Abstract
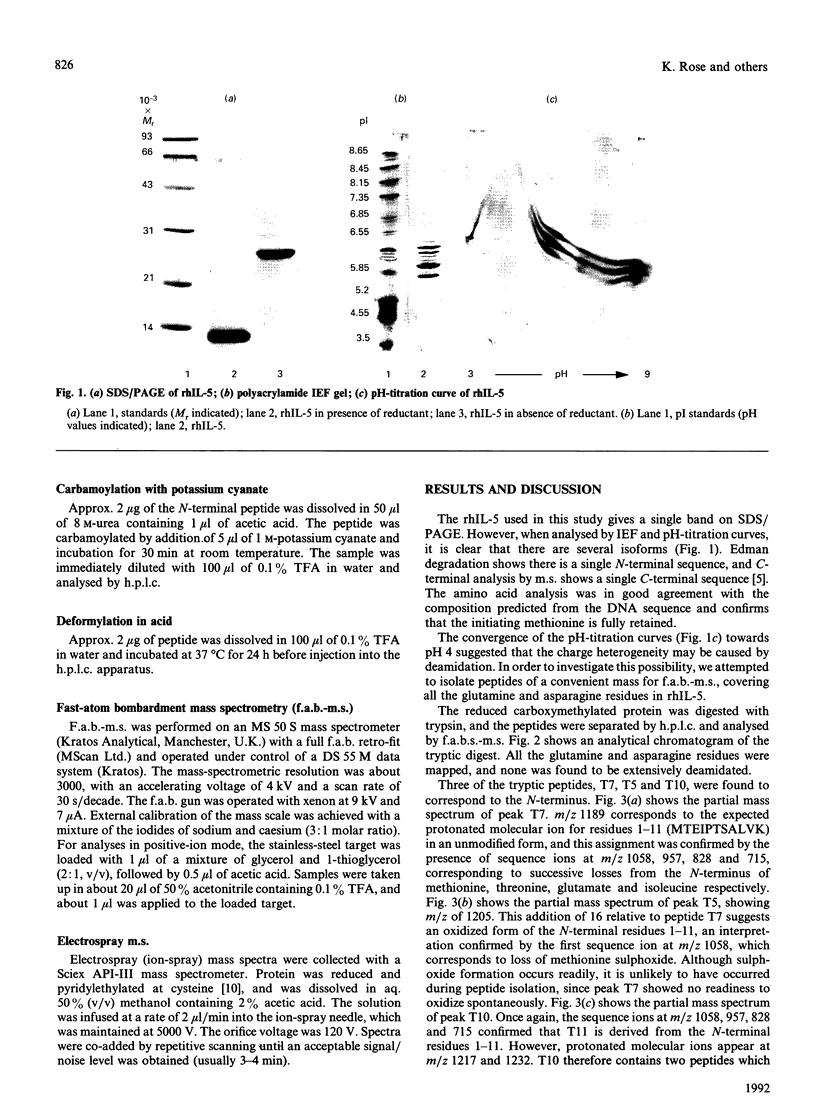
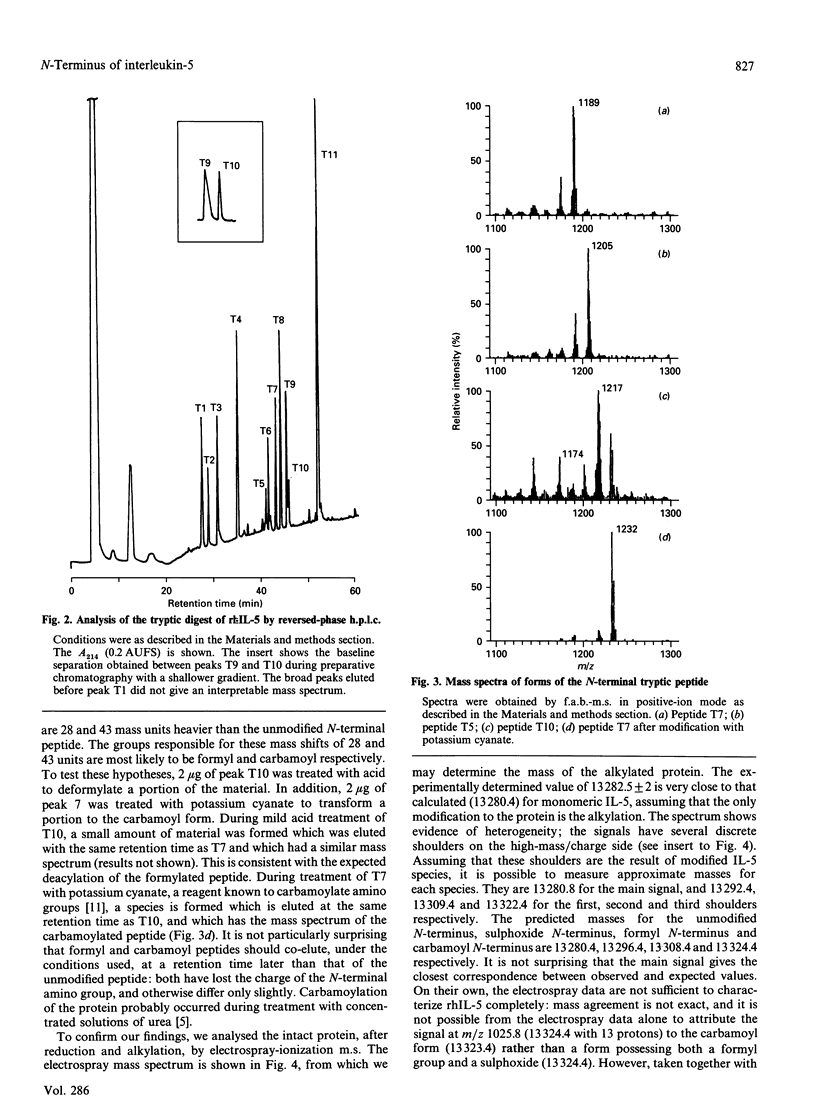
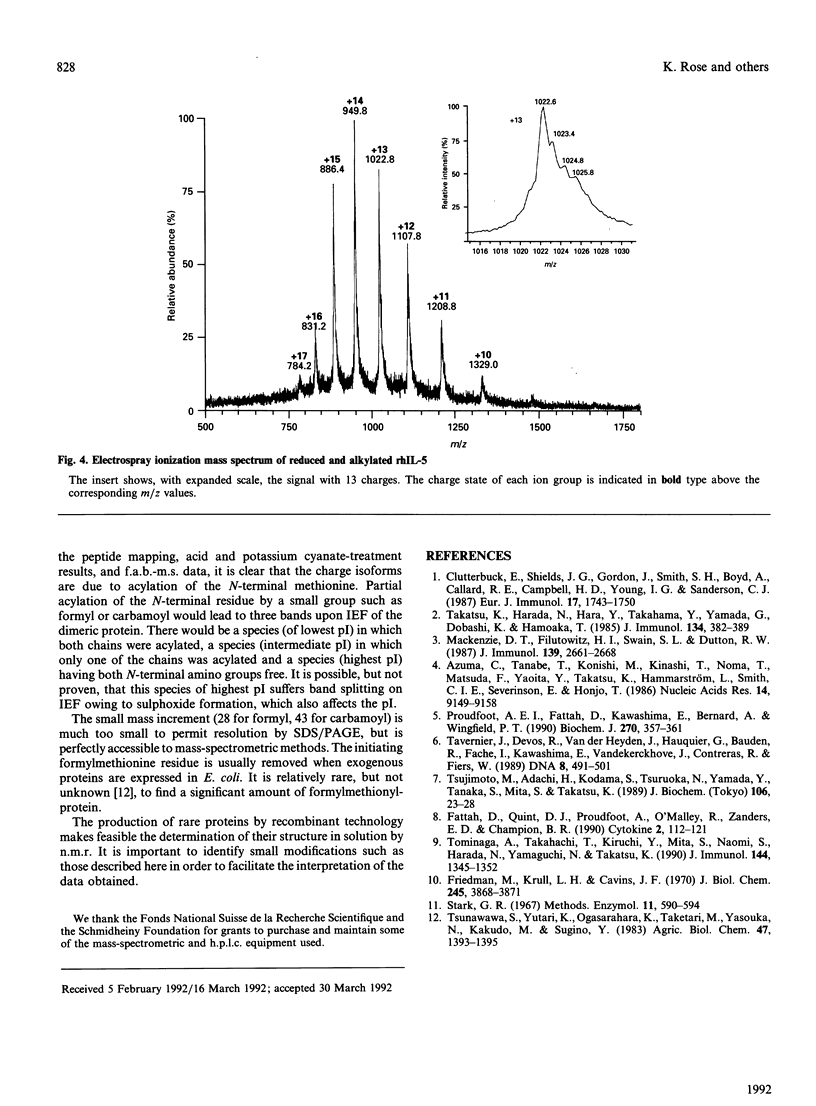
Recombinant human interleukin-5 exists as four major isoforms all possessing N-terminal methionine. Peptide mapping and subsequent analysis by fast-atom-bombardment mass spectrometry (f.a.b.-m.s.) have shown that N-terminal modifications are the cause of the charge heterogeneity. In order of decreasing abundance, these are unmodified methionine, retention of N-terminal formyl group, oxidation of N-terminal methionine to sulphoxide and carbamoylation of the N-terminus. These results were confirmed by analysis of the reduced and alkylated intact protein by electrospray-ionization mass spectrometry. The implications of these findings for the production and characterization of recombinant proteins are briefly discussed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma C., Tanabe T., Konishi M., Kinashi T., Noma T., Matsuda F., Yaoita Y., Takatsu K., Hammarström L., Smith C. I. Cloning of cDNA for human T-cell replacing factor (interleukin-5) and comparison with the murine homologue. Nucleic Acids Res. 1986 Nov 25;14(22):9149–9158. doi: 10.1093/nar/14.22.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck E., Shields J. G., Gordon J., Smith S. H., Boyd A., Callard R. E., Campbell H. D., Young I. G., Sanderson C. J. Recombinant human interleukin 5 is an eosinophil differentiation factor but has no activity in standard human B cell growth factor assays. Eur J Immunol. 1987 Dec;17(12):1743–1750. doi: 10.1002/eji.1830171210. [DOI] [PubMed] [Google Scholar]
- Fattah D., Quint D. J., Proudfoot A., O'Malley R., Zanders E. D., Champion B. R. In vitro and in vivo studies with purified recombinant human interleukin 5. Cytokine. 1990 Mar;2(2):112–121. doi: 10.1016/1043-4666(90)90005-e. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- McKenzie D. T., Filutowicz H. I., Swain S. L., Dutton R. W. Purification and partial sequence analysis of murine B cell growth factor II (interleukin 5). J Immunol. 1987 Oct 15;139(8):2661–2668. [PubMed] [Google Scholar]
- Proudfoot A. E., Fattah D., Kawashima E. H., Bernard A., Wingfield P. T. Preparation and characterization of human interleukin-5 expressed in recombinant Escherichia coli. Biochem J. 1990 Sep 1;270(2):357–361. doi: 10.1042/bj2700357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Harada N., Hara Y., Takahama Y., Yamada G., Dobashi K., Hamaoka T. Purification and physicochemical characterization of murine T cell replacing factor (TRF). J Immunol. 1985 Jan;134(1):382–389. [PubMed] [Google Scholar]
- Tavernier J., Devos R., Van der Heyden J., Hauquier G., Bauden R., Fache I., Kawashima E., Vandekerckhove J., Contreras R., Fiers W. Expression of human and murine interleukin-5 in eukaryotic systems. DNA. 1989 Sep;8(7):491–501. doi: 10.1089/dna.1.1989.8.491. [DOI] [PubMed] [Google Scholar]
- Tominaga A., Takahashi T., Kikuchi Y., Mita S., Naomi S., Harada N., Yamaguchi N., Takatsu K. Role of carbohydrate moiety of IL-5. Effect of tunicamycin on the glycosylation of IL-5 and the biologic activity of deglycosylated IL-5. J Immunol. 1990 Feb 15;144(4):1345–1352. [PubMed] [Google Scholar]
- Tsujimoto M., Adachi H., Kodama S., Tsuruoka N., Yamada Y., Tanaka S., Mita S., Takatsu K. Purification and characterization of recombinant human interleukin 5 expressed in Chinese hamster ovary cells. J Biochem. 1989 Jul;106(1):23–28. doi: 10.1093/oxfordjournals.jbchem.a122812. [DOI] [PubMed] [Google Scholar]