Abstract
In the past, hydrogen sulfide (H2S) was recognized as a toxic and dangerous gas; in recent years, with increased research, we have discovered that H2S can act as an endogenous regulatory transmitter. In mammals, H2S‐catalyzing enzymes, such as cystathionine‐β‐synthase, cystathionine‐γ‐lyase, and 3‐mercaptopyruvate sulfurtransferase, are differentially expressed in a variety of tissues and affect a variety of biological functions, such as transcriptional and posttranslational modification of genes, activation of signaling pathways in the cell, and metabolic processes in tissues, by producing H2S. Various preclinical studies have shown that H2S affects physiological and pathological processes in the body. However, a detailed systematic summary of these roles in health and disease is lacking. Therefore, this review provides a thorough overview of the physiological roles of H2S in different systems and the diseases associated with disorders of H2S metabolism, such as ischemia–reperfusion injury, hypertension, neurodegenerative diseases, inflammatory bowel disease, and cancer. Meanwhile, this paper also introduces H2S donors and novel release modes, as well as the latest preclinical experimental results, aiming to provide researchers with new ideas to discover new diagnostic targets and therapeutic options.
Keywords: antioxidant, apoptosis, cancer, hydrogen sulfide, inflammation
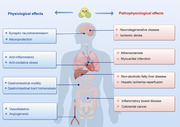
1. INTRODUCTION
Over the years, hydrogen sulfide (H2S) has been known for its rotten egg‐like odor, toxicity, and environmental hazard. The toxicity of H2S is mainly due to the inhibition of cytochrome c oxidase (COX) in mitochondria, leading to chemical asphyxia of cells. 1 , 2 COX is a vital electron transmitter in the respiratory chain, which participates in cellular respiration. Its activity is inhibited, which reduces oxygen utilization in mitochondria, leading to cell hypoxia. 3 , 4 In recent years, human understanding of H2S has gradually shifted from toxic substances to gas transmitters with therapeutic drug potential. In 1989, H2S was proven to exist in the human brain and may play a certain physiological role. 5 In 1996, Japanese scientists demonstrated that H2S is a potential signaling molecule that can be produced by cystathionine‐β‐synthase (CBS) and is involved in neurotransmission. 6 The following year, they discovered that cystathionine‐γ‐lyase (CSE) is another enzyme that produces. 7 Subsequently, Wang et al. 8 confirmed that H2S is the third physiological signaling molecule, except for carbon monoxide (CO) and nitric oxide (NO). Since then, the field of sulfide research has developed rapidly, and the research results have become richer; in a 2005 paper, Blackstone et al. 9 reported in a pioneering manner that H2S can induce a reversible pseudo‐death‐like state in mice. They hypothesized that H2S‐mediated induction of pseudo‐death may have beneficial medical applications, such as ischemia–reperfusion injury (IRI) or organ preservation after trauma. 9
In addition to the cytoprotective effects shown in IRI, H2S can act as an endogenous regulatory transmitter in other physiological states, including vasodilation, neuronal activity, gastrointestinal motility, and so forth. 10 , 11 , 12 , 13 , 14 , 15 A few pathologies are also strongly associated with H2S, such as the IRIs mentioned above, neurodegenerative diseases (ND), pain, inflammatory bowel disease (IBD), and cancer. 16 , 17 , 18 , 19 For exogenous H2S therapy, the manner of H2S delivery is important. At present, the main methods of H2S delivery include direct inhalation and H2S donor. Yet, all these delivery methods have certain limitations. Although recent studies have identified many small molecule H2S donors, they still perform poorly regarding stability, solubility, targeting, and half‐life. Nowadays, the main way to constitute an H2S delivery system is to combine H2S donors with polymers by covalent linkage and physical entrapment. The delivery systems optimize the therapeutic efficacy with higher stability and bioavailability compared with small molecule H2S donors.
In this article, we will focus on the role of H2S in human health and disease. The multiple physiological and pathophysiologic functions of H2S will be discussed systemically and comprehensively. We will delve into the novel H2S donors in anticipation of expanding new therapeutic ideas for researchers.
2. PHYSICOCHEMICAL PROPERTIES, PRODUCTION, AND METABOLISM OF H2S
2.1. General physicochemical properties of H2S
H2S is a colorless and highly toxic flammable gas with a unique rotten egg or sewage odor. Its molecular weight is 34.08, and its vapor density is heavier than air, making it easier to diffuse at lower points. 10 , 20 As a binary weak acid, hydrosulfuric acid is an aqueous solution of H2S that can reach dissociation equilibrium at room temperature (25°C). The solution concentration in a saturated state is 0.11 mol dm−3, and its pH value is approximately 4.0. At 37°C and pH 7.4, pK α1 = 6.76, there is about 20% H2S, 80% HS − and a small amount of negligible S2‐ in extracellular fluid. 21 At the same time, H2S is also a small gas molecule with high lipophilicity, which allows it to freely pass through the lipid bilayer structure of the cell membrane. 22 H2S is a compound similar to water molecules that can be oxidized into sulfur dioxide, sulfate, thiosulfate, and elemental sulfur. In the body, H2S can be oxidized to sulfates and thiosulfates, excreted in the urine. Some studies suggest that urinary thiosulfates can serve as one of the biomarkers of H2S poisoning. 20 , 23
2.2. Production of endogenous H2S
In mammalian cells, enzyme catalysis and nonenzyme catalysis are two ways to produce endogenous H2S. Some studies have shown that enzyme catalysis is the main production route, and CBS, CSE, and 3‐mercaptopyruvate sulfurtransferase (3‐MST) are the three key enzymes of this route. 24 , 25 , 26
Both CBS and CSE use pyridoxal phosphate (also known as vitamin B6) as cofactors, and their concentrations vary in different tissues. 27 , 28 CBS mainly exists in the central nervous system (CNS) (cerebellum, hippocampus) and liver tissue. 29 CSE mainly regulates H2S in the cardiovascular system and respiratory system. 15 Only present in the cytoplasm, these two enzymes catalyze the conversion of homocysteine to cysteine, generating H2S through the reverse sulfur conversion pathway. Research has found that 3‐MST is an enzyme involved in endogenous H2S production. 30 Unlike the two enzymes mentioned earlier, the cofactor of 3‐MST is zinc. 20 It often cocatalyzes with cysteine aminotransferase (CAT) in mitochondria to produce H2S, L‐cysteine, and α‐Ketoglutaric acid generates 3‐mercaptopyruvate (3‐MP) under the catalysis of CAT, and then generates H2S and pyruvic acid under the action of 3‐MST. 31 In 2013, Japanese scientists proposed a new enzyme catalysis pathway. 32 This pathway occurs in the peroxisome. d‐Amino acid oxidase catalyzes d‐cysteine to produce 3‐MP, and then the product is transported to mitochondria through vesicles to participate in the next reaction. 33 , 34 , 35 Endogenous H2S enzymatic generation pathway (as shown in Figure 1). Some studies have found that when the human body is under oxidative stress or hyperglycemia, the H2S produced through nonenzyme catalysis will significantly increase. In red blood cells, the reduction equivalent produced by glucose oxidation can be utilized to reduce elemental sulfur or polysulfides to H2S. 20
FIGURE 1.
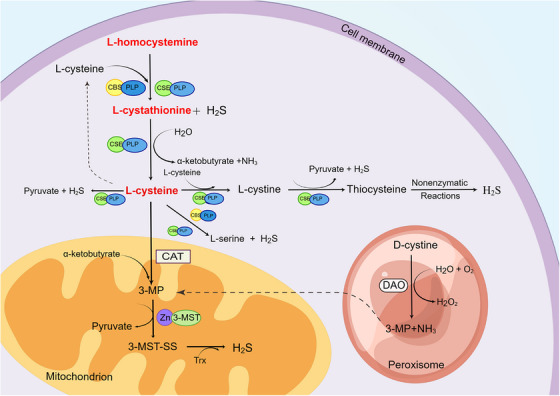
Enzyme catalysis of H2S production. Endogenous H2S can be produced by two ways: enzyme catalysis and nonenzyme catalysis. Enzyme catalysis is the main way and is catalyzed by four enzymes, such as CBS, CSE, 3‐MST and DAO. By Figdraw. H2S, hydrogen sulfide; CBS, cystathionine β‐synthase; CSE, cystathionine γ‐lyase; PLP, pyridoxal‐5′‐phosphate; 3‐MST, 3‐mercaptopyruvate sulfurtransferase; 3‐MP, 3‐methylpyridine; CAT, cysteine aminotransferase; DAO, D‐amino acid oxidase.
2.3. Metabolism of endogenous H2S
In mammals, the excretion of H2S varies in different systems. In the respiratory tract, H2S is directly excreted as gaseous molecules. Through the urinary tract, H2S is mainly excreted as thiosulfate or free sulfate in the urine. However, in the gastrointestinal tract, most H2S is still excreted as free sulfide in the feces. 20 H2S mainly has the following three metabolic pathways: (1) The elimination of H2S through the mitochondrial sulfide oxidation pathway, with sulfide quinone oxidoreductase (SQOR) being the key enzyme in this reaction (as shown in Figure 2). First, H2S is oxidized to thiosulfate under the catalysis of SQOR. 36 , 37 In this reaction, the primary sulfur acceptor is glutathione (GSH), and the resulting product is glutathione persulfide (GSSH). 38 , 39 In the next step of the reaction, rhodanese (or thiosulfate sulfurtransferase) plays a crucial role as a sulfur transferase that can further oxidize thiosulfate to sulfite or sulfate. 40 , 41 However, due to the rapid oxidation of sulfite to sulfate, H2S is ultimately expelled from the body in the form of thiosulfate or sulfate through this pathway. 20 , 41 (2) Research has found that methylation occurring in the cytoplasm is another metabolic pathway for H2S. 26 Thiol‐S‐methyltransferase (TSMT) can catalyze the conversion of H2S to methyl mercaptan and dimethyl sulfide. TSMT is commonly present in cells in the human body but has high activity in mucosal cells of the colon and cecum. 42 Compared with the sulfide oxidation pathway, the metabolic process of sulfide methylation is slower. In a study, the rate of sulfide methylation in mammalian colon mucosal cells was approximately 10,000 times slower than the oxidation rate of H2S. 43 (3) H2S can be cleared by methemoglobin, metal‐containing, or sulfur‐containing macromolecules. Methemoglobin and myoglobin can promote the binding of H2S and iron by regulating the reactivity of iron, accelerating the oxidation rate of H2S. 44
FIGURE 2.
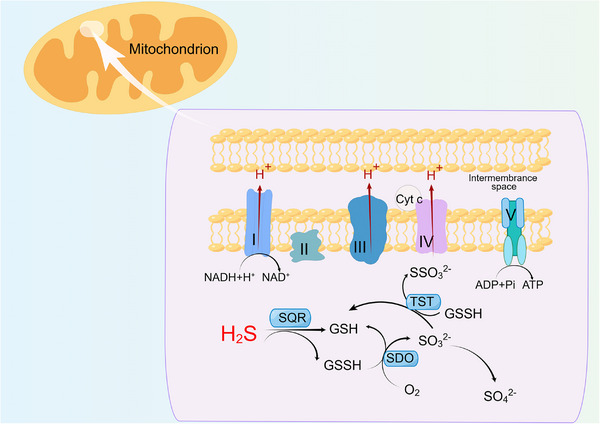
The oxidation pathway of endogenous H2S. The H2S oxidation pathway in mitochondria is mainly catalyzed by sulfuroquinone oxidoreductase. Finally, H2S is discharged from the body in the form of Thiosulfate or sulfate through this pathway. By Figdraw. ADP, adenosine diphosphate; ATP, adenosine triphosphate; Cyt c, cytochrome c; GSH, glutathione; GSSH, glutathione disulfide; H2S, hydrogen sulfide; NADH, nicotinamide adenine dinucleotide; SQR, sulfur quinone oxidoreductase; SDO, sulfide dioxygenase; TST, rhodanese.
3. PHYSIOLOGICAL FUNCTIONS OF H2S
3.1. H2S and cardiovascular regulation
Initially, H2S was thought to have a similar role to NO in relaxing blood vessels. As it has been studied more intensively, H2S can maintain endothelial cell function by several mechanisms, such as increasing NO bioavailability and stabilizing the translation of endothelial nitric oxide synthase (eNOS). 45 , 46 Early researchers found that H2S can cause concentration‐dependent vasodilation by opening adenosine triphosphate (ATP)‐sensitive potassium (KATP) channels in vascular smooth muscle and decreasing extracellular calcium (Ca2+) in flow. 47 Later experimental results have shown that glibenclamide can partially inhibit this vasodilation. Nevertheless, the molecular mechanism of how endogenous H2S activates KATP channels remains unclear, and recent studies may fill this gap. It was found that the KATP protein Kir6.1 undergoes S‐Sul hydration when CSE is overexpressed, and another study found that Kir6.1, which undergoes S‐sulfation at the Cys43 site, reduces the synthesis of ATP and enhances the function of the KATP channel, promoting vasodilation. 48 , 49 Meanwhile, H2S has been investigated as a possible endothelium‐derived relaxing factor, which combines with eNOS to produce S and NO that can activate transient receptor potential (TRP) ankyrin‐ 1 channels, leading to hyperpolarization and vasodilation in endothelial cells and smooth muscle cells. 49 Therefore, H2S has potential therapeutic possibilities for targeting diseases of the vascular system, but more in‐depth mechanistic studies are still needed to determine this. In addition, various mechanisms have been implicated in H2S‐induced vasodilation, such as the protein kinase G (PKG) pathway, Cl−/HCO3 − channel, and TRP channel. 50 , 51 , 52
H2S also plays a vital role in angiogenesis. Angiogenesis is an essential physiological process in mammals that maintains normal life activities, and it consists of several successive stages, among which the migration of vascular endothelial cells is most important. In earlier studies, researchers found that CSE inhibitors reduced the length of blood vessels in chicken chorioallantoic membrane. 53 In another study, exogenous administration of sodium hydrosulfide (NaHS) promoted endothelial cell proliferation and migration, which may be related to the phosphoinositide 3‐kinase (PI3K)/protein kinase B (Akt) signaling pathway. 54 However, these results were not strong evidence that endogenous H2S regulates angiogenesis. The researchers then did several more studies, and they found that mice knocked down for CSE had more rapid angiogenesis and wound healing in response to vascular endothelial growth factor (VEGF) stimulation compared with wild‐type mice. 53 In terms of mechanisms, a growing number of studies have found that both H2S and NO play important roles in angiogenesis. 55 For example, H2S and NO share a common downstream molecule, silent information regulator‐1 (SIRT1), the activation of which increases the expression levels of VEGF and cyclic guanosine 3′, 5′‐monophosphate (cGMP) and thus participates in the regulation of angiogenesis. In the available reports, the mechanisms by which H2S and NO promote cGMP expression are also different, with H2S degrading cGMP by inhibiting phosphodiesterase 5. However, NO promotes soluble guanylyl cyclase production of the cGMP. 56 As described above, H2S activates Akt phosphorylation and may increase eNOS phosphorylation at the activation site Ser1177. 57 Therefore, H2S is a physiologically important factor in angiogenesis.
3.2. H2S and neuromodulation
In an early study in 1989, researchers serendipitously discovered that H2S could be detected in the brains of rats even when NaHS was not administered and was prevalent in several regions of the brainstem, cerebellum, hippocampus, and striatum. 5 Since then, researchers have studied the interactions between endogenous H2S and the nervous system. In 1996, researchers discovered an interesting phenomenon that endogenous H2S can enhance N‐methyl‐d‐aspartate (NMDA) receptors, thus contributing to learning and memory processes. 6 They have shown that knockdown of CBS or 3‐MST leads to significant impairment of physiological memory formation in mice. 58 Another study showed that direct infusion of various CBS inhibitors into the lateral amygdala impaired long‐term enhancement of this region, which led to inhibition of cued fear memory formation in rats. 59 The concept that H2S participates in synaptic neurotransmission has existed since the early 21st century. 60 , 61 Investigators have demonstrated the involvement of CBS‐derived H2S in nucleus tractus solitarius excitability by using a combination of molecular and pharmacological approaches, providing evidence for the role of endogenous H2S in excitatory neurotransmission. 62 This may indicate that H2S acts presynaptically and involves enhancing intracellular Ca2+ channels. Overall, most of the studies discussed above were performed in vitro using high (millimolar) NaHS concentrations and could not draw definitive conclusions about the potential physiological relevance of these effects. Therefore, future studies that aim to determine the physiological effects of H2S should be conducted using donors in the low micromolar range.
3.3. H2S and gastrointestinal regulation
In the gastrointestinal tract, under physiological conditions, H2S is produced endogenously and exogenously; endogenous is the enzyme origin we mentioned earlier since the two enzymes, CBS and CSE, are present in almost the entire gastrointestinal tract of mammals. 63 Regarding the exogenous production of H2S, it is mainly the microbiota in the gastrointestinal tract responsible, with sulfate‐reducing bacteria being one of the leading producers. Microbial metabolism breaks down proteins in the gastrointestinal tract into amino acids, which include cysteine, H2S, and other sulfur‐containing compounds. 64 , 65 So, does H2S present in the gastrointestinal tract influence gastrointestinal motility? Past studies have given us the answer that the effect of H2S on gastrointestinal motility is nonunique. Generally, in vitro studies show that NaHS inhibits smooth muscle contraction in the gastrointestinal tract of different species. For example, in ex vivo experiments in rabbits, guinea pigs, mice, and rats, NaHS concentration‐dependently inhibited spontaneous and agonist‐induced contractions of the small bowel and colon, and ileal contractions in guinea pigs were slowly augmented using the inhibitor pregnancy‐associated glycoprotein (PAG). 63 , 66 , 67 , 68 , 69 , 70 However, NaHS has a dual effect on spontaneous contractions of gastric smooth muscle in guinea pigs and mice, that is, high concentrations of NaHS inhibit the amplitude of gastric smooth muscle contractions. In contrast, low concentrations increase basal tone. 71 , 72 From the above studies, we can find that the effects of H2S on smooth muscle may vary depending on the species and the location of the digestive tract, so the mechanisms also diverge. The inhibitory effects of H2S reported so far may be related to KATP channels, L‐type voltage‐dependent calcium channels, cGMP/PKG pathway, and sodium channels. 67 , 73 , 74 , 75 , 76
3.4. H2S and inflammation
Among the many controversial areas of H2S research, the role of H2S in inflammatory processes is undoubtedly an example. H2S has been reported to have proinflammatory and anti‐inflammatory effects. 77 , 78 , 79 , 80 , 81 , 82 For example, the upregulation of CSE induced by lipopolysaccharide (LPS) or proinflammatory cytokines and the consequent increase in H2S production can be viewed as a proinflammatory effect or an anti‐inflammatory response as a compensatory protective mechanism. 78 , 83 One study found that injection of the H2S donor NaHS in rats inhibited leukocyte infiltration and adhesion. 84 On the other hand, H2S synthesis inhibitors increase leukocyte adhesion, leukocyte infiltration, and edema formation. In animals suffering from acute lung injury caused by burns and smoke inhalation tissue, tissue interleukin (IL)‐1 levels were decreased, IL‐10 levels were increased in inflamed lungs, and protein oxidation was attenuated after NaHS injection. 82 Experimental evidence suggests that H2S is a proinflammatory factor in various animal models, including acute pancreatitis, LPS‐induced endotoxemia, cecum ligation, and puncture‐induced sepsis. 78 , 85 One possible scenario for the proinflammatory mechanism of H2S is that H2S stimulates sensory nerve endings, releasing endogenous tachykinins such as substance P, calcitonin gene‐related peptide, and neurokinin A, leading to neurogenic inflammation. Many factors are involved in determining whether H2S is proinflammatory or anti‐inflammatory, and the concentration of H2S and route of administration may produce different inflammatory outcomes.
3.5. H2S and aging
The earliest suggestion that the endogenous H2S pathway may be regulated during aging was made by researchers Finkelstein and Benevenga, who found that the metabolic capacity of hepatic homogenates to produce volatile sulfur compounds (H2S and/or methanethiols) from 3‐methylthiopropionate was a product of the metabolism transamination pathway of methionine. All three enzymes, CBS, CSE, and 3‐MST, have been reported to show significant age‐related downregulation in the brains of aging rats. 86 Although some H2S‐producing enzymes may be upregulated in some tissues of aging animal models, plasma H2S levels decline with age in rats and mice; moreover, H2S donation produces organ‐protective and beneficial effects. 87 , 88 , 89 , 90 A large body of experimental evidence suggests that endogenous or exogenous H2S can modulate many core mechanisms implicated in the aging process. These mechanisms include regulation of genome stability, telomere maintenance, epigenetic regulatory mechanisms, mitochondrial function/dysfunction, stem cell depletion, and protein inhibitory processes.
4. PATHOPHYSIOLOGIC ROLE OF H2S IN DISEASE
4.1. H2S and IRI
According to the progression of diseases, IRI can be divided into two stages: ischemia and reperfusion. It is generally believed that the degree of cell dysfunction, injury, and necrosis is related to the severity and duration of ischemia. Therefore, the main idea for treating IRI is to restore blood flow to the ischemic site as soon as possible. 91 However, the sensitivity of different organs to ischemic manifestations also varies, such as the brain, heart, and other organs with poor tolerance to ischemia and hypoxia, and differences in organ tolerance can also affect the degree of cell damage. In addition, although the reperfusion recovery can provide oxygen and nutrients to cells, it will further strengthen the damage after ischemia, activate cell death and immune response, and so on. 92 On the other hand, inflammatory mediators will also be transported to the distal organs with the recovery of reperfusion, which is also the reason for multiorgan failure in the later stage of IRI. 93 , 94 , 95 IRI is a dynamic process with significant organ differences, so a deeper understanding of its molecular mechanisms can help us find better treatment methods.
4.1.1. Ca2+ overload
When ischemia occurs, ATP in cells is rapidly depleted, ATP synthesis decreases, sodium pump activity decreases, intracellular Na+ content increases, and sodium–Ca2+ exchange proteins are activated, leading to reverse transport of Na+ to the extracellular space and an increase in intracellular Ca2+. 96 , 97 On the other hand, due to hypoxia and anaerobic metabolism, the production of H+ increases, and the pH of extracellular fluid and cytoplasm decreases. When tissue perfusion resumes, the pH of extracellular fluid increases, but the pH of cytoplasm is still very low. In order to reduce the accumulation of H+ in cells, H+–Na+ exchange protein and Na+–Ca2+ exchange protein is activated, increasing Ca2+ overload. 96 When the body is in a state of stress, releasing a large amount of catecholamines activates protein kinase C (PKC) through a signaling pathway, promotes H+–Na+ exchange, and also increases intracellular Ca2+. Due to the massive accumulation of Ca2+, the endoplasmic reticulum and mitochondria damage intensifies. The complete opening of the mitochondrial mitochondrial permeability transition pore (mPTP) will have a more negative impact on cells. 98 Figure 3 illustrates the mechanism of Ca2+ overload during the ischemia and reperfusion phases caused by ion exchange.
FIGURE 3.
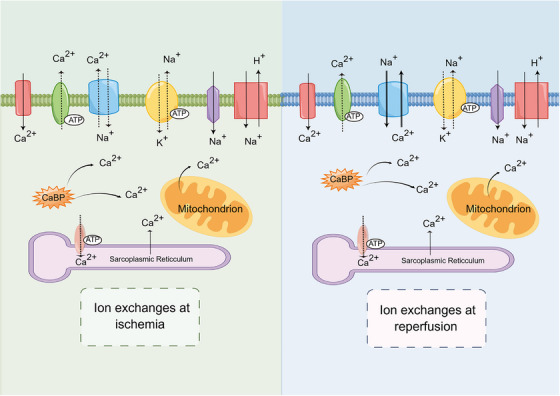
The mechanism of ion exchange leading to Ca2+ overload during IRI. In IRI, abnormalities in Na+–Ca2+ exchange are associated with the following three aspects. First, high intracellular Na+ directly activates natriuretic proteins during ischemic injury. Second, high intracellular H+ decreases pH, which indirectly activates natriuretic proteins. At last, activation of PKC and increased release of catecholamines during reperfusion further promotes Na+–Ca2+ exchange. By Figdraw. CaBP, calcium binding protein; Ca2+, calcium ion; H+, hydrogen ion; Na+, sodium ion; K+, potassium ion.
4.1.2. ROS
When ischemic tissue undergoes reperfusion, blood brings oxygen and nutrients to the tissue. At the same time, due to the low concentration of antioxidants in cells, the production of reactive oxygen species (ROS) increases. In the I/R process of biology, ROS will be produced in many ways, including mitochondrial electron transfer chain (ETC), xanthine oxidase system (XOD), NADPH oxidase system, nitric oxide synthase (NOS), and so on. 99 The first three are related to oxidative stress in multiple organs, such as the heart, brain, lungs, liver, pancreas, kidneys, and gastrointestinal tract. 100 NOS mainly acts as an oxidative stress factor in vascular endothelial cells. 101 During the metabolism of normal mitochondria, the respiratory chain complex on the inner mitochondrial membrane can produce a small amount of ROS. 102 As mentioned earlier, when IRI occurs due to hypoxia, changes in ATP, pH, and Ca2+ overload occur in cells, which can lead to mitochondrial damage and produce more ROS. However, ROS further exacerbates oxidative stress, leading to a vicious cycle of cells. 96 , 102
The XOD system is an important pathway for ROS production. Under ischemia, ATP synthesis is reduced, and xanthine dehydrogenase is converted into XOD. At the same time, ATP degradation products (ADP, AMP, hypoxanthine) accumulate. When reperfusion is resumed, a large amount of oxygen molecules enters the tissue with the blood. XOD catalyzes the conversion of hypoxanthine into xanthine and uric acid, producing a large amount of ROS. 103 The oxygen free radicals generated by this pathway have chemotactic effects, attracting and activating many white blood cells to aggregate. When the tissue resumes its oxygen supply, the activated white blood cells’ oxygen consumption increases sharply, producing a large amount of oxygen free radicals, causing cell damage.
The NOx/Deox family of NADPH oxidase mainly includes seven subtypes, such as Nox‐1, Nox‐2, Nox‐3, Nox‐4, Nox‐5, Duox‐1, and Duox‐2; these enzymes have the ability to produce ROS. 104 Under hypoxic conditions, hypoxia inhibitory factor‐1α. Promote the activation of NOX enzyme, and after reperfusion, cells will release some chemical factors to activate further NADPH oxidases, such as phospholipase A2, tumor necrosis factor‐α (TNF‐α), interleukin‐1 beta (IL‐1β), interferon‐γ, angiotensin II (Ang II), and so on. Overexpression of NADPH oxidase after activation enhances ROS production. 105 106
In addition to the pathways above, NOS is also an important pathway for generating ROS. Tetrahydrobiopterin (BH4) is a cofactor of the NOS enzyme. In IRI, oxidative stress oxidizes BH4 to BH2, decreasing the BH4 cell level and uncoupling NOS, thereby promoting ROS production. 107
4.1.3. Cell death
The IRI process of organisms is dynamic, and the mechanism for producing ROS is also complex. The ROS produced by the above pathways may accumulate in cells during the ischemic stage and inhibit antioxidants. However, after tissue restoration of blood supply, if ischemia is severe, ROS‐induced oxidative stress may further cause cell damage and even cell death. 108
Apoptosis is a process of programmed cell death, mainly caused by Ca2+ overload and ROS activation in IRI. Two cell apoptosis pathways can interact with the endogenous and exogenous apoptosis pathways. The endogenous pathway is also known as the mitochondrial pathway. In the cells injured by ischemia/reperfusion, a large amount of calcium influx and ROS production will cause the opening of mitochondrial mPTP and the activation of apoptosis promoting B‐cell lymphoma‐2 (Bcl‐2) family, increase the permeability of mitochondrial membrane, release cytochrome c into the cytoplasm, and then combine with apoptosis protease activating factor 1 (APAF‐1) to activate Caspase‐9 and form a complex, and then trigger the apoptosis cascade reaction to promote apoptosis. 109 The exogenous pathway, or the death receptor pathway, is mainly activated by death factors or receptors. Important death factors include TNF‐α, Fas ligands, tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand, and tumor necrosis factor‐like cytokine 1A. As mentioned earlier, during IRI, reperfusion cells release TNF‐α and activate the c‐Jun N‐terminal kinase (JNK) pathway to stimulate the production of ROS. The accompanying oxidative stress will further stabilize the phosphorylation of JNK and accelerate cell death. 110 If TNF‐α persistent increase will induce the TNFR‐related death domain (TRADD) to combine with it to synthesize TNF α‐TRADD. TNF α‐TRADD and Fas FADD can induce and activate caspase 8 and 10, then enzymolysis activates downstream caspase 3, 6, and 10, and then starts the cell apoptosis. 111 However, cell apoptosis in IRI is not as typical as the necrosis mentioned below.
Necrotizing apoptosis is also programmed cell death, but its impact on organisms completely differs from previous cell apoptosis. The main characteristics of necrotic apoptosis are cell swelling, organelle disintegration, and leakage of intracellular components, which often cause severe inflammatory reactions in ischemic tissues. 112 , 113 As a regulatory mode of death, the main factors triggering necrotic apoptosis are the interacting serine threonine‐kinase 3 (RIPK3) and mixed lineage kinase‐like domain (MLKL). 114 RIP3 can enable TNF‐α driven cell death changes from apoptosis to necrotic apoptosis. When Caspase‐8 is depleted or the inhibitor of apoptosis protein is deficient, TNF receptor I will promote necrotic apoptosis. 113 The assembly of the necrotic complex is mainly related to RIPK1/RIPK3 interaction and MLKL activation. RIPK3 induces phosphorylation of MLKL, leading to oligomerization and translocation of MLKL to the lobules within the plasma membrane, ultimately increasing plasma membrane permeability and cell death. 114 ROS‐induced DNA damage also promotes the formation of necrotic complexes by activating poly (ADP‐ribose) polymerase (PARP), further accelerating cell death. Due to the close relationship between necrotic apoptosis and the occurrence of inflammation in the human body, understanding the molecular mechanism and pathophysiological significance of necrotic apoptosis remains an essential goal of therapeutic IRI research.
The role of autophagy in IRI is bidirectional, which can both protect cells and disrupt them. Appropriate mitochondrial autophagy can clear partially damaged mitochondria during ischemia and reduce subsequent damage. 115 During the reperfusion phase, the levels of Ca2+ and ROS increase in the cells, while oxidative stress inhibits the activity of rapamycin mechanistic target of rapamycin (mTOR), leading to the formation of UNC51‐like kinase 1 complexes and PI3K III class, which induce autophagy. However, autophagy cannot clear all damaged mitochondria, and when autophagy clearance capacity is exceeded, it can lead to cell death. 116 , 117 Figure 4 provides a detailed description of ROS‐induced cell death mechanism.
FIGURE 4.
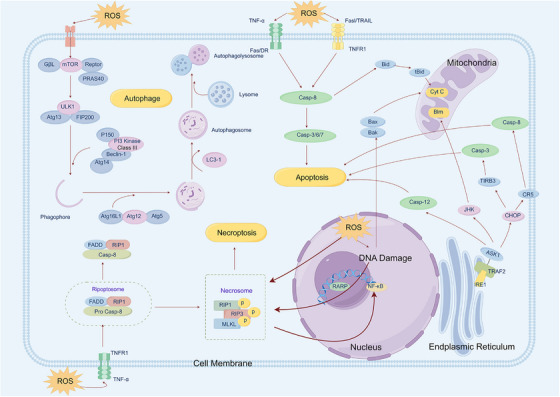
ROS‐induced cell death mechanism. ROS induced oxidative stress may further cause cell damage, even cell death, such as apoptosis, autophagy, and programmed cell death. By Figdraw. APAF‐1, apoptosis protease activating factor 1; Cyt c, cytochrome c; FADD, Fas‐associating protein with a novel death domain; MLKL, mixed lineage kinase like domain; NF‐κB, nuclear factor‐kappa B; RARP, poly(ADP‐ribose) polymerase; ROS, reactive oxygen species; TNF‐α, tumor necrosis factor‐α; TRADD, TNFR‐related death domain.
4.1.4. Inflammation
During reperfusion, producing a large amount of ROS activates the nuclear factor‐κappa B (NF‐κB) gene, further stimulating the secretion of TNF‐α by endothelial cells and macrophages. 118 Conversely, TNF‐α can induce cell apoptosis through a sphingosine‐dependent mechanism. On the other hand, it can also cause leukocyte infiltration in damaged tissues, increase the permeability of vascular endothelial cells, produce a no‐reflow phenomenon, and aggravate reperfusion injury. 119 , 120 At the same time, activated cells release many inflammatory factors, such as IL‐1, IL‐6, IL‐8, IL‐10, IL‐18, and so on. 121
4.1.5. Nephroprotective effects of H2S
Renal IRI is a major predisposing factor for the development and progression of acute kidney injury (AKI). 122 , 123 AKI is a complex clinical syndrome characterized by a rapid decline in renal function, such as decreased glomerular filtration rate (GFR) with increased creatinine and urea nitrogen, water‐electrolyte disturbances, acid–base imbalance, oliguria, or even anuria. 124 , 125 AKI is often associated with severe complications, and the high mortality rate places a significant burden on the healthcare system. 126 Some recent studies have shown that H2S can improve renal function during IRI to prevent AKI, associated with decreased ROS expression. 127 , 128 , 129 In addition to antioxidant effects, H2S may exert renoprotective effects through several other mechanisms. First, H2S can induce vascular relaxation by opening KATP channels in endothelial and renal vascular smooth muscle cells, thereby increasing renal blood flow. 47 , 130 Second, H2S can potentially protect renal function by inhibiting Ang II in the renin‐angiotensin‐aldosterone system. 131 In addition, some investigators have also found that AP39, a mitochondrial‐targeting H2S donor, can reduce ROS levels, protect mitochondrial function, and reduce renal epithelial cell injury. However, this protective effect is dose‐dependent. 81 , 132
4.1.6. Hepatoprotective effects of H2S
Ischemic liver tissue is extremely susceptible to more severe liver dysfunction and failure after reperfusion occurs. 133 , 134 More seriously, hepatic ischemia–reperfusion (HIR) can also affect the success of liver resection or transplantation and increase the risk of death for the operator. 133 , 135 The risk of death is increased. There is a lot of experimental data to demonstrate that H2S can effectively protect liver tissue in HIRI and is expected to be a new way to reduce the morbidity and mortality of HIRI complications. 136 , 137 , 138 Some experiments have shown that the expression levels of endogenous H2S and CSE are elevated in the tissues of HIR rats, and the researchers speculate that this may be due to the self‐protective response of the organism induced by HIR. Meanwhile, after using the exogenous H2S donor NaHS in HIR rats, the investigators found that NaHS could attenuate IRI‐induced liver injury 136 , 139 At present, there has been a large amount of data demonstrating that H2S can play a role in reducing liver injury through various mechanisms, such as inhibition of oxidative stress, antiapoptosis, anti‐inflammation, protection of mitochondrial function, and regulation of autophagy. 140 , 141 , 142 , 143 , 144 , 145 However, it has also been found that endogenous H2S may exacerbate HIR‐induced liver injury in the context of insulin resistance, so H2S should be used with caution in this situation. 146
4.1.7. Retinal protective effect of H2S
IRI to the retina is the cause of many retinal vascular diseases, such as diabetic retinopathy (DR), glaucoma, retinal artery occlusion, and so on. 147 It is mainly caused by generating and accumulating large amounts of ROS during ischemia and reperfusion. It causes a series of oxidative stress and inflammatory responses that promote irreversible damage to retinal ganglion cells, which may eventually lead to vision loss or even blindness. 148 In a study more than a decade ago, researchers injected an H2S donor (ACS67) into the vitreous humor of rats with retinal IRI. Subsequently, they found that ACS67 could regulate GSH levels and inhibit apoptosis of retinal ganglion cell‐5 (RGC‐5) cells induced by oxidative stress, thus exerting a protective effect. 149 Another experiment found that direct inhalation of H2S for pretreatment prior to retinal IRI in rats reduced the mortality of RGC. 150 In a 2016 study, it was first proposed that enzymes involved in the generation of H2S and related pathways are activated during retinal IRI and may have the ability to induce retinal neovascularization. 151 In addition, H2S may also protect retinal ganglion cells by inhibiting the production of inflammatory factors and activating signaling pathways involved in mediating the protection of mitochondrial function and diastolic vascularity. 152 , 153 , 154
4.1.8. Testicular protective effect of H2S
Testicular torsion is a urological emergency that occurs in children and requires immediate surgical treatment; however, despite successful surgical intervention, the incidence of associated complications (such as testicular atrophy and infertility) ranges from 40 to 60%. 155 , 156 Postoperative IRI is the main cause of testicular damage, and previous studies have demonstrated that testicular IRI is closely related to excessive production of ROS, with subsequent massive production of inflammatory factors, oxidative stress, and apoptosis further exacerbating tissue damage. 157 , 158 The subsequent high production of inflammatory factors, oxidative stress, and apoptosis further aggravate the tissue damage. In the last 2 years, studies have revealed that H2S may have potential therapeutic effects in protecting testicular tissue. 159 , 160 Bozkurt et al. 160 first investigated the role of H2S in IRI in testicular torsion. They found that H2S administration inhibited oxidative stress and suppressed the expression of TNF‐α, APAF‐1, and iNOS to reduce tissue damage. 160 Myeloperoxidase (MPO), malonaldehyde, and advanced oxidation protein products (AOPP) are markers of lipid peroxidation, and Yuksel et al. 159 found that NaHS could effectively reduce the expression levels of MPO and AOPP. Meanwhile, Johnson scores were significantly higher in the H2S administration group, suggesting that H2S can improve spermatogenic function in IRI testes. 159 However, there are still relatively few related studies. The mechanism of the protective effect of H2S in testicular IRI is still unclear, and we need to conduct more in‐depth studies.
4.1.9. H2S and organ transplantation
Organ transplantation is the treatment modality of choice for organ failure or severe lesions. The current clinically accepted standard for transplantation is static cold storage (SCS), which essentially involves soaking the donor organ in a variety of preservation solutions, such as University of Wisconsin (UW) solution or histidine–tryptophan–ketoglutaratesolution, and subsequently storing it on ice at 4°C. 161 Short‐term SCS reduces the graft donor metabolic demand and cellular activity; however, long‐term SCS leads to severe cold IRI, which may cause severe rejection and reduce graft success. 162 , 163 , 164
Researchers have tried adding additives to the graft preservation solution to discourage cold IRI. Among them, richer results have been achieved in adding H2S donors. Previous studies have shown that supplementing graft preservation fluids with appropriate doses of H2S donors such as NaHS, 10‐oxo‐10‐(4‐(3‐thioxo‐3H‐1,2‐dithiol‐5yl)phenoxy)decyl (AP39), and morpholin‐4‐ium 4 methoxyphenyl (morpholino) phosphinodithioate (GYY4137) can reduce the damage of IRI to kidney donors in rats and pigs and improve donor function. 128 , 165 , 166 , 167 , 168 At the same time, H2S donors protect other donor organs from cold IRI, such as the heart, liver, lungs, and pancreas. 169 , 170 , 171 , 172 This protective function mainly relies on antioxidant, apoptosis, anti‐inflammatory, and vascular mechanisms. So far, in the study of H2S donor amelioration of cold IRI, AP39 has shown a high potential to target the mitochondria of donor organs and promote H2S entry. Nevertheless, AP39 is an experimental donor and is not clinically licensed, so it is important to find an H2S donor that is clinically licensed, which will further the process of moving H2S donors from the lab to the clinic. Previously, we mentioned sodium thiosulfate‐supplemented (STS) as an antidote that the United States Food and Drug Administration (US FDA) had approved. Some researchers have found that supplementation of STS in the UW solution in which kidneys are stored attenuates tubular necrosis and apoptosis, increases transplant survival, and improves transplant function. 173 However, there are fewer reports on the inhibition of cold IRI by STS in SCS, and the specific molecular mechanisms still need to be determined. However, adding STS to the preservation solution is a more economical and convenient method, preventing cold IRI in SCS and improving the grafting success rate.
4.2. H2S and cardiovascular health
4.2.1. Anti‐inflammatory effects of H2S in the cardiovascular system
Inflammation is another hallmark of endothelial dysfunction, and under pathological conditions, transcriptional activation of inflammatory adhesion factors leads to leukocyte and macrophage enrichment of the vascular lining, which induces a range of cardiovascular diseases. 174 It has been reported in recent years that H2S may be an inflammatory modulator and play an anti‐inflammatory role in the cardiovascular system. 175 , 176 In a 2006 study, we reported that blocking endogenous H2S synthesis promoted leukocyte adhesion, which the administration of exogenous H2S donors conversely inhibited, and that this may be achieved by activating KATP channels. 84 Later reports support the idea that H2S has an anti‐inflammatory effect. It has been reported that the endogenous H2S synthase CSE may be involved in the pathogenesis of atherosclerosis. When specifically absent, CSE enhances monocyte adhesion and accelerates endothelial damage and the atherosclerotic process. It is noteworthy that the use of H2S donors had the opposite effect. The underlying mechanism may be related to CSE‐H2S‐induced persulfuration at the Cys‐13 position. 177 Another study in the same year demonstrated the anti‐inflammatory ability of H2S from a different perspective, as the investigators found that the H2S donors NaHS and GYY4137 increased the expression of mRNA for Sirtuin‐1 in arteries and induced its persulfuration, thereby inhibiting arterial inflammation and AS. 178 TNF‐α has an obvious proinflammatory effect, and it can promote vascular inflammation by inducing endothelial cells to produce adhesion mediators such as monocyte chemotactic protein‐1 (MCP‐1). It has been found that H2S can inhibit the shedding of TNF‐α and the release of MCP‐1, thereby inhibiting inflammation. 179 In addition to the above mechanisms, H2S has been found to exert anti‐inflammatory effects through various signaling pathways, such as inhibiting the JNK/NF‐κB signaling pathway and preventing the activation of NLPR3 inflammasome. 180 , 181 , 182
4.2.2. Myocardial protective effect of H2S
When the blood perfusion and oxygen supply of the myocardium are severely reduced, extensive cell death may occur, leading to myocardial infarction. 183 , 184 As mentioned earlier, although restoring blood supply can alleviate ischemia to a certain extent, it can also lead to a series of more serious reactions, such as oxidative stress, cell damage, and even death. In current research on IRI, increasing evidence suggests that endogenous H2S regulation or exogenous H2S donors may be involved in the pathogenesis of ischemic cardiomyopathy, improving cardiac function, controlling structural lesions, and reducing related complications. 185 , 186 , 187 We found that H2S may have a protective effect on myocardial cells through the following five mechanisms.
Massively production of ROS during reperfusion is the main cause of oxidative stress responses. However, H2S can inhibit its production by regulating ROS signaling pathways, such as inhibiting NF‐κB and JAK2–STAT3 pathways to reduce ROS levels. 188 At the same time, H2S can also increase the expression levels of superoxide dismutase (SOD) and GSH in IRI tissues, both of which are antioxidant enzymes that protect cardiomyocytes by converting peroxides (H2O2). 16 , 189 In addition, H2S can promote nuclear translocation of nuclear‐factor‐E2‐related factor‐2 (Nrf2), an important antioxidant transcription factor that increases transcription of antioxidant proteins and reduces apoptosis and mitochondrial damage. 190
In the process of apoptosis, Bcl‐2 plays a crucial role. It has been reported that H2S is able to reduce the proportion of apoptotic cells in the myocardium of IRI mice by upregulating the expression level of Bcl‐2 and decreasing the expression of Bax and cysteine 3. 191 Apoptotic proteins (IAPs) can block the apoptotic cascade response, and it has been found that H2S can inhibit apoptosis by affecting the phosphorylation of IAPs and cysteine aspartase recruitment structural domains.
H2S protects cardiomyocytes through a bidirectional action in autophagy. 192 Tissue or cellular ischemia leads to the development of cellular autophagy, and moderate cellular autophagy facilitates the repair of damaged cells. It has been demonstrated that H2S can exert cytoprotective effects by promoting autophagy, which is associated with NLRP3 inflammatory vesicles. Excessive autophagy exacerbates cellular IRI, and H2S can activate the PI3K/serum glucocorticoid‐regulated kinase 1 (SGK1)/glycogen kinase synthase 3β (GSK3β) signaling pathway and PI3K/Akt/mTOR signaling pathway to inhibit autophagy and provide protection for IRI cardiomyocytes. 193 , 194
In the current study, H2S can inhibit the inflammatory response of cardiomyocytes, which is one of the important mechanisms for its cardioprotective effect. 195 , 196 In earlier years, some researchers found that certain H2S donors can reduce leukocyte adhesion and infiltration, and this effect seems to be related to the activation of KATP channels. 84 , 197 In addition, administration of H2S treatment before the ischemic tissue regains blood supply also prevents the activation of NF‐κB and reduces the production of proinflammatory mediators, with the most significant reduction of IL‐1 and IL‐6. 198 , 199 Increased expression levels of TNF‐α during reperfusion promote interaction between leukocytes and endothelial cells, resulting in increased infiltration of inflammatory cells in the IRI region of the myocardium, which leads to more severe myocardial injury, so inhibition of TNF‐α expression may attenuate myocardial injury. It has been found that H2S can inhibit the adhesion of inflammatory cells and release associated inflammatory factors caused by TNF‐α activation, significantly reducing the expression levels of MCP‐1, adhesion factors, and so on. 179
The role of mitochondria is particularly important in mammalian growth and development, providing energy for the basic metabolism of the body. When myocardial IRI occurs, the function of mitochondria is severely impaired, leading to further myocardial damage. It has been found that NaHS can reduce mitochondrial malondialdehyde levels in ischemic cardiomyocytes while elevating the activities of SOD and glutathione peroxidase, allowing the preservation of mitochondrial function. 200 In addition, H2S also increased the efficiency of complexes I and II of the oxidative respiratory chain in mitochondria. It inhibited cytochrome oxidase, reducing the metabolism of cardiomyocytes to a preconditioned state, thereby reducing cardiomyocyte damage. 201 , 202
4.3. H2S and the nervous system
4.3.1. H2S and neurodegenerative diseases
H2S plays an important role in the CNS. In early times, H2S was recognized as a neuromodulator that played a role in enhancing long‐term memory in the hippocampal region of the brain in particular. 6 Several studies have found that abnormal expression levels of endogenous H2S and the transsulfuration pathway may be associated with neurodegenerative diseases. ND, which includes Alzheimer's disease (AD), Parkinson's disease, Huntington's chorea, amyotrophic lateral sclerosis, and spinal cerebellar ataxia, is characterized by a pathological condition in which proteins are misfolded and aggregated, resulting in neuronal damage or even loss, which can in turn lead to cognitive and motor dysfunction. 203 , 204 We mentioned above that CSE and 3‐MST are predominantly found in neurons, and several studies have reported reduced or absent CSE expression in different NDs, with or without reduced protein persulfate translation. 86 , 205 , 206 Oversulfuration modification may be a potential redox switch, and a growing number of studies suggest that H2S‐mediated oversulfuration modification may be involved in regulating oxidative stress, apoptosis, autophagy, and other processes to maintain normal physiological functions of cells. 16 , 207
The current study found that appropriate concentrations of H2S exert neuroprotective effects in ND, and in AD, phosphorylated Tau protein aggregates and misfolded β‐amyloid are its main features. 208 H2S has been shown to cause peroxidative modification of GSK3β and inhibit phosphorylation of Tau proteins. At the same time, H2S has been shown to inhibit the expression of amyloid precursor protein cleaving enzyme 1 (BACE1), which, in turn, reduces β‐amyloid activity and prevents the development of AD. 206 , 209 In Parkinson's disease, H2S has been found to sulfurize the Parkin protein to promote E3 ligase expression and also induces peroxisulfuric modification of SIRT1 to increase mitochondrial activity, reduce neuronal damage, and further exert neuroprotective effects on neuronal cells. 205 , 210 H2S‐induced S‐sulfonylation of Nrf2 in Huntington's disease inhibits oxidative stress, thereby promoting autophagy of misfolded proteins and suppressing Huntington's disease progression. 211 In summary, H2S has the role of a neuromodulatory transmitter. It can exert anti‐inflammatory, antiapoptotic, and antioxidative stress effects in neuronal cells, so it can be used as a potential neuroprotective agent in neurodegenerative diseases. However, high concentrations of H2S accelerate the progression of ND and aggravate neuronal cell damage. Therefore, our attitude regarding the research on using H2S as a neuroprotective agent should be cautious and in‐depth.
4.3.2. Neuroprotective effects of H2S
IRI of the brain is an important cause of ischemic stroke, mainly manifested by necrosis or softening of ischemic brain tissue and focal neuronal damage. 212 , 213 Stroke is the most common cause of disability in developed countries, and its high morbidity and mortality pose a great threat to the health of the whole population 214 , 215 Therefore, researchers need to find ways to detect and prevent ischemic strokes early. Fortunately, it has been found that appropriate concentrations of H2S have a neuroprotective effect on IRI in brain tissue. 216 , 217 Many experimental data suggest that the use of H2S donors to provide low concentrations of H2S can reduce infarct size and restore neurological function in brain tissue through mechanisms such as antioxidant, anti‐inflammatory, antiapoptotic, modulation of autophagy, protection of mitochondrial function, and vasodilation and angiogenesis. 218 , 219 , 220 , 221 The detailed mechanism is shown in Figure 5. In addition, it has been reported that H2S can also reduce infarct size and restore neurological function by modulating the expression of NMDA receptor expression levels, thereby activating the CREB pathway and improving neuronal cell survival. 222 , 223 However, another study showed that high concentrations of H2S inhibited COX activity in experimental mice, leading to brainstem toxicity and respiratory depression. 224 These suggest that H2S plays a dual biological role in the brain. 225 , 226 Although the potential mechanisms of H2S in neuroprotection are poorly well understood and refined, there is no doubt that as a multitargeted neuromodulator, H2S has a very bright application in treating ischemic stroke.
FIGURE 5.
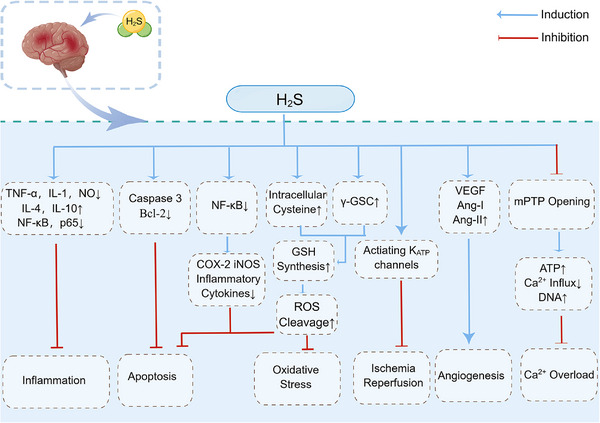
Mechanisms of neuroprotection exerted by H2S. H2S can reduce the infarct size of cerebellar tissue and restore neurological function through mechanisms such as antioxidant, anti‐inflammatory, antiapoptotic, regulating autophagy, protecting mitochondrial function, and vasodilation and generation. By Figdraw. COX‐2, cytochrome oxidase subunit 2; γ‐GCS, γ‐glutamyl cysteine synthetase; iNOS, inducible nitric oxide synthase; mPTP, mitochondrial permeability transition pore; NF‐κB, nuclear factor‐kappa B; VEGF, vascular endothelial growth factor.
4.4. H2S and the digestive system
4.4.1. Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease, which is mainly related to the abnormal accumulation of triglycerides in hepatocytes. 227 CSE, CBS, and 3‐MST are all expressed in the liver, which contribute to the endogenous production of H2S in the liver, and the disruption of the metabolism of H2S may be related to the development of liver disease. In mice given a high‐fat diet (HFD), researchers found that the HFD induced lipid accumulation in the liver and disrupted normal liver structure compared with the normal diet group. However, the H2S‐treated group reversed this phenomenon, improving liver structure, reducing triglyceride and total cholesterol accumulation, decreasing fatty acid synthase expression, and increasing SOD and glutathione peroxidase activity. This experiment showed that H2S alleviated fatty liver by improving lipid metabolism and increasing antioxidant capacity. 228 It has also been shown that plasma H2S levels are positively correlated with HDL cholesterol and negatively correlated with LDL cholesterol. 229 Regarding the mechanism by which H2S affects NAFLD, it is still unclear. Still, a previous study by our group found that H2S reduced apoptosis and promoted autophagy through the ROS‐mediated PI3K/AKT/ mTOR signaling pathway, thereby improving HFD‐induced NAFLD. 230
4.4.2. H2S and IBD
IBD is a chronic inflammatory condition of the gastrointestinal tract that includes two main types: ulcerative colitis and Crohn's disease. 231 Inflammatory response and oxidative stress are the main features of IBD. 232 When inflammation occurs, neutrophils infiltrate in large numbers, generating ROS, inducing the formation of neutrophil extracellular traps (NETs), and initiating chromatin polymerization. 233 , 234 It has been found that H2S donors can induce neutrophil apoptosis and prevent the formation of colitis NETs, thus exerting an anti‐inflammatory effect. 235 In addition, another study found that H2S donor treatment reduced the expression level of the oxidative stress marker 3‐NT and increased the expression of the antioxidants GSSH and SOD in a chemically induced IBD model. This suggests that H2S may play a cytoprotective role in reducing oxidative stress in IBD by inducing the production of antioxidants. 236 In colitis, H2S reduces neutrophil infiltration and decreases the expression of several proinflammatory factors, such as IL‐6, IL‐1β, TNF‐α, and NO. 237 , 238 In addition to acting alone, H2S can also act as an anti‐inflammatory in conjunction with NO or CO. 8 , 239 Although most studies have demonstrated that H2S exerts an anti‐inflammatory effect, some experiments have found that higher concentrations of H2S increase cytotoxic effects and exacerbate the effects of IBD. 64 , 240 Therefore, the relationship between H2S and IBD is complicated, and the effect on IBD is related to the concentration of H2S, the type of donor, and the release rate, for which we should carry out a more in‐depth and accurate study.
4.5. H2S and cancer
Cellular metabolic reprogramming is one of the hallmarks of cancer and an inevitable requirement for the survival and proliferation of cancer cells, which helps them to adapt more rapidly to their environment and gain energy. 241 In recent years, protein S‐persulfidation (P‐SSH) has gradually entered the field of vision of researchers, expanding a new way of thinking in cancer research. H2S is the most widely studied sulfur‐containing gaseous transmitter, and its role in cancer is known to be a double‐edged sword, with low concentrations of H2S promoting the development of cancer and high concentrations having the opposite effect. 242 So there are two options for using H2S to treat cancer, that is, inhibition of endogenous H2S synthesis or exogenous donor supplementation of H2S. 19 Currently, the molecular mechanisms of H2S in cancer are uncertain, but one more recognized mechanisms is the persulfidation modification of cysteine residues. 243 A deeper understanding of P‐SSH's mechanism can help us better develop strategies to inhibit endogenous H2S and thus treat cancer.
Overcoming oxidative stress is particularly important for the development of cancer cells. Cysteine is a precursor for the synthesis of GSH and is also involved in the antioxidant response. Therefore, ensuring sufficient cysteine is also important for the development of cancer. 244 , 245 P‐SSH protects Cys residues from oxidative damage and reduces them to natural thiols under certain conditions, thus preserving protein function. 246 In basal‐like breast cancer (BLCL), CBS overexpression has been reported to increase P‐SSH, which shields BLBC cells from damage caused by oxidative stress and promotes tumor cell proliferation and migration. 247
Alterations in signaling pathways are also one of the characteristics of cancer, and it has been found that several key molecules of signaling pathways are already sulfur‐modified in different cancer types. For example, PTEN, an oncogenic factor of the PI3K/Akt signaling pathway, and protein tyrosine phosphatase 1B (PTPIB). 248 , 249 It has been reported that under the induction of H2S, PTEN undergoes P‐SSH modification at Cys‐71 or Cys‐124 and PTPIB at the Cys‐215 site. P‐SSH of these two molecules would inhibit themselves, activating the PI3K/Akt pathway and enhancing tumor cell proliferation. 248 , 249 Activation of the Akt pathway by exogenous H2S or donor administration has been reported in many tumor cell lines, for example, in colon cancer cells, thyroid cancer cells, and hepatocellular carcinoma cells. 250 , 251 , 252
Epithelial–mesenchymal transition (EMT) is mandatory for tumors to undergo migration and invasion. 253 , 254 It has been found that silencing the endogenous H2S synthase CBS inhibits the EMT process in pancreatic ductal adenocarcinoma cells (PDAC). 255 The experimental results showed that silencing CBS inhibited the migration and colony‐forming ability of PDAC. Meanwhile, cells silenced with CBS showed decreased expression of WNT5A, SANIL and decreased phosphorylation level of STAT3. STAT3 is an upstream molecule required for the Wnt signaling pathway, so the decrease in phosphorylation may further inhibit the Wnt pathway, thus hindering the EMT process in tumor cells. 256 To dig deeper, the researchers did a metabolomic analysis. They found that silencing CBS leads to suppressed levels of protein persulfate, loss of oxidative protection of STAT3, and decreased levels of phosphorylation. 257 , 258
In summary, cancer cells can utilize P‐SSH to increase their ability to proliferate and migrate, are less susceptible to oxidative stress, and can better adapt to their environment. 259 However, it is currently difficult to distinguish between H2S‐mediated P‐SSH and the organism's RSSH, making further mechanism exploration difficult. In addition to the endogenous H2S blocking therapies we mentioned above, providing exogenous H2S donors is also an effective option in suppressing cancer. GYY4137 inhibited the proliferation and migration of colorectal and hepatocellular carcinoma, and HA‐ADT inhibited the development of breast and esophageal squamous carcinoma. 260 , 261 As the most common H2S donor, the cancer‐inhibitory effects of high‐dose NaHS have been widely reported in such cancers as gastric cancer, melanoma, esophageal cancer, oral cavity cancer, thyroid cancer, prostate cancer, and neuroblastoma. 251 , 262 , 263 , 264 , 265 , 266
5. THERAPEUTIC POTENTIAL OF H2S AND H2S DONORS
5.1. H2S donors
5.1.1. Sulfates
Sulfates are currently the most common H2S donors in biological research, such as sodium sulfide and NaHS, and have been shown to have protective effects on cells in disease states in multiple studies. 238 Both sodium sulfide and NaHS exhibit a crystalline powder appearance, are easily soluble in water, and can provide H2S more directly. In early studies, Zhao et al. used NaHS aqueous solution to release H2S and found that it can reduce systemic arterial pressure, indicating that H2S has the characteristic of relaxing blood vessels. 47 This has been verified in the research of Yoo et al.; in addition, they also found that the reduction of H2S donors will lead to the reduction of cardiac output, which will lead to the reduction of systemic arterial pressure. 267 This phenomenon does not depend on the regulation of the CNS. 267 In multiple studies, H2S released by exogenous donor NaHS can protect against organ damage, such as myocardial damage, 268 , 269 liver, 270 brain, 271 kidney, 269 and so on. However, the chemical properties of sulfide salts are not stable, and the dosage and speed of H2S produced after direct dissolution in water are uncontrollable. The release of a large amount of H2S can cause a sudden drop in blood pressure, which has adverse effects on experimental animals.
5.1.2. Lawesson reagent and their derivatives
Lawesson reagent (LR) is a common and readily available sulfur ion agent that can be an H2S releaser. The molecule of LR contains a quaternary ring with alternating sulfur and phosphorus atoms. Under high‐temperature conditions, the sulfur/phosphorus quaternary ring opens to form two unstable RCheicalbook‐PS2 (R‐PS2), which decompose and release H2S. 11 , 272 Compared with sulfide salts, LR releases H2S more slowly. 11 However, LR's detailed release molecular dynamics still need to be clarified, and it lacks water solubility, so it has not been widely used as an H2S donor. GYY4137 is a new water‐soluble H2S donor synthesized based on LR reagent, which can slowly release H2S. Some studies have found that GYY4137 has the function of dilating blood vessels to resist hypertension. 273 Not only that, it can also exert myocardial protection and prevent myocardial ischemia and reperfusion injury by inhibiting inflammation, reducing cell apoptosis, and reducing ROS production. 274 , 275 In addition to its myocardial protective effect, some scholars have found that in IRI, GYY4137 increases antioxidant activity by activating the Nrf2 signaling pathway, which can effectively alleviate renal injury. 276 This protective effect has also been reported in testicular torsion and intestinal injury. 277 , 278 In addition, GYY4137 has been found to inhibit the proliferation and migration of colorectal and hepatocellular carcinomas, and it also relieves the pain caused by certain chemotherapeutic drugs such as paclitaxel. 279 , 280 , 281 It can also relieve inflammation and reduce intestinal dysfunction when the intestinal barrier is compromised. 282
5.1.3. Sodium thiosulfate‐supplemented
Sodium thiosulfate is also a water‐soluble H2S donor with minimal side effects, and its chemical formula is Na2S203. STS is an antidote that has been approved by the US FDA and is currently mainly used in clinical practice to treat calcification reactions and toxic reactions (such as cisplatin poisoning, CO poisoning, cyanide poisoning, etc.). 283 , 284 , 285 As mentioned earlier, in the metabolism of H2S, H2S can be oxidized to thiosulfate, and in turn, STS can also become a source of H2S. When the body is in a state of hypoxia, H2S can be regenerated in thiosulfate through 3‐MST and rhodase. 286 In addition to releasing H2S, STS is also an effective antioxidant that has been proven to have strong protective effects in different organ injuries, such as acute liver injury, 287 acute lung injury, 288 brain injury caused by IRI, 289 myocardial injury, 290 kidney injury, 173 and so on.
Studies have shown that STS may have anti‐inflammatory effects and protect vascular endothelial cells. H2S seems inhibit the NF‐κB signaling pathway and exerts anti‐inflammatory effects. 291 Because of this cytoprotection on IRI, STS therapy has excellent potential in organ transplantation. The organ preservation solution added with STS is expected to become a simple, cheap, and safe new treatment strategy, which can reduce the transplant sequelae and improve the success rate. 292
5.1.4. Natural H2S donors and derivatives
Some natural foods can also serve as donors of H2S. Garlic is considered a good preventive food and has been found to have great medicinal research value. 293 , 294 Allicin is a metabolic active substance in garlic, which can be decomposed to produce diallyl polysulfides, such as diallyl sulfides, diallyl disulfides, and diallyl trisulfides (DATS). 295 Moreover, these sulfides can react with GSH to produce H2S. 296 However, due to the rapid release of H2S in water by DATS, some laboratories have utilized exploiting mesoporous silica nanoparticles (MSN) as a carrier to construct a new H2S release system (DATS‐MSN). 189 DATS‐MSN can release H2S more slowly and controllably. In this study, compared with traditional H2S donors (NaHS, DATS, and GYY4137), DATS‐MSN showed better cardioprotective effects. 189
5.1.5. AP39
In addition, scientists have synthesized an H2S donor targeting mitochondria (AP39). AP39 can reduce intracellular oxidative stress and proinflammatory factor gene expression, maintain cell vitality, ensure mitochondrial energy and DNA integrity, and play an anti‐inflammatory and antioxidant cytoprotection. 297 In mouse heart transplantation experiments, studies have found that adding AP39 to an organ preservation solution can significantly improve cell viability and reduce cold IRI and tissue fibrosis. 169 AP39 can significantly reduce ROS production in mouse pancreatic transplantation experiments and improve pancreatic island function. 172 These studies undoubtedly demonstrate the significant potential of AP39 in preventing and treating IRI in organ transplantation. As an H2S donor, in addition to protecting cells, AP39 can induce vascular relaxation by stimulating NO signaling and activating KATP channels. 298 The development of AP39 shows that the development of specific target donors of H2S in subcellular organelles has great potential in future biological research.
5.2. Potential and future directions of H2S therapy
As we mentioned above, H2S therapy can be divided into endogenous and exogenous; endogenous inhibition of H2S production can be achieved not only by using H2S synthase inhibitors A0AA, PAG, or L‐ASP but also by using targeted drugs to prevent persulfuration modification, which can contribute to the oxidative damage of the cancer cells and make them more susceptible to death. 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 For exogenous H2S therapy, the manner of H2S delivery is important. At present, the main methods of H2S delivery include direct inhalation and H2S donor. However, all these delivery methods have certain limitations. Gaseous H2S has a pungent odor and poor safety, and the concentration is difficult to control and cannot be delivered in a targeted manner. Hence, data stability could be improved and applied to human beings. 308 Inorganic sulfates are currently the main H2S donors in biology and clinical trials, but they are still poorly targeted and require large amounts to be administered, which can lead to adverse reactions. 309 Although recent studies have identified many small molecule H2S donors, they still perform poorly regarding stability, solubility, targeting, and half‐life. 309 Therefore, designing safe, controllable, and stable targeting methods for H2S delivery is particularly important. Nowadays, the main way to constitute an H2S delivery system is to combine H2S donors with polymers by covalent linkage and physical entrapment. Most of these polymers, such as micelles, 310 , 311 hydrogels, 312 liposomes, 313 and nanoparticles, have good biocompatibility. The delivery systems optimize the therapeutic efficacy with higher stability and bioavailability compared with small molecule H2S donors.
To further improve the efficacy of H2S therapy, H2S delivery systems with intelligent properties have been carried out in recent years. Intelligent delivery systems allow for a stable and controlled release of H2S by specifically targeting and responding to the pathological microenvironment and external stimuli. In addition, the system monitors the release of H2S through the use of materials with imaging capability. 308 Zhao et al. 314 , 315 designed an N‐mercapto (N‐SH)‐based H2S delivery system that not only releases H2S in a controlled manner, but also displays potent myocardial protection in a mouse model of myocardial IRI. Takatani‐Nakase et al. 316 found that ADT nano micelle could release H2S in an in vitro ischemia model and were more effective in blocking cardiomyocyte apoptosis than NaHS and ADT‐OH. Sun et al 317 . constructed a targetable H2S delivery system (DATS@MION–PEG–LF) based on mesoporous iron oxide nanoparticles (MION). The system is mainly composed of MION, DATS, polyethylene glycol (PEG), and lactoferrin (LF). To prolong circulation time, the researchers modified PEG into MIONs, while LF helps the system target the brain through the blood–brain barrier. The study found that DATS@MION–PEG–LF can be taken up by neuronal cells and cardiomyocytes, protecting the brain and heart by exerting antiapoptotic and oxidative effects in IRI. More notably, this process can also be visualized by MRI. 317 Hsieh et al. prepared a nanoparticle system loaded with DATS (DATS@MPs). 318 Compared with free DATS, DATS@MPs can release H2S more slowly and for a more extended period. In a mouse model of hind limb ischemia, DATS@MPs can limit apoptosis, protect cells, and promote angiogenesis, which is beneficial for hind limb recovery. 318
In addition to its cytoprotective role in IRI, researchers have developed smart delivery systems for H2S that have therapeutic effects in other diseases such as IBD, angiogenesis, disc degeneration, and rheumatoid arthritis. 319 , 320 , 321 , 322 , 323 He et al. used bovine serum albumin (BSA) as a template to design MnS@BSA. MnS@BSA dissociates in a weakly acidic environment, releasing H2S and Mn2+. H2S can be used for gas therapy for cancer, and Mn2+ can not only be used for MRI, but it produces H2O2 that can have a synergistic anticancer effect with H2S. 324 In exception to this, other smart delivery systems with anticancer effects have also been reported in large numbers. 310 , 313 , 325 , 326 , 327 , 328
We summarize the progress of the H2S smart delivery system in preclinical studies (summarized in Table 1) with the aim of more clearly and unambiguously describing the therapeutic potential of H2S and donors.
TABLE 1.
Summary of advances in H2S donors and smart delivery systems in preclinical studies.
| Therapeutic potential | H2S donors/polymeric carriers | Experimental models | Proposed mechanisms | References |
|---|---|---|---|---|
| Myocardial protection | NaHS | Neonatal cardiomyocytes IR model(rat) | Decrease of ROS level via downregulation of NF‐κB and JAK2/STAT3 pathways | 188 |
| NaHS | Infarction model(mice) | Upregulation of Bcl‐2, demoted expression of Bax, IL‐1β, and Caspase 3 | 191 | |
| NaHS | Myocardial I/R model(mice) | Antioxidant and antiapoptotic | 190 | |
| NaHS | Neonatal cardiomyocytes IR model(rat) | Inhibition of autophagy through regulation of the PI3K/SGK1/GSK3β signaling pathway | 193, 194 | |
| NaHS | Myocardial I/R model(mice) | Mitochondrial KATP channel opening | 329 | |
| GYY4137 | SICM model(mice) | Inhibition of inflammatory response and reduction of ROS generation via NLRP3 pathway | 274 | |
| STS | Myocardial I/R model(rat) | Antioxidant and antiapoptotic | 290 | |
| DATS‐MSN | Myocardial I/R model and cardiomyocytes(rat) | Antioxidant, anti‐inflammatory, and antiapoptotic | 189 | |
| AP39 | Heart transplant model(mice) | Blocked prolonged cold IRI and reduced tissue damage and fibrosis | 169 | |
| ADT micelle | Ischemic cardiomyocytes(mice) | Antiapoptotic | 316 | |
| DATS@MION–PEG–LF | Hypoxia/reoxygenation model and CA/CPR model(mice) | Antioxidant, anti‐inflammatory and antiapoptotic | 317 | |
| Antiatherogenic | NaHS | Apolipoprotein‐E K.O. model (mice) | Inhibition of ICAM‐1 and TNF‐α signaling pathway | 330, 331 |
| APA/SATO | HUVEC | Improved cell proliferation and migration | 332, 333 | |
| Relief of pulmonary arterial | ACS14 MSs | PAH model(rat), HPAEC | Suppressed NF‐κB–Snail pathway | 334 |
| Relief of ND | GYY4137 | 3xTg‐AD mice | Prevented hyperphosphorylation of Tau by sulfhydrating GSK3β | 206 |
| NaHS | PD mice | Increased SIRT1 expression and sulfhydration | 210 | |
| Relief of ischemic stroke | NaHS | Cerebral I/R model(rat) | Improved SOD activity, removed oxygen free radicals, inhibited lipid peroxidation, and downregulated the expression of HSP70 | 219 |
| Relief of NAFLD | GYY4137 | NAFLD model(mice) | Inhibition of hepatic ER stress through the SIRT1/FoxO1/PCSK9 pathway | 335 |
| Relief of IBD | Lawesson's reagent and SASP | Colitis model(rat) | Inhibition of NETs formation to exert anti‐inflammatory effects | 235 |
| CAP‐w‐FC | Colitis model(rat) | Anti‐inflammatory | 319 | |
| Anticancer | NaHS | A549 cell | Regulation of the TGF‐β1/Smad2/Smad3 pathway inhibits EMT protein and migratory capacity | 264 |
| GYY4137 | Colon cancer cell | Downregulation of CD44 inhibits tumor cell proliferation and metastasis | 280 | |
| GYY4137 | Subcutaneous HepG2 xenograft model(mice) | Blocked STAT3 pathway | 281 | |
| mPEG‐SSS‐cholesteryl (T) | Coculture model of fibroblasts and breast cancer cells | Reduced ROS levels | 325 | |
| SATO‐functionalized micelle | Colon cancer cell | Targeted cell toxicity | 310 | |
| MnS@BSA | 4T1 cell, xenograft model(mice) | Inhibition of tumor growth | 324 | |
| FeS@BSA | Huh7 cell, xenograft model(mice) | Inhibition of tumor growth | 328 |
However, smart H2S delivery systems are still in their infancy, and there is still a long way to go from the lab to the clinic. First, the pharmacological research of H2S needs to be more profound, and the detailed molecular mechanism and targets of its action in vivo need to be clarified. Second, it is not easy to monitor the location and concentration of H2S accurately using current commonly used technologies. Therefore, H2S delivery systems rely on more in‐depth biological research. However, we believe that through innovation and improvement in all aspects, the intelligent H2S delivery system will eventually unleash its great potential.
6. DISCUSSION
There is increasing evidence that reasonable concentrations of H2S can have protective effects in physiological and pathological conditions, possibly through antiapoptosis, regulation of autophagy, and inhibition of oxidative stress and inflammation. The growing understanding of the important biological effects of H2S, such as vasodilatory, cytoprotective antioxidant, and anti‐inflammatory effects, as well as its signaling pathway mechanisms, has facilitated the translation of the highly promising cytoprotective functions of H2S into more viable clinical therapeutic modalities.
Key to this is the effective design of H2S donors to deliver the desired therapeutic effects. As discussed earlier, designing stable, controlled H2S donors that maintain a stable and slow release of H2S over time is preferable for clinical applications, and much of the physiological utility of H2S is derived from its redox properties. The uncontrolled and rapid release of H2S donors rapidly alters the redox state of cells, which has a much greater impact on cells than its beneficial physiological functions. With rapidly increasing H2S concentrations, the distribution of each different oxidation state sulfide is vastly different from the normal physiological state, yet each sulfide has its unique physiological properties.
The volatility of H2S and its rapid metabolism makes the development of H2S donors uniquely challenging compared with the development of other small molecule donors, which are highly volatile and are always in a dynamic, volatile‐soluble equilibrium. In addition, many of the current H2S donors are polysulfides, both the donor itself and the by‐products of H2S fraction production, so it is often difficult to distinguish whether the physiological effects of such donors are derived from H2S or other polysulfides. Another difficulty in H2S research is how to quantify the range of endogenous H2S concentrations during human circulation and the changes in H2S concentrations during treatment. This is mainly due to the reactive chemical nature of H2S and the complex environment of sulfides in vivo. The inability to accurately monitor H2S concentrations in the circulatory system or target organs will make it difficult to assess the exact relationship between H2S and physiological effects. Therefore, it is important to develop methods to quantitatively detect H2S concentrations in vivo for H2S research.
In conclusion, although sulfide generators are not new drugs to date, there is precedent for reducing metabolism, thus providing protection against IRI in humans. For example, hypothermia therapy has been shown to be beneficial for outcomes in a variety of situations, including out‐of‐hospital cardiac arrest and during myocardial revascularization. Although many issues still need to be addressed, these critical issues must be resolved to move into clinical treatment. However, future multidisciplinary collaborations involving nanomaterials, chemistry, pharmaceutical, and biological disciplines may finally offer a possibility for H2S therapy, and we look forward to seeing more exciting studies in this area.
AUTHOR CONTRIBUTIONS
Dong‐Dong Wu, Zhi‐Guang Ren, and Xin‐Ying Ji: conceived and supervised the study. Yu‐Qing Jin, Hang Yuan, Ya‐Fang Liu, Yi‐Wen Zhu, Yan Wang, Xiao‐Yi Liang, and Wei Gao drafted the manuscript and prepared the figures. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT AND CONSENT TO PARTICIPATE
Not applicable.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (No. 81802718), the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038), the Natural Science Foundation of Education Department of Henan Province, China (Nos. 21A310003, 24B310001), and the Foundation of Science & Technology Department of Henan Province, China (Nos. 222102310490, 222102310495, 232102310507).
Jin Y‐Q, Yuan H, Liu Y‐F, et al. Role of hydrogen sulfide in health and disease. MedComm. 2024;5:e661. 10.1002/mco2.661
Contributor Information
Zhi‐Guang Ren, Email: renzhiguang66@126.com.
Xin‐Ying Ji, Email: 10190096@vip.henu.edu.cn.
Dong‐Dong Wu, Email: ddwubiomed2010@163.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40(5):533‐539. [DOI] [PubMed] [Google Scholar]
- 2. Nicholls P, Marshall DC, Cooper CE, Wilson MT. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans. 2013;41(5):1312‐1316. [DOI] [PubMed] [Google Scholar]
- 3. Ramzan R, Dolga AM, Michels S, et al. Cytochrome c oxidase inhibition by ATP decreases mitochondrial ROS production. Cells. 2022;11(6):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brischigliaro M, Zeviani M. Cytochrome c oxidase deficiency. Biochim Biophys Acta Bioenerg. 2021;1862(1):148335. [DOI] [PubMed] [Google Scholar]
- 5. Warenycia MW, Goodwin LR, Benishin CG, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38(6):973‐981. [DOI] [PubMed] [Google Scholar]
- 6. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237(3):527‐531. [DOI] [PubMed] [Google Scholar]
- 8. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792‐1798. [DOI] [PubMed] [Google Scholar]
- 9. Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation‐like state in mice. Science. 2005;308(5721):518. [DOI] [PubMed] [Google Scholar]
- 10. Łowicka E, Bełtowski J. Hydrogen sulfide (H2S) ‐ the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59(1):4‐24. [PubMed] [Google Scholar]
- 11. Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H(2)S) donors: Chemistry and potential therapeutic applications. Biochem Pharmacol. 2018;149:110‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3‐mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146(5):623‐626. [DOI] [PubMed] [Google Scholar]
- 13. Han Y, Qin J, Chang X, Yang Z, Tang X, Du J. Hydrogen sulfide may improve the hippocampal damage induced by recurrent febrile seizures in rats. Biochem Biophys Res Commun. 2005;327(2):431‐436. [DOI] [PubMed] [Google Scholar]
- 14. Qu K, Chen CP, Halliwell B, Moore PK, Wong PT. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke. 2006;37(3):889‐893. [DOI] [PubMed] [Google Scholar]
- 15. Kawabata A, Ishiki T, Nagasawa K, et al. Hydrogen sulfide as a novel nociceptive messenger. Pain. 2007;132(1‐2):74‐81. [DOI] [PubMed] [Google Scholar]
- 16. Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18(10):1165‐1167. [DOI] [PubMed] [Google Scholar]
- 17. Guo J, Li G, Yang L. Role of H(2)S in pain: Growing evidences of mystification. Eur J Pharmacol. 2020;883:173322. [DOI] [PubMed] [Google Scholar]
- 18. Guo FF, Yu TC, Hong J, Fang JY. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front Physiol. 2016;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu X, Xiao Y, Sun J, et al. New possible silver lining for pancreatic cancer therapy: Hydrogen sulfide and its donors. Acta Pharm Sin B. 2021;11(5):1148‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791‐896. [DOI] [PubMed] [Google Scholar]
- 21. Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radical Biol Med. 2009;47(10):1346‐1353. [DOI] [PubMed] [Google Scholar]
- 22. Mathai JC, Missner A, Kügler P, et al. No facilitator required for membrane transport of hydrogen sulfide. Proc Nat Acad Sci USA. 2009;106(39):16633‐16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kangas J, Savolainen H. Urinary thiosulphate as an indicator of exposure to hydrogen sulphide vapour. Clin Chim Acta. 1987;164(1):7‐10. [DOI] [PubMed] [Google Scholar]
- 24. Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3‐mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27(2):601‐611. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta‐synthase and gamma‐cystathionase to H2S biogenesis via alternative trans‐sulfuration reactions. J Biol Chem. 2009;284(33):22457‐22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olson KR. H(2)S and polysulfide metabolism: Conventional and unconventional pathways. Biochem Pharmacol. 2018;149:77‐90. [DOI] [PubMed] [Google Scholar]
- 27. Gregory JF, DeRatt BN, Rios‐Avila L, Ralat M, Stacpoole PW. Vitamin B6 nutritional status and cellular availability of pyridoxal 5'‐phosphate govern the function of the transsulfuration pathway's canonical reactions and hydrogen sulfide production via side reactions. Biochimie. 2016;126:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bełtowski J. [Hydrogen sulfide as a biologically active mediator in the cardiovascular system]. Postepy Hig Med Dosw. 2004;58:285‐291. [PubMed] [Google Scholar]
- 29. Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S‐propargyl‐cysteine, a novel hydrogen sulfide‐modulated agent, attenuates lipopolysaccharide‐induced spatial learning and memory impairment: involvement of TNF signaling and NF‐κB pathway in rats. Brain Behav Immun. 2011;25(1):110‐119. [DOI] [PubMed] [Google Scholar]
- 30. Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 31. Shibuya N, Tanaka M, Yoshida M, et al. 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11(4):703‐714. [DOI] [PubMed] [Google Scholar]
- 32. Shibuya N, Koike S, Tanaka M, et al. A novel pathway for the production of hydrogen sulfide from D‐cysteine in mammalian cells. Nat Commun. 2013;4:1366. [DOI] [PubMed] [Google Scholar]
- 33. Gould SJ, Keller GA, Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988;107(3):897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4‐10. [DOI] [PubMed] [Google Scholar]
- 35. Schumann U, Subramani S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008;18(6):253‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275(13):3352‐3361. [DOI] [PubMed] [Google Scholar]
- 37. Jackson MR, Melideo SL, Jorns MS. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51(34):6804‐6815. [DOI] [PubMed] [Google Scholar]
- 38. Libiad M, Yadav PK, Vitvitsky V, Martinov M, Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem. 2014;289(45):30901‐30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Landry AP, Ballou DP, Banerjee R. H(2)S oxidation by nanodisc‐embedded human sulfide quinone oxidoreductase. J Biol Chem. 2017;292(28):11641‐11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libiad M, Sriraman A, Banerjee R. Polymorphic Variants of Human Rhodanese Exhibit Differences in Thermal Stability and Sulfur Transfer Kinetics. J Biol Chem. 2015;290(39):23579‐23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landry AP, Ballou DP, Banerjee R. Hydrogen sulfide oxidation by sulfide quinone oxidoreductase. Chembiochem. 2021;22(6):949‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pacifici GM, Romiti P, Santerini S, Giuliani L. S‐methyltransferases in human intestine: differential distribution of the microsomal thiol methyltransferase and cytosolic thiopurine methyltransferase along the human bowel. Xenobiotica. 1993;23(6):671‐679. [DOI] [PubMed] [Google Scholar]
- 43. Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999;104(8):1107‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bostelaar T, Vitvitsky V, Kumutima J, et al. Hydrogen sulfide oxidation by myoglobin. J Am Chem Soc. 2016;138(27):8476‐8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res. 2014;114(4):730‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kolluru GK, Shackelford RE, Shen X, Dominic P, Kevil CG. Sulfide regulation of cardiovascular function in health and disease. Nat Rev Cardiol. 2023;20(2):109‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008‐6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Altaany Z, Ju Y, Yang G, Wang R. The coordination of S‐sulfhydration, S‐nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal. 2014;7(342):ra87. [DOI] [PubMed] [Google Scholar]
- 49. Mustafa AK, Sikka G, Gazi SK, et al. Hydrogen sulfide as endothelium‐derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109(11):1259‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bucci M, Papapetropoulos A, Vellecco V, et al. cGMP‐dependent protein kinase contributes to hydrogen sulfide‐stimulated vasorelaxation. PLoS One. 2012;7(12):e53319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kiss L, Deitch EA, Szabó C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 2008;83(17‐18):589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White BJ, Smith PA, Dunn WR. Hydrogen sulphide‐mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br J Pharmacol. 2013;168(4):785‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Nat Acad Sci USA. 2009;106(51):21972‐21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29‐40. [DOI] [PubMed] [Google Scholar]
- 55. Kolluru GK, Bir SC, Yuan S, et al. Cystathionine γ‐lyase regulates arteriogenesis through NO‐dependent monocyte recruitment. Cardiovasc Res. 2015;107(4):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Szabo C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: mechanisms and implications. Am J Physiol Cell Physiol. 2017;312(1):C3‐C15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coletta C, Papapetropoulos A, Erdelyi K, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium‐dependent vasorelaxation. Proc Nat Acad Sci USA. 2012;109(23):9161‐9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo H, Wu PF, Han QQ, et al. Reactive sulfur species emerge as gliotransmitters to support memory via sulfuration‐dependent gating of NR2A‐containing N‐methyl‐d‐aspartate subtype glutamate receptor function. Antioxid Redox Signal. 2019;30(16):1880‐1899. [DOI] [PubMed] [Google Scholar]
- 59. Chen HB, Wu WN, Wang W, et al. Cystathionine‐β‐synthase‐derived hydrogen sulfide is required for amygdalar long‐term potentiation and cued fear memory in rats. Pharmacol Biochem Behav. 2017;155:16‐23. [DOI] [PubMed] [Google Scholar]
- 60. Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 61. Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105‐131. [DOI] [PubMed] [Google Scholar]
- 62. Austgen JR, Hermann GE, Dantzler HA, Rogers RC, Kline DD. Hydrogen sulfide augments synaptic neurotransmission in the nucleus of the solitary tract. J Neurophysiol. 2011;106(4):1822‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Linden DR. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal. 2014;20(5):818‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blachier F, Beaumont M, Kim E. Cysteine‐derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr Opin Clin Nutr Metab Care. 2019;22(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 65. Blachier F, Davila AM, Mimoun S, et al. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39(2):335‐347. [DOI] [PubMed] [Google Scholar]
- 66. Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2010;628(1‐3):179‐186. [DOI] [PubMed] [Google Scholar]
- 67. Gallego D, Clavé P, Donovan J, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20(12):1306‐1316. [DOI] [PubMed] [Google Scholar]
- 68. Gil V, Gallego D, Jiménez M. Effects of inhibitors of hydrogen sulphide synthesis on rat colonic motility. Br J Pharmacol. 2011;164(2b):485‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kasparek MS, Linden DR, Farrugia G, Sarr MG. Hydrogen sulfide modulates contractile function in rat jejunum. J Surg Res. 2012;175(2):234‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137(2):139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao P, Huang X, Wang ZY, et al. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea‐pig. Eur J Pharmacol. 2009;616(1‐3):223‐228. [DOI] [PubMed] [Google Scholar]
- 72. Han YF, Huang X, Guo X, et al. Evidence that endogenous hydrogen sulfide exerts an excitatory effect on gastric motility in mice. Eur J Pharmacol. 2011;673(1‐3):85‐95. [DOI] [PubMed] [Google Scholar]
- 73. Kang M, Hashimoto A, Gade A, Akbarali HI. Interaction between hydrogen sulfide‐induced sulfhydration and tyrosine nitration in the KATP channel complex. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G532‐G539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Quan X, Luo H, Liu Y, Xia H, Chen W, Tang Q. Hydrogen sulfide regulates the colonic motility by inhibiting both L‐type calcium channels and BKCa channels in smooth muscle cells of rat colon. PLoS One. 2015;10(3):e0121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nalli AD, Bhattacharya S, Wang H, Kendig DM, Grider JR, Murthy KS. Augmentation of cGMP/PKG pathway and colonic motility by hydrogen sulfide. Am J Physiol Gastrointest Liver Physiol. 2017;313(4):G330‐G341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Strege PR, Bernard CE, Kraichely RE, et al. Hydrogen sulfide is a partially redox‐independent activator of the human jejunum Na+ channel, Nav1.5. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1105‐G1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clausen T, Wahl MC, Messerschmidt A, et al. Cloning, purification and characterisation of cystathionine gamma‐synthase from Nicotiana tabacum. Biol Chem. 1999;380(10):1237‐1242. [DOI] [PubMed] [Google Scholar]
- 78. Li L, Bhatia M, Zhu YZ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide‐induced inflammation in the mouse. FASEB J. 2005;19(9):1196‐1198. [DOI] [PubMed] [Google Scholar]
- 79. Tamizhselvi R, Moore PK, Bhatia M. Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas. 2008;36(4):e24‐e31. [DOI] [PubMed] [Google Scholar]
- 80. Zhang H, Moochhala SM, Bhatia M. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. J Immunol. 2008;181(6):4320‐4331. [DOI] [PubMed] [Google Scholar]
- 81. Elrod JW, Calvert JW, Morrison J, et al. Hydrogen sulfide attenuates myocardial ischemia‐reperfusion injury by preservation of mitochondrial function. Proc Nat Acad Sci USA. 2007;104(39):15560‐15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Esechie A, Kiss L, Olah G, et al. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci. 2008;115(3):91‐97. [DOI] [PubMed] [Google Scholar]
- 83. Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18(3):557‐559. [DOI] [PubMed] [Google Scholar]
- 84. Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte‐mediated inflammation. FASEB J. 2006;20(12):2118‐2120. [DOI] [PubMed] [Google Scholar]
- 85. Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19(6):623‐625. [DOI] [PubMed] [Google Scholar]
- 86. Zivanovic J, Kouroussis E, Kohl JB, et al. Selective persulfide detection reveals evolutionarily conserved antiaging effects of S‐sulfhydration. Cell Metab. 2019;30(6):1152‐1170. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhan JQ, Zheng LL, Chen HB, et al. Hydrogen sulfide reverses aging‐associated amygdalar synaptic plasticity and fear memory deficits in rats. Front Neurosci. 2018;12:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ma N, Liu HM, Xia T, Liu JD, Wang XZ. Chronic aerobic exercise training alleviates myocardial fibrosis in aged rats through restoring bioavailability of hydrogen sulfide. Can J Physiol Pharmacol. 2018;96(9):902‐908. [DOI] [PubMed] [Google Scholar]
- 89. Srilatha B, Muthulakshmi P, Adaikan PG, Moore PK. Endogenous hydrogen sulfide insufficiency as a predictor of sexual dysfunction in aging rats Aging Male. 2012;15(3):153‐158. [DOI] [PubMed] [Google Scholar]
- 90. Hou CL, Wang MJ, Sun C, et al. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Long. 2016;2016:7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121‐1135. [DOI] [PubMed] [Google Scholar]
- 92. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17(11):1391‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec‐Weglinski JW. Ischaemia‐reperfusion injury in liver transplantation–from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10(2):79‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Al‐Githmi IS, Abdulqader AA, Alotaibi A, et al. Acute kidney injury after open heart surgery. Cureus. 2022;14(6):e25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. O'Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Comprehens Physiol. 2016;7(1):113‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Walkon LL, Strubbe‐Rivera JO, Bazil JN. Calcium overload and mitochondrial metabolism. Biomolecules. 2022;12(12):1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122‐129. [DOI] [PubMed] [Google Scholar]
- 99. Tang SP, Mao XL, Chen YH, Yan LL, Ye LP, Li SW. Reactive oxygen species induce fatty liver and ischemia‐reperfusion injury by promoting inflammation and cell death. Front Immunol. 2022;13:870239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perkins KA, Pershad S, Chen Q, et al. The effects of modulating eNOS activity and coupling in ischemia/reperfusion (I/R). Naunyn‐Schmiedeberg's Arch Pharmacol. 2012;385(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 102. Bhat AH, Dar KB, Anees S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101‐110. [DOI] [PubMed] [Google Scholar]
- 103. Bortolotti M, Polito L, Battelli MG, Bolognesi A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021;41:101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bedard K, Krause KH. The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245‐313. [DOI] [PubMed] [Google Scholar]
- 105. Brandes RP, Weissmann N, Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208‐226. [DOI] [PubMed] [Google Scholar]
- 106. Lassègue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30(4):653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Pascali F, Hemann C, Samons K, Chen CA, Zweier JL. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S‐glutathionylation. Biochemistry. 2014;53(22):3679‐3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sanada S, Kitakaze M. Ischemic preconditioning: emerging evidence, controversy, and translational trials. Int J Cardiol. 2004;97(2):263‐276. [DOI] [PubMed] [Google Scholar]
- 109. Chen X, Zhang X, Xue L, Hao C, Liao W, Wan Q. Treatment with enriched environment reduces neuronal apoptosis in the periinfarct cortex after cerebral ischemia/reperfusion injury. Cell Physiol Biochem. 2017;41(4):1445‐1456. [DOI] [PubMed] [Google Scholar]
- 110. Uehara T, Bennett B, Sakata ST, et al. JNK mediates hepatic ischemia reperfusion injury. J Hepatol. 2005;42(6):850‐859. [DOI] [PubMed] [Google Scholar]
- 111. Tibbetts MD, Zheng L, Lenardo MJ. The death effector domain protein family: regulators of cellular homeostasis. Nat Immunol. 2003;4(5):404‐409. [DOI] [PubMed] [Google Scholar]
- 112. Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia‐reperfusion injury. Am J Transplant. 2013;13(11):2797‐2804. [DOI] [PubMed] [Google Scholar]
- 113. Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4(15):e128834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:103‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride‐induced liver fibrosis. J Pineal Res. 2016;60(4):383‐393. [DOI] [PubMed] [Google Scholar]
- 116. Qin J, Zhou J, Dai X, et al. Short‐term starvation attenuates liver ischemia‐reperfusion injury (IRI) by Sirt1‐autophagy signaling in mice. Am J Transl Res. 2016;8(8):3364‐3375. [PMC free article] [PubMed] [Google Scholar]
- 117. Liu A, Huang L, Guo E, et al. Baicalein pretreatment reduces liver ischemia/reperfusion injury via induction of autophagy in rats. Sci Rep. 2016;6:25042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chen X, Li X, Zhang W, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK‐mediated NF‐κB pathway. Metabolism. 2018;83:256‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ritter LS, Stempel KM, Coull BM, McDonagh PF. Leukocyte‐platelet aggregates in rat peripheral blood after ischemic stroke and reperfusion. Biol Res Nurs. 2005;6(4):281‐288. [DOI] [PubMed] [Google Scholar]
- 120. Teoh NC. Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection‐the good, bad and deadly. J Gastroenterol Hepatol. 2011;26(Suppl 1):180‐187. [DOI] [PubMed] [Google Scholar]
- 121. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428‐435. [DOI] [PubMed] [Google Scholar]
- 122. Xiao C, Zhao H, Zhu H, et al. Tisp40 induces tubular epithelial cell GSDMD‐mediated pyroptosis in renal ischemia‐reperfusion injury via NF‐κB signaling. Front Physiol. 2020;11:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia‐reperfusion injury. Curr Opin Nephrol Hypertens. 2012;21(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Levey AS, James MT. Acute Kidney Injury Acute Kidney Injury. Annals of Internal Medicine. 2017;167(9):Itc66‐itc80. [DOI] [PubMed] [Google Scholar]
- 125. Zhao H, Alam A, Soo AP, George AJT, Ma D. Ischemia‐Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine. 2018;28:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Farrar A. Acute kidney injury. Nurs Clin North Am. 2018;53(4):499‐510. [DOI] [PubMed] [Google Scholar]
- 127. Han SJ, Kim JI, Park JW, Park KM. Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant. 2015;30(9):1497‐1506. [DOI] [PubMed] [Google Scholar]
- 128. Bos EM, Wang R, Snijder PM, et al. Cystathionine γ‐lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24(5):759‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Azizi F, Seifi B, Kadkhodaee M, Ahghari P. Administration of hydrogen sulfide protects ischemia reperfusion‐induced acute kidney injury by reducing the oxidative stress. Ir J Med Sci. 2016;185(3):649‐654. [DOI] [PubMed] [Google Scholar]
- 130. Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma‐lyase. Science. 2008;322(5901):587‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Snijder PM, Frenay AR, Koning AM, et al. Sodium thiosulfate attenuates angiotensin II‐induced hypertension, proteinuria and renal damage. Nitric Oxide. 2014;42:87‐98. [DOI] [PubMed] [Google Scholar]
- 132. Ahmad A, Olah G, Szczesny B, Wood ME, Whiteman M, Szabo C. AP39, a mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock (Augusta, Ga). 2016;45(1):88‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Long. 2014;2014:906965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhou J, Guo L, Ma T, et al. N‐acetylgalactosaminyltransferase‐4 protects against hepatic ischemia/reperfusion injury by blocking apoptosis signal‐regulating kinase 1 N‐terminal dimerization. Hepatology (Baltimore, Md). 2022;75(6):1446‐1460. [DOI] [PubMed] [Google Scholar]
- 135. Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90(4):665‐677. [DOI] [PubMed] [Google Scholar]
- 136. Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia‐reperfusion‐induced injury in rats. Liver Transplant. 2009;15(10):1306‐1314. [DOI] [PubMed] [Google Scholar]
- 137. Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia‐reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circul Physiol. 2008;295(2):H801‐H806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lu M, Jiang X, Tong L, et al. MicroRNA‐21‐regulated activation of the Akt pathway participates in the protective effects of H(2)S against liver ischemia‐reperfusion injury. Biol Pharm Bull. 2018;41(2):229‐238. [DOI] [PubMed] [Google Scholar]
- 139. Kang K, Jiang HC, Zhao MY, Sun XY, Pan SH. [Protection of CSE/H2S system in hepatic ischemia reperfusion injury in rats]. Zhonghua Wai Ke Za Zhi. 2010;48(12):924‐928. [PubMed] [Google Scholar]
- 140. Wu D, Wang J, Li H, Xue M, Ji A, Li Y. Role of hydrogen sulfide in ischemia‐reperfusion injury. Oxid Med Cell Long. 2015;2015:186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Haga S, Remington SJ, Morita N, Terui K, Ozaki M. Hepatic ischemia induced immediate oxidative stress after reperfusion and determined the severity of the reperfusion‐induced damage. Antioxid Redox Signal. 2009;11(10):2563‐2572. [DOI] [PubMed] [Google Scholar]
- 142. Cheng P, Wang F, Chen K, et al. Hydrogen sulfide ameliorates ischemia/reperfusion‐induced hepatitis by inhibiting apoptosis and autophagy pathways. Mediators Inflamm. 2014;2014:935251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu Y, Kalogeris T, Wang M, et al. Hydrogen sulfide preconditioning or neutrophil depletion attenuates ischemia‐reperfusion‐induced mitochondrial dysfunction in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G44‐G54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang Q, Fu H, Zhang H, et al. Hydrogen sulfide preconditioning protects rat liver against ischemia/reperfusion injury by activating Akt‐GSK‐3β signaling and inhibiting mitochondrial permeability transition. PLoS One. 2013;8(9):e74422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Du J, Wang Q, Li QM, Zhang BM, Xie KL, Wang GL. [Alternation of thioredoxin system in postconditioning with hydrogen sulfide against hepatic ischemia‐reperfusion injury in rats]. Zhonghua Yi Xue Za Zhi. 2012;92(37):2607‐2610. [PubMed] [Google Scholar]
- 146. Younis NN, Shaheen MA, Mahmoud MF. Silymarin preconditioning protected insulin resistant rats from liver ischemia‐reperfusion injury: role of endogenous H2S. J Surg Res. 2016;204(2):398‐409. [DOI] [PubMed] [Google Scholar]
- 147. Husain S, Abdul Y, Potter DE. Non‐analgesic effects of opioids: neuroprotection in the retina. Curr Pharm Des. 2012;18(37):6101‐6108. [DOI] [PubMed] [Google Scholar]
- 148. Qin X, Li N, Zhang M, et al. Tetrahedral framework nucleic acids prevent retina ischemia‐reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale. 2019;11(43):20667‐20675. [DOI] [PubMed] [Google Scholar]
- 149. Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del Soldato P. ACS67, a hydrogen sulfide‐releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC‐5 cells in culture. Invest Ophthalmol Vis Sci. 2010;51(1):284‐294. [DOI] [PubMed] [Google Scholar]
- 150. Biermann J, Lagrèze WA, Schallner N, Schwer CI, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011;17:1275‐1286. [PMC free article] [PubMed] [Google Scholar]
- 151. Gersztenkorn D, Coletta C, Zhu S, et al. Hydrogen sulfide contributes to retinal neovascularization in ischemia‐induced retinopathy. Invest Ophthalmol Vis Sci. 2016;57(7):3002‐3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu H, Perumal N, Manicam C, Mercieca K, Prokosch V. Proteomics reveals the potential protective mechanism of hydrogen sulfide on retinal ganglion cells in an ischemia/reperfusion injury animal model. Pharmaceuticals (Basel, Switzerland). 2020;13(9):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Scheid S, Goeller M, Baar W, et al. Hydrogen sulfide reduces ischemia and reperfusion injury in neuronal cells in a dose‐ and time‐dependent manner. Int J Mol Sci. 2021;22(18):10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Scheid S, Goeller M, Baar W, et al. Inhalative as well as intravenous administration of H(2)S provides neuroprotection after ischemia and reperfusion injury in the rats' retina. Int J Mol Sci. 2022;23(10):5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Krarup T. The testes after torsion. Br J Urol. 1978;50(1):43‐46. [DOI] [PubMed] [Google Scholar]
- 156. Aihole JS. Testicular torsion; clinical diagnosis or imaging diagnosis? Radiol Case Rep. 2022;17(8):2665‐2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Abdelzaher WY, Mostafa‐Hedeab G, Sayed AboBakr Ali AH, et al. Idebenone regulates sirt1/Nrf2/TNF‐α pathway with inhibition of oxidative stress, inflammation, and apoptosis in testicular torsion/detorsion in juvenile rats. Hum Exp Toxicol. 2022;41:9603271221102515. [DOI] [PubMed] [Google Scholar]
- 158. Djurhuus JC. Preclinical studies of testicular ischemia‐reperfusion treatment. J Pediatr Urol. 2021;17(2):168. [DOI] [PubMed] [Google Scholar]
- 159. Yuksel S, Erginel B, Bingul I, et al. The effect of hydrogen sulfide on ischemi̇a /reperfusion injury in an experimental testicular torsion model. J Pediatr Urol. 2022;18(1):16. e1‐e7. [DOI] [PubMed] [Google Scholar]
- 160. Bozkurt M, Degirmentepe RB, Polat EC, et al. Protective effect of hydrogen sulfide on experimental testicular ischemia reperfusion in rats. J Pediatr Urol. 2020;16(1):40. e1‐e8. [DOI] [PubMed] [Google Scholar]
- 161. Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. 2019;3(3):Cd011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 163. Dragun D, Hoff U, Park JK, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60(3):1173‐1181. [DOI] [PubMed] [Google Scholar]
- 164. Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long‐term survival of cadaveric renal allografts. Kidney Int. 2004;65(2):713‐718. [DOI] [PubMed] [Google Scholar]
- 165. Lobb I, Davison M, Carter D, et al. Hydrogen sulfide treatment mitigates renal allograft ischemia‐reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol. 2015;194(6):1806‐1815. [DOI] [PubMed] [Google Scholar]
- 166. Lobb I, Mok A, Lan Z, Liu W, Garcia B, Sener A. Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischaemia‐reperfusion injury by mitigating renal graft apoptosis and inflammation. BJU Int. 2012;110(11 Pt C):E1187‐E1195. [DOI] [PubMed] [Google Scholar]
- 167. Hosgood SA, Nicholson ML. Hydrogen sulphide ameliorates ischaemia‐reperfusion injury in an experimental model of non‐heart‐beating donor kidney transplantation. Br J Surg. 2010;97(2):202‐209. [DOI] [PubMed] [Google Scholar]
- 168. Lobb I, Jiang J, Lian D, et al. Hydrogen sulfide protects renal grafts against prolonged cold ischemia‐reperfusion injury via specific mitochondrial actions. Am J Transplant. 2017;17(2):341‐352. [DOI] [PubMed] [Google Scholar]
- 169. Zhu C, Su Y, Juriasingani S, et al. Supplementing preservation solution with mitochondria‐targeted H(2) S donor AP39 protects cardiac grafts from prolonged cold ischemia‐reperfusion injury in heart transplantation. Am J Transplant. 2019;19(11):3139‐3148. [DOI] [PubMed] [Google Scholar]
- 170. Wu J, Wei J, You X, et al. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res. 2013;182(1):e25‐e33. [DOI] [PubMed] [Google Scholar]
- 171. Balaban CL, Rodríguez JV, Tiribelli C, Guibert EE. The effect of a hydrogen sulfide releasing molecule (Na2S) on the cold storage of livers from cardiac dead donor rats. A study in an ex vivo model. Cryobiology. 2015;71(1):24‐32. [DOI] [PubMed] [Google Scholar]
- 172. Nishime K, Miyagi‐Shiohira C, Kuwae K, et al. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am J Transplant. 2021;21(8):2698‐2708. [DOI] [PubMed] [Google Scholar]
- 173. Zhang MY, Dugbartey GJ, Juriasingani S, et al. Sodium thiosulfate‐supplemented UW solution protects renal grafts against prolonged cold ischemia‐reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed Pharmacother. 2022;145:112435. [DOI] [PubMed] [Google Scholar]
- 174. Fang F, Chen D, Yu L, et al. Proinflammatory stimuli engage Brahma related gene 1 and Brahma in endothelial injury. Circ Res. 2013;113(8):986‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signaling. 2012;17(1):141‐185. [DOI] [PubMed] [Google Scholar]
- 176. Lin Y, Zeng H, Gao L, Gu T, Wang C, Zhang H. Hydrogen sulfide attenuates atherosclerosis in a partially ligated carotid artery mouse model via regulating angiotensin converting enzyme 2 expression. Front Physiol. 2017;8:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bibli SI, Hu J, Sigala F, et al. Cystathionine γ lyase sulfhydrates the RNA binding protein human antigen R to preserve endothelial cell function and delay atherogenesis. Circulation. 2019;139(1):101‐114. [DOI] [PubMed] [Google Scholar]
- 178. Du C, Lin X, Xu W, et al. Sulfhydrated sirtuin‐1 increasing its deacetylation activity is an essential epigenetics mechanism of anti‐atherogenesis by hydrogen sulfide. Antioxid Redox Signal. 2019;30(2):184‐197. [DOI] [PubMed] [Google Scholar]
- 179. Perna AF, Sepe I, Lanza D, et al. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17‐dependent TNF‐α activation. J Cell Biochem. 2013;114(7):1536‐1548. [DOI] [PubMed] [Google Scholar]
- 180. Wang XH, Wang F, You SJ, et al. Dysregulation of cystathionine γ‐lyase (CSE)/hydrogen sulfide pathway contributes to ox‐LDL‐induced inflammation in macrophage. Cell Signalling. 2013;25(11):2255‐2262. [DOI] [PubMed] [Google Scholar]
- 181. Li J, Teng X, Jin S, et al. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. J Hypertens. 2019;37(8):1633‐1643. [DOI] [PubMed] [Google Scholar]
- 182. Bourque C, Zhang Y, Fu M, et al. H(2)S protects lipopolysaccharide‐induced inflammation by blocking NFκB transactivation in endothelial cells. Toxicol Appl Pharmacol. 2018;338:20‐29. [DOI] [PubMed] [Google Scholar]
- 183. Ghaderi S, Alidadiani N, Dilaver N, et al. Role of glycogen synthase kinase following myocardial infarction and ischemia‐reperfusion. Apoptosis. 2017;22(7):887‐897. [DOI] [PubMed] [Google Scholar]
- 184. Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72(3):865‐867. [DOI] [PubMed] [Google Scholar]
- 185. Papapetropoulos A, Whiteman M, Cirino G. Pharmacological tools for hydrogen sulphide research: a brief, introductory guide for beginners. Br J Pharmacol. 2015;172(6):1633‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Donnarumma E, Trivedi RK, Lefer DJ. Protective actions of H2S in acute myocardial infarction and heart failure. Comprehens Physiol. 2017;7(2):583‐602. [DOI] [PubMed] [Google Scholar]
- 187. Calvert JW, Elston M, Nicholson CK, et al. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia‐induced heart failure in mice. Circulation. 2010;122(1):11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li L, Li M, Li Y, et al. Exogenous H2S contributes to recovery of ischemic post‐conditioning‐induced cardioprotection by decrease of ROS level via down‐regulation of NF‐κB and JAK2‐STAT3 pathways in the aging cardiomyocytes. Cell Biosci. 2016;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sun X, Wang W, Dai J, et al. A long‐term and slow‐releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci Rep. 2017;7(1):3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Calvert JW, Jha S, Gundewar S, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Wang X, Wang Q, Guo W, Zhu YZ. Hydrogen sulfide attenuates cardiac dysfunction in a rat model of heart failure: a mechanism through cardiac mitochondrial protection. Biosci Rep. 2011;31(2):87‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wu D, Wang H, Teng T, Duan S, Ji A, Li Y. Hydrogen sulfide and autophagy: a double edged sword. Pharmacol Res. 2018;131:120‐127. [DOI] [PubMed] [Google Scholar]
- 193. Jiang H, Xiao J, Kang B, Zhu X, Xin N, Wang Z. PI3K/SGK1/GSK3β signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Exp Cell Res. 2016;345(2):134‐140. [DOI] [PubMed] [Google Scholar]
- 194. Wang H, Zhong P, Sun L. Exogenous hydrogen sulfide mitigates NLRP3 inflammasome‐mediated inflammation through promoting autophagy via the AMPK‐mTOR pathway. Biol Open. 2019;8(7):bio043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Gemici B, Wallace JL. Anti‐inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol. 2015;555:169‐193. [DOI] [PubMed] [Google Scholar]
- 196. Sodha NR, Clements RT, Feng J, et al. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J Thorac Cardiovasc Surg. 2009;138(4):977‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zuidema MY, Korthuis RJ. Intravital microscopic methods to evaluate anti‐inflammatory effects and signaling mechanisms evoked by hydrogen sulfide. Methods Enzymol. 2015;555:93‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hu HJ, Jiang ZS, Zhou SH, Liu QM. Hydrogen sulfide suppresses angiotensin II‐stimulated endothelin‐1 generation and subsequent cytotoxicity‐induced endoplasmic reticulum stress in endothelial cells via NF‐κB. Mol Med Rep. 2016;14(5):4729‐4740. [DOI] [PubMed] [Google Scholar]
- 199. Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108(4):626‐635. [PubMed] [Google Scholar]
- 200. Xie YH, Zhang N, Li LF, et al. Hydrogen sulfide reduces regional myocardial ischemia injury through protection of mitochondrial function. Mol Med Rep. 2014;10(4):1907‐1914. [DOI] [PubMed] [Google Scholar]
- 201. Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61(3):461‐470. [DOI] [PubMed] [Google Scholar]
- 202. Zamzami N, Marchetti P, Castedo M, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182(2):367‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Models Mech. 2017;10(5):499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kritsilis M, VR S, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. 2018;19(10):2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Vandiver MS, Paul BD, Xu R, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Giovinazzo D, Bursac B, Sbodio JI, et al. Hydrogen sulfide is neuroprotective in Alzheimer's disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc Nat Acad Sci USA. 2021;118(4):e2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 208. Graff‐Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Fišar Z. Linking the amyloid, tau, and mitochondrial hypotheses of Alzheimer's disease and identifying promising drug targets. Biomolecules. 2022;12(11):1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Li J, Li M, Wang C, et al. NaSH increases SIRT1 activity and autophagy flux through sulfhydration to protect SH‐SY5Y cells induced by MPP∼. Cell Cycle. 2020;19(17):2216‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Tucci P, Lattanzi R, Severini C, Saso L. Nrf2 pathway in Huntington's disease (HD): what is its role? Int J Mol Sci. 2022;23(23):15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zhu H, Hu S, Li Y, et al. Interleukins and ischemic stroke. Front Immunol. 2022;13:828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Paul S, Candelario‐Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sveinsson OA, Kjartansson O, Valdimarsson EM. [Cerebral ischemia/infarction ‐ epidemiology, causes and symptoms]. Laeknabladid. 2014;100(5):271‐279. [DOI] [PubMed] [Google Scholar]
- 215. Zhao Y, Zhang X, Chen X, Wei Y. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). Int J Mol Med. 2022;49(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Compar Physiol. 2008;294(6):R1930‐R1937. [DOI] [PubMed] [Google Scholar]
- 217. Deng G, Muqadas M, Adlat S, et al. Protective effect of hydrogen sulfide on cerebral ischemia‐reperfusion injury. Cell Mol Neurobiol. 2023;43(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169‐187. [DOI] [PubMed] [Google Scholar]
- 219. Qin H, Gu LZ, Gao L, Guo J. [Protective effect of H2S pretreatment on cerebral ischemia‐reperfusion injury and its mechanisms in rats]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35(3):249‐253. [DOI] [PubMed] [Google Scholar]
- 220. Luo Y, Yang X, Zhao S, et al. Hydrogen sulfide prevents OGD/R‐induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS‐mediated caspase‐3 pathway in cortical neurons. Neurochem Int. 2013;63(8):826‐831. [DOI] [PubMed] [Google Scholar]
- 221. Yin J, Tu C, Zhao J, et al. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti‐oxidative, anti‐inflammatory and anti‐apoptotic effects in rats. Brain Res. 2013;1491:188‐196. [DOI] [PubMed] [Google Scholar]
- 222. Dai HB, Xu MM, Lv J, et al. Mild hypothermia combined with hydrogen sulfide treatment during resuscitation reduces hippocampal neuron apoptosis via NR2A, NR2B, and PI3K‐Akt signaling in a rat model of cerebral ischemia‐reperfusion injury. Mol Neurobiol. 2016;53(7):4865‐4873. [DOI] [PubMed] [Google Scholar]
- 223. Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000;267(1):129‐133. [DOI] [PubMed] [Google Scholar]
- 224. Santana Maldonado CM, Kim DS, Purnell B, et al. Acute hydrogen sulfide‐induced neurochemical and morphological changes in the brainstem. Toxicology. 2023;485:153424. [DOI] [PubMed] [Google Scholar]
- 225. Dou Y, Wang Z, Chen G. The role of hydrogen sulfide in stroke. Med Gas Res. 2016;6(2):79‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang J, Zhang S, Shan H, Zhang M. Biologic effect of hydrogen sulfide and its role in traumatic brain injury. Oxid Med Cell Long. 2020;2020:7301615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99‐114. [DOI] [PubMed] [Google Scholar]
- 228. Wu D, Zheng N, Qi K, et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med Gas Res. 2015;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jain SK, Micinski D, Lieblong BJ, Stapleton T. Relationship between hydrogen sulfide levels and HDL‐cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis. 2012;225(1):242‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Wu D, Zhong P, Wang Y, et al. Hydrogen sulfide attenuates high‐fat diet‐induced non‐alcoholic fatty liver disease by inhibiting apoptosis and promoting autophagy via reactive oxygen species/phosphatidylinositol 3‐kinase/AKT/mammalian target of rapamycin signaling pathway. Front Pharmacol. 2020;11:585860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12(2):113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease–radicals or ridiculous? Aliment Pharmacol Ther. 2002;16(12):1997‐2015. [DOI] [PubMed] [Google Scholar]
- 233. Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Carvalho FA, Barnich N, Sivignon A, et al. Crohn's disease adherent‐invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med. 2009;206(10):2179‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Török S, Almási N, Valkusz Z, Pósa A, Varga C, Kupai K. Investigation of H(2)S donor treatment on neutrophil extracellular traps in experimental colitis. Int J Mol Sci. 2021;22(23):12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Török S, Almási N, Veszelka M, Börzsei D, Szabó R, Varga C. Protective effects of H(2)S donor treatment in experimental colitis: a focus on antioxidants. Antioxidants (Basel, Switzerland). 2023;12(5):1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137(2):569‐578, e1. [DOI] [PubMed] [Google Scholar]
- 238. Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide‐induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12(10):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Oh GS, Pae HO, Lee BS, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor‐kappaB via heme oxygenase‐1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Rad Biol Med. 2006;41(1):106‐119. [DOI] [PubMed] [Google Scholar]
- 240. Attene‐Ramos MS, Nava GM, Muellner MG, Wagner ED, Plewa MJ, Gaskins HR. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen. 2010;51(4):304‐314. [DOI] [PubMed] [Google Scholar]
- 241. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 242. Chen HJ, Li K, Qin YZ, et al. Recent advances in the role of endogenous hydrogen sulphide in cancer cells. Cell Prolif. 2023;56(9):e13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S‐sulfhydration. Sci Signal. 2009;2(96):ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Harris IS, Treloar AE, Inoue S, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27(2):211‐222. [DOI] [PubMed] [Google Scholar]
- 245. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715‐748. [DOI] [PubMed] [Google Scholar]
- 246. Dóka É, Pader I, Bíró A, et al. A novel persulfide detection method reveals protein persulfide‐ and polysulfide‐reducing functions of thioredoxin and glutathione systems. Sci Adv. 2016;2(1):e1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Erdélyi K, Ditrói T, Johansson HJ, et al. Reprogrammed transsulfuration promotes basal‐like breast tumor progression via realigning cellular cysteine persulfidation. Proc Nat Acad Sci USA. 2021;118(45):e2100050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S‐Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal. 2011;4(203):ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Greiner R, Pálinkás Z, Bäsell K, et al. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal. 2013;19(15):1749‐1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Cai WJ, Wang MJ, Ju LH, Wang C, Zhu YC. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int. 2010;34(6):565‐572. [DOI] [PubMed] [Google Scholar]
- 251. Wu D, Li J, Zhang Q, et al. Exogenous hydrogen sulfide regulates the growth of human thyroid carcinoma cells. Oxid Med Cell Long. 2019;2019:6927298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Wu D, Li M, Tian W, et al. Hydrogen sulfide acts as a double‐edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP‐2 and PTEN/AKT signaling pathways. Sci Rep. 2017;7(1):5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed Pharmacother. 2021;133:110909. [DOI] [PubMed] [Google Scholar]
- 254. Akhmetkaliyev A, Alibrahim N, Shafiee D, Tulchinsky E. EMT/MET plasticity in cancer and Go‐or‐Grow decisions in quiescence: the two sides of the same coin? Mol Cancer. 2023;22(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Czikora Á, Erdélyi K, Ditrói T, et al. Cystathionine β‐synthase overexpression drives metastatic dissemination in pancreatic ductal adenocarcinoma via inducing epithelial‐to‐mesenchymal transformation of cancer cells. Redox Biol. 2022;57:102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Li L, Cheung SH, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70(20):8222‐8232. [DOI] [PubMed] [Google Scholar]
- 257. Sobotta MC, Liou W, Stöcker S, et al. Peroxiredoxin‐2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol. 2015;11(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 258. Li L, Shaw PE. A STAT3 dimer formed by inter‐chain disulphide bridging during oxidative stress. Biochem Biophys Res Commun. 2004;322(3):1005‐1011. [DOI] [PubMed] [Google Scholar]
- 259. Cortese‐Krott MM, Koning A, Kuhnle GGC, et al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27(10):684‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Dong Q, Yang B, Han JG, et al. A novel hydrogen sulfide‐releasing donor, HA‐ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019;455:60‐72. [DOI] [PubMed] [Google Scholar]
- 261. Duan SF, Zhang MM, Dong Q, et al. A water‐soluble hydrogen sulfide donor suppresses the growth of hepatocellular carcinoma via inhibiting the AKT/GSK‐3β/β‐catenin and TGF‐β/Smad2/3 signaling pathways. J Oncol. 2023;2023:8456852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Cui X, Liu R, Duan L, Zhang Q, Cao D, Zhang A. Exogenous hydrogen sulfide (H(2)S) exerts therapeutic potential in triple‐negative breast cancer by affecting cell cycle and DNA replication pathway. Biomed Pharmacother. 2023;161:114488. [DOI] [PubMed] [Google Scholar]
- 263. Zhang L, Qi Q, Yang J, et al. An anticancer role of hydrogen sulfide in human gastric cancer cells. Oxid Med Cell Long. 2015;2015:636410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Ye M, Yu M, Yang D, et al. Exogenous hydrogen sulfide donor NaHS alleviates nickel‐induced epithelial‐mesenchymal transition and the migration of A549 cells by regulating TGF‐β1/Smad2/Smad3 signaling. Ecotoxicol Environ Saf. 2020;195:110464. [DOI] [PubMed] [Google Scholar]
- 265. Zhang S, Bian H, Li X, et al. Hydrogen sulfide promotes cell proliferation of oral cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep. 2016;35(5):2825‐2832. [DOI] [PubMed] [Google Scholar]
- 266. Lei Y, Zhen Y, Zhang W, et al. Exogenous hydrogen sulfide exerts proliferation, anti‐apoptosis, angiopoiesis and migration effects via activating HSP90 pathway in EC109 cells. Oncol Rep. 2016;35(6):3714‐3720. [DOI] [PubMed] [Google Scholar]
- 267. Yoo D, Jupiter RC, Pankey EA, et al. Analysis of cardiovascular responses to the H2S donors Na2S and NaHS in the rat. Am J Physiol Heart circul Physiol. 2015;309(4):H605‐H614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Gong W, Zhang S, Chen Y, et al. Protective role of hydrogen sulfide against diabetic cardiomyopathy via alleviating necroptosis. Free Rad Biol Med. 2022;181:29‐42. [DOI] [PubMed] [Google Scholar]
- 269. Bibli SI, Andreadou I, Chatzianastasiou A, et al. Cardioprotection by H2S engages a cGMP‐dependent protein kinase G/phospholamban pathway. Cardiovasc Res. 2015;106(3):432‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Ibrahim SA, Abdel‐Gaber SA, Ibrahim MA, Amin EF, Mohammed RK, Abdelrahman AM. Nitric oxide modulation as a potential molecular mechanism underlying the protective role of NaHS in liver ischemia reperfusion injury. Curr Mol Pharmacol. 2022;15(4):676‐682. [DOI] [PubMed] [Google Scholar]
- 271. Yu Q, Lu Z, Tao L, et al. ROS‐dependent neuroprotective effects of NaHS in ischemia brain injury involves the PARP/AIF pathway. Cell Physiol Biochem. 2015;36(4):1539‐1551. [DOI] [PubMed] [Google Scholar]
- 272. Ozturk T, Ertas E, Mert O. Use of Lawesson's reagent in organic syntheses. Chem Rev. 2007;107(11):5210‐5278. [DOI] [PubMed] [Google Scholar]
- 273. Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water‐soluble hydrogen sulfide‐releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351‐2360. [DOI] [PubMed] [Google Scholar]
- 274. Zhou T, Qian H, Zheng N, Lu Q, Han Y. GYY4137 ameliorates sepsis‐induced cardiomyopathy via NLRP3 pathway. Biochim Biophys Acta Mol Basis Dis. 2022;1868(12):166497. [DOI] [PubMed] [Google Scholar]
- 275. Meng G, Wang J, Xiao Y, et al. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29(3):203‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Zhao H, Qiu Y, Wu Y, Sun H, Gao S. Protective effects of GYY4137 on renal ischaemia/reperfusion injury through Nrf2‐mediated antioxidant defence. Kidney Blood Press Res. 2021;46(3):257‐265. [DOI] [PubMed] [Google Scholar]
- 277. Cui N, Luo H, Zhao Y. Protective effect of GYY4137, a water‑soluble hydrogen sulfide‑releasing molecule, on intestinal ischemia‑reperfusion. Mol Med Rep. 2020;21(3):1633‐1639. [DOI] [PubMed] [Google Scholar]
- 278. Chen LJ, Ning JZ, Cheng F, et al. Comparison of intraperitoneal and intratesticular GYY4137 therapy for the treatment of testicular ischemia reperfusion injury in rats. Curr Med Sci. 2020;40(2):332‐338. [DOI] [PubMed] [Google Scholar]
- 279. Qabazard B, Masocha W, Khajah M, Phillips OA. H(2)S donor GYY4137 ameliorates paclitaxel‐induced neuropathic pain in mice. Biomed Pharmacother. 2020;127:110210. [DOI] [PubMed] [Google Scholar]
- 280. Zhang Y, Chen S, Zhu J, et al. Overexpression of CBS/H(2)S inhibits proliferation and metastasis of colon cancer cells through downregulation of CD44. Cancer Cell Int. 2022;22(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Lu S, Gao Y, Huang X, Wang X. GYY4137, a hydrogen sulfide (H₂S) donor, shows potent anti‐hepatocellular carcinoma activity through blocking the STAT3 pathway. Int J Oncol. 2014;44(4):1259‐1267. [DOI] [PubMed] [Google Scholar]
- 282. Zhao H, Yan R, Zhou X, Ji F, Zhang B. Hydrogen sulfide improves colonic barrier integrity in DSS‐induced inflammation in Caco‐2 cells and mice. Int Immunopharmacol. 2016;39:121‐127. [DOI] [PubMed] [Google Scholar]
- 283. Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton, Vic). 2018;23(7):669‐675. [DOI] [PubMed] [Google Scholar]
- 284. Tsang RY, Al‐Fayea T, Au HJ. Cisplatin overdose: toxicities and management. Drug Saf. 2009;32(12):1109‐1122. [DOI] [PubMed] [Google Scholar]
- 285. Bebarta VS, Brittain M, Chan A, et al. Sodium nitrite and sodium thiosulfate are effective against acute cyanide poisoning when administered by intramuscular injection. Ann Emerg Med. 2017;69(6):718‐725. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Olson KR, Deleon ER, Gao Y, et al. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Compar Physiol. 2013;305(6):R592‐R603. [DOI] [PubMed] [Google Scholar]
- 287. Shirozu K, Tokuda K, Marutani E, Lefer D, Wang R, Ichinose F. Cystathionine γ‐lyase deficiency protects mice from galactosamine/lipopolysaccharide‐induced acute liver failure. Antioxid Redox Signal. 2014;20(2):204‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Sakaguchi M, Marutani E, Shin HS, et al. Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology. 2014;121(6):1248‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Marutani E, Yamada M, Ida T, et al. Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc. 2015;4(11):e002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Ravindran S, Jahir Hussain S, Boovarahan SR, Kurian GA. Sodium thiosulfate post‐conditioning protects rat hearts against ischemia reperfusion injury via reduction of apoptosis and oxidative stress. Chem Biol Interact. 2017;274:24‐34. [DOI] [PubMed] [Google Scholar]
- 291. Tokuda K, Kida K, Marutani E, et al. Inhaled hydrogen sulfide prevents endotoxin‐induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal. 2012;17(1):11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Zhang MY, Dugbartey GJ, Juriasingani S, Sener A. Hydrogen sulfide metabolite, sodium thiosulfate: clinical applications and underlying molecular mechanisms. Int J Mol Sci. 2021;22(12):6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. DeLeon ER, Gao Y, Huang E, Olson KR. Garlic oil polysulfides: H2S‐ and O2‐independent prooxidants in buffer and antioxidants in cells. Am J Physiol Regul Integr Compar Physiol. 2016;310(11):R1212‐R1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ried K, Fakler P. Potential of garlic (Allium sativum) in lowering high blood pressure: mechanisms of action and clinical relevance. Integrd Blood Press Control. 2014;7:71‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3 Suppl):716s‐725s. [DOI] [PubMed] [Google Scholar]
- 296. Rose P, Moore PK, Zhu YZ. Garlic and gaseous mediators. Trends Pharmacol Sci. 2018;39(7):624‐634. [DOI] [PubMed] [Google Scholar]
- 297. Szczesny B, Módis K, Yanagi K, et al. AP39, a novel mitochondria‐targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014;41:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. da Costa Marques LA, Teixeira SA, de Jesus FN, et al. Vasorelaxant activity of AP39, a mitochondria‐targeted H(2)S donor, on mouse mesenteric artery rings in vitro. Biomolecules. 2022;12(2):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Teng H, Yang B, Su Y, et al. Aminooxyacetic acid hemihydrochloride leads to decreased intracellular ATP levels and altered cell cycle of prostate cancer cells by suppressing energy metabolism. Biomed Pharmacother. 2023;167:115605. [DOI] [PubMed] [Google Scholar]
- 300. Chao C, Zatarain JR, Ding Y, et al. Cystathionine‐beta‐synthase inhibition for colon cancer: Enhancement of the efficacy of aminooxyacetic acid via the prodrug approach. Mol Med. 2016;22:361‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ye F, Li X, Sun K, et al. Inhibition of endogenous hydrogen sulfide biosynthesis enhances the anti‐cancer effect of 3,3'‐diindolylmethane in human gastric cancer cells. Life Sci. 2020;261:118348. [DOI] [PubMed] [Google Scholar]
- 302. Untereiner AA, Pavlidou A, Druzhyna N, Papapetropoulos A, Hellmich MR, Szabo C. Drug resistance induces the upregulation of H(2)S‐producing enzymes in HCT116 colon cancer cells. Biochem Pharmacol. 2018;149:174‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Wang D, Yang H, Zhang Y, et al. Inhibition of cystathionine β‐synthase promotes apoptosis and reduces cell proliferation in chronic myeloid leukemia. Signal Transd Target Ther. 2021;6(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Wang HG, Wang D, Sarfraz M, et al. Endogenous hydrogen sulfide inhibition suppresses tumor growth by promoting apoptosis and pyroptosis in esophageal cancer cells. Transl Oncol. 2023;38:101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Peleli M, Antoniadou I, Rodrigues‐Junior DM, et al. Cystathionine gamma‐lyase (CTH) inhibition attenuates glioblastoma formation. Redox Biol. 2023;64:102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Wang DY, Zhang J, Li HX, et al. Inhibition of endogenous hydrogen sulfide production suppresses the growth of nasopharyngeal carcinoma cells. Mol Carcinog. 2023;62(5):652‐664. [DOI] [PubMed] [Google Scholar]
- 307. Khan NH, Wang D, Wang W, et al. Pharmacological inhibition of endogenous hydrogen sulfide attenuates breast cancer progression. Molecules. 2022;27(13):4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Rong F, Wang T, Zhou Q, et al. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioactive Mater. 2023;19:198‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Kaur K, Carrazzone RJ, Matson JB. The benefits of macromolecular/supramolecular approaches in hydrogen sulfide delivery: a review of polymeric and self‐assembled hydrogen sulfide donors. Antioxid Redox Signal. 2020;32(2):79‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Foster JC, Radzinski SC, Zou X, Finkielstein CV, Matson JB. H(2)S‐releasing polymer micelles for studying selective cell toxicity. Mol Pharm. 2017;14(4):1300‐1306. [DOI] [PubMed] [Google Scholar]
- 311. Ercole F, Mansfeld FM, Kavallaris M, et al. Macromolecular hydrogen sulfide donors trigger spatiotemporally confined changes in cell signaling. Biomacromolecules. 2016;17(1):371‐383. [DOI] [PubMed] [Google Scholar]
- 312. Zhou M, Qian Y, Zhu Y, Matson J. Elastase‐triggered H(2)S delivery from polymer hydrogels. Chem Commun (Camb). 2020;56(7):1085‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Gazzano E, Buondonno I, Marengo A, et al. Hyaluronated liposomes containing H2S‐releasing doxorubicin are effective against P‐glycoprotein‐positive/doxorubicin‐resistant osteosarcoma cells and xenografts. Cancer Lett. 2019;456:29‐39. [DOI] [PubMed] [Google Scholar]
- 314. Zhao Y, Yang C, Organ C, et al. Design, synthesis, and cardioprotective effects of N‐mercapto‐based hydrogen sulfide donors. J Med Chem. 2015;58(18):7501‐7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Zhao Y, Bhushan S, Yang C, et al. Controllable hydrogen sulfide donors and their activity against myocardial ischemia‐reperfusion injury. ACS Chem Biol. 2013;8(6):1283‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Takatani‐Nakase T, Katayama M, Matsui C, et al. Hydrogen sulfide donor micelles protect cardiomyocytes from ischemic cell death. Mol Biosyst. 2017;,13(9):1705‐1708. [DOI] [PubMed] [Google Scholar]
- 317. Sun X, Wang Y, Wen S, et al. Novel controlled and targeted releasing hydrogen sulfide system exerts combinational cerebral and myocardial protection after cardiac arrest. J Nanobiotechnol. 2021;19(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Hsieh MH, Tsai HW, Lin KJ, et al. An in situ slow‐releasing H(2)S donor depot with long‐term therapeutic effects for treating ischemic diseases. Mater Sci Eng C Mater Biol Appl. 2019;104:109954. [DOI] [PubMed] [Google Scholar]
- 319. Lin WC, Pan WY, Liu CK, et al. In situ self‐spray coating system that can uniformly disperse a poorly water‐soluble H(2)S donor on the colorectal surface to treat inflammatory bowel diseases. Biomaterials. 2018;182:289‐298. [DOI] [PubMed] [Google Scholar]
- 320. Lee J, Yang C, Ahn S, Choi Y, Lee K. Enhanced NO‐induced angiogenesis via NO/H(2)S co‐delivery from self‐assembled nanoparticles. Biomater Sci. 2021;9(15):5150‐5159. [DOI] [PubMed] [Google Scholar]
- 321. Chen JJY, van der Vlies AJ, Hasegawa U. Hydrogen sulfide‐releasing micelles for promoting angiogenesis. Polym Chem. 2020;11:4454‐4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Zheng Z, Chen A, He H, et al. pH and enzyme dual‐responsive release of hydrogen sulfide for disc degeneration therapy. J Mater Chem B. 2019;7(4):611‐618. [DOI] [PubMed] [Google Scholar]
- 323. Yu Y, Wang Z, Ding Q, et al. The preparation of a novel poly(lactic acid)‐based sustained H(2)S releasing microsphere for rheumatoid arthritis alleviation. Pharmaceutics. 2021;13(5):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. He T, Qin X, Jiang C, et al. Tumor pH‐responsive metastable‐phase manganese sulfide nanotheranostics for traceable hydrogen sulfide gas therapy primed chemodynamic therapy. Theranostics. 2020;10(6):2453‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Dao NV, Ercole F, Urquhart MC, et al. Trisulfide linked cholesteryl PEG conjugate attenuates intracellular ROS and collagen‐1 production in a breast cancer co‐culture model. Biomater Sci. 2021;9(3):835‐846. [DOI] [PubMed] [Google Scholar]
- 326. Liu Y, Yang F, Yuan C, et al. Magnetic nanoliposomes as in situ microbubble bombers for multimodality image‐guided cancer theranostics. ACS Nano. 2017;11(2):1509‐1519. [DOI] [PubMed] [Google Scholar]
- 327. Liu Y, Li J, Chen H, et al. Magnet‐activatable nanoliposomes as intracellular bubble microreactors to enhance drug delivery efficacy and burst cancer cells. Nanoscale. 2019;11(40):18854‐18865. [DOI] [PubMed] [Google Scholar]
- 328. Xie C, Cen D, Ren Z, et al. FeS@BSA nanoclusters to enable H(2)S‐amplified ROS‐based therapy with MRI guidance. Adv Sci. 2020;7(7):1903512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia‐reperfusion injury. Eur J Pharmacol. 2008;587(1‐3):1‐7. [DOI] [PubMed] [Google Scholar]
- 330. Wang Y, Zhao X, Jin H, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29(2):173‐917. [DOI] [PubMed] [Google Scholar]
- 331. Pan LL, Liu XH, Gong QH, Wu D, Zhu YZ. Hydrogen sulfide attenuated tumor necrosis factor‐α‐induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS One. 2011;6(5):e19766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 332. Kaur K, Wang Y, Matson JB. Linker‐regulated H(2)S release from aromatic peptide amphiphile hydrogels. Biomacromolecules. 2020;21(3):1171‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Longchamp A, Kaur K, Macabrey D, et al. Hydrogen sulfide‐releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomater. 2019;97:374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Zhang H, Hao LZ, Pan JA, et al. Microfluidic fabrication of inhalable large porous microspheres loaded with H(2)S‐releasing aspirin derivative for pulmonary arterial hypertension therapy. J Control Rel. 2021;329:286‐298. [DOI] [PubMed] [Google Scholar]
- 335. Cui X, Yao M, Feng Y, et al. Exogenous hydrogen sulfide alleviates hepatic endoplasmic reticulum stress via SIRT1/FoxO1/PCSK9 pathway in NAFLD. FASEB J. 2023;37(8):e23027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


