 2). Exploratory nonlinear regression analysis of the three different SJW doses and the individual increase of eCLmet suggests a dose close to the highest dose used required for half‐maximum induction (ED50 = 1720 mg) and a maximum possible induction of 1039% (=11.4‐fold increase in eCLmet; Figure S1). Analysis of the individual SJW dose and eCLmet increase showed convergence in seven out of 11 study participants (Figures S2 and S3). The single participant carrying the CYP3A5*1/*3 genotype had eCLmet values very similar to the population average.
TABLE 2.
Geometric mean (95% confidence interval) of AUC2‐4 and partial metabolic clearance of midazolam after oral administration of 30 μg oral midazolam at baseline, at 300, 900, and 1800 mg St. John's wort, and at 1800 mg St. John's wort plus 600 mg rifampin in 11 healthy volunteers.
| Treatment | Duration (days) | AUC2‐4 (h pg mL−1) | eCLmet (mL min−1) | GMR (90% CI) of eCLmet |
|---|---|---|---|---|
| Baseline | – | 65.7 (51.2–84.3) | 843 (657–1081) | – |
| 300 mg/day St. John's wort | 14 | 33.6* (26.3–42.8) | 1649* (1290–2104) | 1.957 (1.661–2.305) |
| 900 mg/day St. John's wort | 14 | 17.0*,# (13.9–20.9) | 3257*,# (2653–3999) | 3.864 (3.393–4.400) |
| 1800 mg/day St. John's wort | 14 | 11.7*,# (9.14–15.0) | 4739*,# (3705–6061) | 5.621 (4.815–6.562) |
| 1800 mg/day St. John's wort + 600 mg/day rifampin | 7 | 3.76*,# (3.00–4.71) | 14,752*,# (11,766–18,495) | 17.50 (15.41–19.87) |
Note: Test versus baseline: *p < 0.001; Test versus next lower St. John's Wort dose: # p < 0.001.
Abbreviations: AUC2‐4, area under the concentration–time curve from 2 to 4 h; CI, confidence interval; eCLmet, estimated midazolam partial metabolic clearance; GMR, geometric mean ratio.
FIGURE 4.
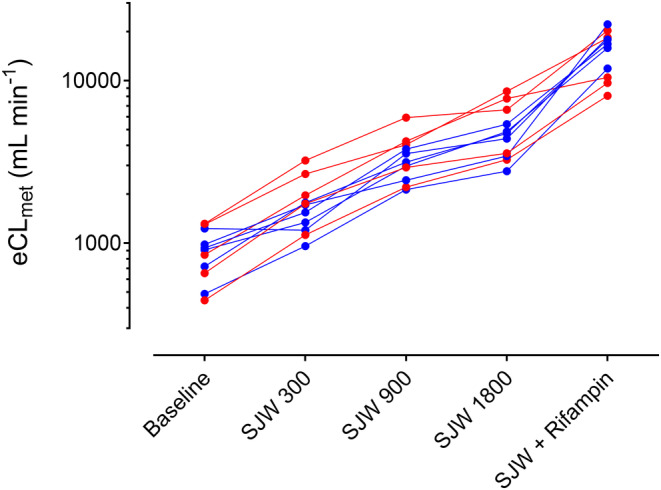
Individual estimated metabolic midazolam clearance (eCLmet) in relation to different induction conditions (baseline; SJW 300 = St. John's wort 300 mg for 14 days; SJW 900 = 900 mg for 14 days; SJW 1800 = 1800 mg for 14 days; SJW + Rifampin = 1800 mg SJW together with 600 mg rifampin for 7 days), calculated by means of the limited‐sampling strategy in 11 healthy volunteers (female = red symbol; male = blue symbol).
Adverse events
Of 12 participants enrolled, one dropped out due to allodynia and erythrodermia, 25 two had no adverse events, and 11 had 1–5 adverse events (hypophosphatemia (n = 6), paresthesia (4), headache (2), nausea (2), vomiting (1), myalgia (1), hyperpigmentation (1), drop in hemoglobin (2), and pruritus (1)). All adverse events were mild‐to‐moderate in severity and transient. No adverse event was serious.
DISCUSSION
The data acquired in this clinical pharmacokinetic experiment in healthy volunteers provide insights into three areas: First, we have addressed important methodological aspects. It has been shown earlier 22 that the midazolam AUC obtained with an LSS after oral administration closely correlates with an extended pharmacokinetic profile. Our findings now (i) confirmed that this is also the case after administration of a microdose in the absence of a perpetrator. Our trial further showed (ii) that also during profound enzyme induction of the key metabolizing enzyme (CYP3A4), AUC2‐4 correlates well with AUC∞. In this study, each dose of the paradigm inducer SJW was administered for 14 days, which has been shown to be certainly long enough to reach an induction steady state. 26 After induction of CYP3A activity with SJW administered in ascending doses, the AUC ratios correspond well with values reported in earlier trials that used the same SJW brand. 26 , 27 As an example, in our trial, eCLmet increased 3.86 times during daily administration of 900 mg SJW whereas the fold increase with a regular midazolam dose was 4.22. 26 We further evaluated (iii) how well the semi‐simultaneous method first reported by Lee and co‐workers, 19 which allows us to estimate CLsys, CL/F, and absolute F, works with midazolam microdoses. The results were comparable with the data reported by others 19 , 28 and suggest that microdoses can also be used in this way.
Second, in this experiment, we provide insights into the dose dependence of SJW on PXR‐mediated CYP3A induction. It has previously been shown that induction of different SJW formulations correlates with their content in hyperforin but not hypericin, 29 , 30 and our trial revealed that subtherapeutic SJW doses (300 mg q.d.) approximately doubled midazolam eCLmet and higher doses further but less than linearly increased eCLmet suggesting that a plateau will be reached. This is in line with observations with other PXR activators such as rifapentine 31 or trials with rifampin that have shown that autoinduction is saturable 32 and maximum induction is already reached at daily doses of 300–600 mg. 33
The trial clearly confirmed that also induction by SJW is dose‐dependent and estimates that the maximum increase in midazolam metabolism is approximately elevenfold, but requires doses outside the approved range and beyond good tolerance. While the induction by rifampin exceeded this estimated maximum achievable by SJW (11.4‐fold increase) by ~50% (17.5‐fold increase of eCLmet from baseline) and occurred in all participants (Figure 4), this result should be interpreted with caution as it is based on extrapolated values from only three observations per participant. In vitro, maximum effects of hyperforin were similar (PXR activation 34 ), higher (CYP3A4 promoter activation 35 ), or lower than the effects of rifampin (gene expression, 36 CY3A4 RNA expression 37 ). In contrast, in vivo, the effect on the exposure of paradigm CYP3A4 substrates (ciclosporin, 38 nifedipine, 39 tacrolimus 40 ) was substantially more pronounced with rifampin than with SJW (ciclosporin, 41 nifedipine, 42 tacrolimus 43 ), thus well matching the results of this trial. The reason for this apparent discrepancy is not known; considering that the molecular weight of hyperforin is 536.8 g/Mol, the molar concentration of hyperforin was 83.8 μM in the 200 mL drinking water, which is ~9‐fold lower than the corresponding molar concentrations of rifampin (729 μM, molecular weight: 822.9 g/Mol) and for both compounds exceeds EC50 of their binding to PXR by orders of magnitude. It appears therefore unlikely that the numerically higher rifampin concentrations will displace relevant amounts of the more tightly bound hyperforin from PXR. Much more likely is that free rifampin concentrations in blood and tissue 44 exceed free hyperforin concentrations by orders of magnitude.
Estimation of the intestinal contribution of enzyme induction to overall metabolism revealed that the induction was predominantly due to an increase in intestinal CL. Our calculation may even slightly underestimate the true contribution of intestinal metabolism, as a small proportion of circulating midazolam is also cleared extrahepatically. 45
Interaction trials thoroughly evaluating the combined effect of two enzyme‐inducing agents are rare. A trial with therapeutic doses of bosentan in healthy volunteers revealed that the bosentan‐induced (auto)induction of CYP3A4 could be further increased by 47% by adding the same dose (900 mg/day) and brand of SJW as administered in this trial. 46 This was also observed with rifampin, which also increased the (auto)induction of therapeutic bosentan doses, 47 but not beyond a range observed with supratherapeutic bosentan doses alone. 48 In another trial in healthy volunteers, 900 mg SJW of another brand had no effect on carbamazepine‐induced (auto)induction 49 but reduced exposure to single carbamazepine doses by 21% in the absence of autoinduction. 50
When comparing the dose‐normalized data of an earlier rifampin–midazolam interaction trial with our microdose trial, similar results were obtained. 51 Thus, these results suggest that the combination of the two PXR activators did not result in a stronger induction than rifampin alone and that the maximal induction differs between the two compounds. In addition to the possibility that this is a consequence of different binding properties at the corresponding PXR pocket, 13 , 17 it could also be caused by modifications further downstream of the signaling cascade that modify PXR translocation from the cytoplasm into the nucleus 52 or by post‐translational changes of the hinge region of PXR 53 that alter the interaction with the DNA.
The findings of this trial have several implications. First, this study clearly shows that the extent of CYP3A4 induction induced by SJW does not only depend on the specific brand and its hyperforin content 29 but also on the administered dose. Although this could be anticipated from in vitro studies, 15 this has not been thoroughly investigated before and only assessed with two doses of hypericum powder. 54 Our trial also showed that the maximum CYP3A4 induction is not reached with the maximum recommended dose of this brand (900 mg/day), suggesting that administration of brands with higher hyperforin content (e.g., Neuroplant®, Dr. Willmar Schwabe GmbH, Karlsruhe, Germany, hyperforin content 12.43 mg/300 mg 21 ) will cause even stronger induction and more pronounced drug–drug interactions. The results of interaction studies with the highest approved dose of a certain SJW brand are therefore not readily transferable to other phytotherapeutic generics because their composition can differ considerably. This also shows that it is important to report the specific phytotherapeutic brand administered together with its content of relevant constituents.
Second, this trial also suggests that compounds that induce CYP via the same mechanism (PXR) may differ significantly in the magnitude of induction they exert and that SJW is a weaker inducer than rifampin despite the higher affinity of hyperforin to the ligand‐binding pocket. Whether this is due to fundamental differences in the activation of the PXR cascade needs to be clarified in vitro. Together with the results of our trial, these data indicate that SJW is less potent inducer than rifampin, carbamazepine, and bosentan, and suggest that when SJW is combined with these inducers, the effects of the stronger inducers will determine the magnitude of induction.
Finally, the pharmacokinetic changes observed with microdoses of midazolam correspond well with the changes observed when regular doses were administered with SJW, suggesting that microdoses reflect the interaction well even in an induced state. In addition, the close correlation with abbreviated sampling protocols suggests that the LSS can be used also for this purpose.
The limitations of this trial are (i) the short duration of rifampin treatment, which was, however, considered long enough to achieve maximum induction, 55 (ii) the absence of a control group receiving rifampin alone, and (iii) that no information on the effect of rifampin on the pharmacokinetics and thus exposure of hyperforin was obtained, which is a substrate of the CYP2C and CYP3A enzyme families. 56 Finally, (iv) a change in the volume of distribution of midazolam after i.v. administration was noted in our trial, which has not been observed previously 57 and is most likely due to a decrease in serum albumin levels from 46.0 g/L (95% CI: 44.6–47.5) at screening to 41.4 g/L (39.5–43.3; p < 0.0001) at the end of the study, which may significantly increase the volume of distribution of midazolam 58 and is likely due to the withdrawal of slightly more than 400 mL of blood during the trial.
In conclusion, we have shown in this trial in healthy volunteers that the inducing effect of SJW is dose‐dependent and can be substantially enhanced by the addition of rifampin, suggesting that the two inducers may differ mechanistically. At the same time, we have shown that the pharmacokinetics of midazolam microdoses also reflect the pharmacokinetic changes during the administration of CYP3A inducers and compare well with the known changes reported after the administration of regular doses of midazolam.
AUTHOR CONTRIBUTIONS
N.H., G.M., and W.E.H. wrote the manuscript. N.H., G.M., and W.E.H. designed the research. N.H., A.S.F., A.B., and G.M. performed the research. G.M., J.B., and N.H. analyzed the data.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Figures S1‐S3
ACKNOWLEDGMENTS
The authors would like to thank the staff of the Early Clinical Trial Unit (KliPS) and the Analytical Chemistry Laboratory of Internal Medicine IX, Department of Clinical Pharmacology and Pharmacoepidemiology, Heidelberg University Hospital, for their excellent support in conducting this trial. For the publication fee, we acknowledge financial support from Heidelberg University. All authors declare no financial or nonfinancial interest in the subject matter or materials discussed in this manuscript. Open Access funding enabled and organized by Projekt DEAL.
Hohmann N, Friedrichs AS, Burhenne J, Blank A, Mikus G, Haefeli WE. Dose‐dependent induction of CYP3A activity by St. John's wort alone and in combination with rifampin. Clin Transl Sci. 2024;17:e70007. doi: 10.1111/cts.70007
Gerd Mikus and Walter E. Haefeli contributed equally to this manuscript.
REFERENCES
- 1. Hohmann N, Haefeli WE, Mikus G. CYP3A activity: towards dose adaptation to the individual. Expert Opin Drug Metab Toxicol. 2016;12:479‐497. doi: 10.1517/17425255.2016.1163337 [DOI] [PubMed] [Google Scholar]
- 2. Lappin G, Garner RC. The utility of microdosing over the past 5 years. Expert Opin Drug Metab Toxicol. 2008;4:1499‐1506. doi: 10.1517/17425250802531767 [DOI] [PubMed] [Google Scholar]
- 3. Lappin G, Kuhnz W, Jochemsen R, et al. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther. 2006;80:203‐215. doi: 10.1016/j.clpt.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 4. EMA . Guideline on the investigation of drug interactions. 2012. Accessed June 20, 2024. https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐investigation‐drug‐interactions‐revision‐1_en.pdf
- 5. U.S. Food & Drug Administration . Drug development and drug interactions|table of substrates, inhibitors and inducers. 2023. Accessed June 20, 2024. https://www.fda.gov/drugs/drug‐interactions‐labeling/drug‐development‐and‐drug‐interactions‐table‐substrates‐inhibitors‐and‐inducers
- 6. Halama B, Hohmann N, Burhenne J, Weiss J, Mikus G, Haefeli WE. A nanogram dose of the CYP3A probe substrate midazolam to evaluate drug interactions. Clin Pharmacol Ther. 2013;93:564‐571. doi: 10.1038/clpt.2013.27 [DOI] [PubMed] [Google Scholar]
- 7. Nassar YM, Hohmann N, Michelet R, et al. Quantification of the time course of CYP3A inhibition, activation, and induction using a population pharmacokinetic model of microdosed midazolam continuous infusion. Clin Pharmacokinet. 2022;61:1595‐1607. doi: 10.1007/s40262-022-01175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hohmann N, Reinhard R, Schnaidt S, et al. Treatment with rilpivirine does not alter plasma concentrations of the CYP3A substrates tadalafil and midazolam in humans. J Antimicrob Chemother. 2016;71:2241‐2247. doi: 10.1093/jac/dkw125 [DOI] [PubMed] [Google Scholar]
- 9. Breithaupt MH, Krohmer E, Taylor L, et al. Time course of CYP3A activity during and after metamizole (dipyrone) in healthy volunteers. Br J Clin Pharmacol. 2023;89:2458‐2464. doi: 10.1111/bcp.15720 [DOI] [PubMed] [Google Scholar]
- 10. Hohmann N, Halama B, Siller N, Mikus G, Haefeli WE. Response to “can CYP3A activity be evaluated for drug interaction using a nanogram dose of probe drug?”: evaluation of CYP3A activity with microdoses of midazolam. Clin Pharmacol Ther. 2014;95:490‐491. doi: 10.1038/clpt.2014.28 [DOI] [PubMed] [Google Scholar]
- 11. Yamazaki H, Nakajima M, Nakamura M, et al. Enhancement of cytochrome P‐450 3A4 catalytic activities by cytochrome b(5) in bacterial membranes. Drug Metab Dispos. 1999;27:999‐1004. [PubMed] [Google Scholar]
- 12. Gutmann H, Poller B, Buter KB, Pfrunder A, Schaffner W, Drewe J. Hypericum perforatum: which constituents may induce intestinal MDR1 and CYP3A4 mRNA expression? Planta Med. 2006;72:685‐690. doi: 10.1055/s-2006-931585 [DOI] [PubMed] [Google Scholar]
- 13. Chrencik JE, Orans J, Moore LB, et al. Structural disorder in the complex of human pregnane X receptor and the macrolide antibiotic rifampicin. Mol Endocrinol. 2005;19:1125‐1134. doi: 10.1210/me.2004-0346 [DOI] [PubMed] [Google Scholar]
- 14. Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45:60‐72. doi: 10.3109/03602532.2012.746363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore LB, Goodwin B, Jones SA, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500‐7502. doi: 10.1073/pnas.130155097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Raymond K. Roles of rifampicin in drug‐drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob. 2006;5:3. doi: 10.1186/1476-0711-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin W, Huber AD, Poudel S, et al. Structure‐guided approach to modulate small molecule binding to a promiscuous ligand‐activated protein. Proc Natl Acad Sci USA. 2023;120:e2217804120. doi: 10.1073/pnas.2217804120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ngan CH, Beglov D, Rudnitskaya AN, Kozakov D, Waxman DJ, Vajda S. The structural basis of pregnane X receptor binding promiscuity. Biochemistry. 2009;48:11572‐11581. doi: 10.1021/bi901578n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JI, Chaves‐Gnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF. Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther. 2002;72:718‐728. doi: 10.1067/mcp.2002.129068 [DOI] [PubMed] [Google Scholar]
- 20. Hafner V, Jäger M, Matthée AK, et al. Effect of simultaneous induction and inhibition of CYP3A by St John's wort and ritonavir on CYP3A activity. Clin Pharmacol Ther. 2010;87:191‐196. doi: 10.1038/clpt.2009.206 [DOI] [PubMed] [Google Scholar]
- 21. Wurglics M, Westerhoff K, Kaunzinger A, et al. Comparison of German St. John's wort products according to hyperforin and total hypericin content. J Am Pharm Assoc. 2001;41:560‐566. doi: 10.1016/s1086-5802(16)31280-3 [DOI] [PubMed] [Google Scholar]
- 22. Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther. 2011;90:666‐673. doi: 10.1038/clpt.2011.164 [DOI] [PubMed] [Google Scholar]
- 23. Burhenne J, Halama B, Maurer M, et al. Quantification of femtomolar concentrations of the CYP3A substrate midazolam and its main metabolite 1′‐hydroxymidazolam in human plasma using ultra performance liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402:2439‐2450. doi: 10.1007/s00216-011-5675-y [DOI] [PubMed] [Google Scholar]
- 24. PKanalix version 2020R1. 2020. Accessed June 20, 2024. http://lixoft.com/products/PKanalix/
- 25. Hohmann N, Maus A, Carls A, Haefeli WE, Mikus GS. John's wort treatment in women bears risks beyond pharmacokinetic drug interactions. Arch Toxicol. 2016;90:1013‐1015. doi: 10.1007/s00204-015-1532-7 [DOI] [PubMed] [Google Scholar]
- 26. Markert C, Kastner IM, Hellwig R, et al. The effect of induction of CYP3A4 by St John's wort on ambrisentan plasma pharmacokinetics in volunteers of known CYP2C19 genotype. Basic Clin Pharmacol Toxicol. 2015;116:423‐428. doi: 10.1111/bcpt.12332 [DOI] [PubMed] [Google Scholar]
- 27. Huppertz A, Werntz L, Meid AD, et al. Rivaroxaban and macitentan can be coadministered without dose adjustment but the combination of rivaroxaban and St John's wort should be avoided. Br J Clin Pharmacol. 2018;84:2903‐2913. doi: 10.1111/bcp.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikus G, Heinrich T, Bödigheimer J, et al. Semisimultaneous midazolam administration to evaluate the time course of CYP3A activation by a single oral dose of efavirenz. J Clin Pharmacol. 2017;57:899‐905. doi: 10.1002/jcph.879 [DOI] [PubMed] [Google Scholar]
- 29. Mueller SC, Majcher‐Peszynska J, Uehleke B, et al. The extent of induction of CYP3A by St. John's wort varies among products and is linked to hyperforin dose. Eur J Clin Pharmacol. 2006;62:29‐36. doi: 10.1007/s00228-005-0061-3 [DOI] [PubMed] [Google Scholar]
- 30. Zahner C, Kruttschnitt E, Uricher J, et al. No clinically relevant interactions of St. John's wort extract Ze 117 low in hyperforin with cytochrome P450 enzymes and P‐glycoprotein. Clin Pharmacol Ther. 2019;106:432‐440. doi: 10.1002/cpt.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keung A, Reith K, Eller MG, McKenzie KA, Cheng L, Weir SJ. Enzyme induction observed in healthy volunteers after repeated administration of rifapentine and its lack of effect on steady‐state rifapentine pharmacokinetics: part I. Int J Tuberc Lung Dis. 1999;3:426‐436. [PubMed] [Google Scholar]
- 32. Svensson RJ, Aarnoutse RE, Diacon AH, et al. A population pharmacokinetic model incorporating saturable pharmacokinetics and autoinduction for high rifampicin doses. Clin Pharmacol Ther. 2018;103:674‐683. doi: 10.1002/cpt.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohnhaus EE, Brockmeyer N, Dylewicz P, Habicht H. The effect of antipyrine and rifampin on the metabolism of diazepam. Clin Pharmacol Ther. 1987;42:148‐156. doi: 10.1038/clpt.1987.125 [DOI] [PubMed] [Google Scholar]
- 34. Kandel BA, Ekins S, Leuner K, Thasler WE, Harteneck C, Zanger UM. No activation of human pregnane X receptor by hyperforin‐related phloroglucinols. J Pharmacol Exp Ther. 2014;348:393‐400. doi: 10.1124/jpet.113.209916 [DOI] [PubMed] [Google Scholar]
- 35. Kim S, Dinchuk JE, Anthony MN, et al. Evaluation of cynomolgus monkey pregnane X receptor, primary hepatocyte, and in vivo pharmacokinetic changes in predicting human CYP3A4 induction. Drug Metab Dispos. 2010;38:16‐24. doi: 10.1124/dmd.109.029637 [DOI] [PubMed] [Google Scholar]
- 36. Xiao L, Nickbarg E, Wang W, et al. Evaluation of in vitro PXR‐based assays and in silico modeling approaches for understanding the binding of a structurally diverse set of drugs to PXR. Biochem Pharmacol. 2011;81:669‐679. [DOI] [PubMed] [Google Scholar]
- 37. Watkins RE, Maglich JM, Moore LB, et al. 2.1 a crystal structure of human PXR in complex with the St. John's wort compound hyperforin. Biochemistry. 2003;42:1430‐1438. doi: 10.1021/bi0268753 [DOI] [PubMed] [Google Scholar]
- 38. Hebert MF, Roberts JP, Prueksaritanont T, Benet LZ. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther. 1992;52:453‐457. doi: 10.1038/clpt.1992.171 [DOI] [PubMed] [Google Scholar]
- 39. Holtbecker N, Fromm MF, Kroemer HK, Ohnhaus EE, Heidemann H. The nifedipine‐rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos. 1996;24:1121‐1123. [PubMed] [Google Scholar]
- 40. Hebert MF, Fisher RM, Marsh CL, Dressler D, Bekersky I. Effects of rifampin on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol. 1999;39:91‐96. doi: 10.1177/00912709922007499 [DOI] [PubMed] [Google Scholar]
- 41. Mai I, Bauer S, Perloff ES, et al. Hyperforin content determines the magnitude of the St John's wort‐cyclosporine drug interaction. Clin Pharmacol Ther. 2004;76:330‐340. doi: 10.1016/j.clpt.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 42. Wang XD, Li JL, Su QB, et al. Impact of the haplotypes of the human pregnane X receptor gene on the basal and St John's wort‐induced activity of cytochrome P450 3A4 enzyme. Br J Clin Pharmacol. 2009;67:255‐261. doi: 10.1111/j.1365-2125.2008.03344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hebert MF, Park JM, Chen YL, Akhtar S, Larson AM. Effects of St. John's wort (Hypericum perforatum) on tacrolimus pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2004;44:89‐94. doi: 10.1177/0091270003261078 [DOI] [PubMed] [Google Scholar]
- 44. Asaumi R, Toshimoto K, Tobe Y, et al. Comprehensive PBPK model of rifampicin for quantitative prediction of complex drug‐drug interactions: CYP3A/2C9 induction and OATP inhibition effects. CPT Pharmacometrics Syst Pharmacol. 2018;7:186‐196. doi: 10.1002/psp4.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paine MF, Shen DD, Kunze KL, et al. First‐pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14‐24. doi: 10.1016/S0009-9236(96)90162-9 [DOI] [PubMed] [Google Scholar]
- 46. Markert C, Ngui P, Hellwig R, et al. Influence of St. John's wort on the steady‐state pharmacokinetics and metabolism of bosentan. Int J Clin Pharmacol Ther. 2014;52:328‐336. doi: 10.5414/CP202048 [DOI] [PubMed] [Google Scholar]
- 47. van Giersbergen PL, Treiber A, Schneiter R, Dietrich H, Dingemanse J. Inhibitory and inductive effects of rifampin on the pharmacokinetics of bosentan in healthy subjects. Clin Pharmacol Ther. 2007;81:414‐419. doi: 10.1038/sj.clpt.6100075 [DOI] [PubMed] [Google Scholar]
- 48. Dingemanse J, van Giersbergen PL. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet. 2004;43:1089‐1115. doi: 10.2165/00003088-200443150-00003 [DOI] [PubMed] [Google Scholar]
- 49. Burstein AH, Horton RL, Dunn T, Alfaro RM, Piscitelli SC, Theodore W. Lack of effect of St John's wort on carbamazepine pharmacokinetics in healthy volunteers. Clin Pharmacol Ther. 2000;68:605‐612. doi: 10.1067/mcp.2000.111530 [DOI] [PubMed] [Google Scholar]
- 50. Burstein AH, Piscitelli SC, Alfaro RM, Theodore W. Effect of St John's wort on carbamazepine single‐dose pharmacokinetics. Epilepsia. 2001;42(Suppl 7):253. [Google Scholar]
- 51. Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59:7‐13. doi: 10.1016/S0009-9236(96)90018-1 [DOI] [PubMed] [Google Scholar]
- 52. Sugatani J, Uchida T, Kurosawa M, et al. Regulation of pregnane X receptor (PXR) function and UGT1A1 gene expression by posttranslational modification of PXR protein. Drug Metab Dispos. 2012;40:2031‐2040. doi: 10.1124/dmd.112.046748 [DOI] [PubMed] [Google Scholar]
- 53. Pasquel D, Doricakova A, Li H, et al. Acetylation of lysine 109 modulates pregnane X receptor DNA binding and transcriptional activity. Biochim Biophys Acta. 2016;1859:1155‐1169. doi: 10.1016/j.bbagrm.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mueller SC, Uehleke B, Woehling H, et al. Effect of St John's wort dose and preparations on the pharmacokinetics of digoxin. Clin Pharmacol Ther. 2004;75:546‐557. doi: 10.1016/j.clpt.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 55. Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819‐850. doi: 10.2165/00003088-200342090-00003(56) [DOI] [PubMed] [Google Scholar]
- 56. Hokkanen J, Tolonen A, Mattila S, Turpeinen M. Metabolism of hyperforin, the active constituent of St. John's wort, in human liver microsomes. Eur J Pharm Sci. 2011;42:273‐284. doi: 10.1016/j.ejps.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 57. Wang Z, Gorski JC, Hamman MA, Huang SM, Lesko LJ, Hall SD. The effects of St John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70:317‐326. [PubMed] [Google Scholar]
- 58. Vree TB, Shimoda M, Driessen JJ, et al. Decreased plasma albumin concentration results in increased volume of distribution and decreased elimination of midazolam in intensive care patients. Clin Pharmacol Ther. 1989;46:537‐544. doi: 10.1038/clpt.1989.182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S3


