Abstract
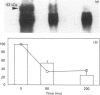
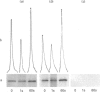
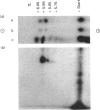
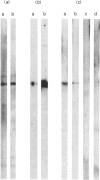
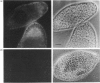
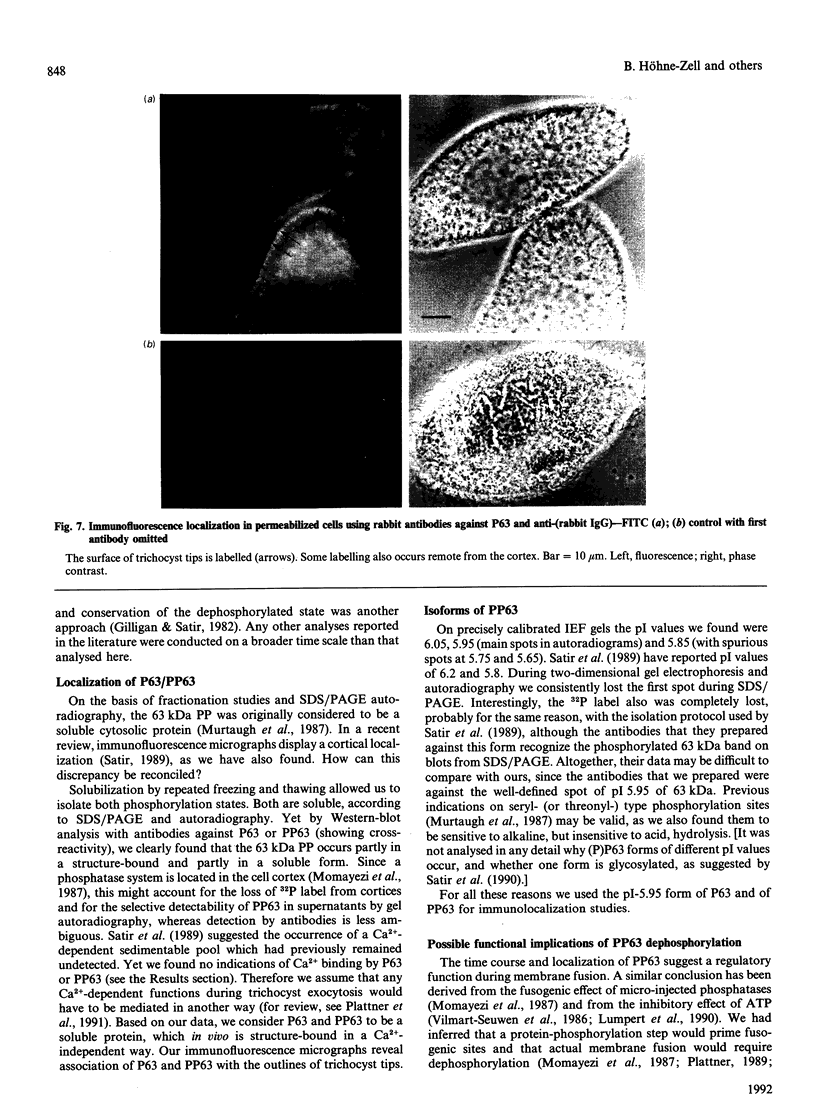
We had previously shown that a phosphoprotein of 63 kDa ('PP63') is rapidly and selectively dephosphorylated during synchronous (less than or equal to 1 s) trichocyst exocytosis in Paramecium cells and then rephosphorylated within less than or equal to 1 min [Zieseniss & Plattner (1985) J. Cell Biol. 101, 2028-2035]. Using a new quenched-flow device, we now find a strict correlation between PP63 dephosphorylation and the process of membrane fusion, both occurring within 80 ms. Uptake of 32P over 90 min, followed by exocytosis and rephosphorylation for 1 min, results in a rather selective phosphorylation of the dephosphorylated form, P63, to PP63. Solubilization by repeated freezing and thawing allows isolations of P63 and PP63. On isoelectric focusing autoradiograms they have pI values of 6.05, 5.95 (major spots), 5.85 and 5.75. All spots are sensitive to alkaline, but not to acidic, hydrolysis (except for the pI-6.05 spot). On two-dimensional-gel autoradiograms the most prominent spot, of pI 5.95, is most extensively de- and re-phosphorylated. This spot, from de- and re-phosphorylated samples, was used to produce monospecific antibodies. A cortical localization of PP63 was revealed by producing Western blots from isolated cell-surface fragments ('cortices') and by immunofluorescence labelling. We assume that both P63 and PP63 are attached to cortical structures, e.g. around trichocysts, though they are partly soluble. This localization and the strict correlation of PP63 dephosphorylation with exocytotic membrane fusion suggests a role in fusion regulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Exocytosis. Annu Rev Physiol. 1990;52:607–624. doi: 10.1146/annurev.ph.52.030190.003135. [DOI] [PubMed] [Google Scholar]
- Beisson J., Lefort-Tran M., Pouphile M., Rossignol M., Satir B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J Cell Biol. 1976 Apr;69(1):126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa C., Chapdelaine A., Roberts K. D., Chevalier S. Enhancement of the detection of alkali-resistant phosphoproteins in polyacrylamide gels. Anal Biochem. 1988 Mar;169(2):356–362. doi: 10.1016/0003-2697(88)90295-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Treml S., Brooks M. Thiophosphorylation prevents catecholamine secretion by chemically skinned chromaffin cells. Life Sci. 1984 Jul 30;35(5):569–574. doi: 10.1016/0024-3205(84)90251-0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991 Jul 22;1071(2):174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Churcher Y., Kramer K. M., Gomperts B. D. Evidence for protein dephosphorylation as a permissive step in GTP-gamma-S-induced exocytosis from permeabilized mast cells. Cell Regul. 1990 Jun;1(7):523–530. doi: 10.1091/mbc.1.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Smith D. E. Affinity purification of antibodies using antigens immobilized on solid supports. Biochem Soc Trans. 1988 Apr;16(2):134–138. doi: 10.1042/bst0160134. [DOI] [PubMed] [Google Scholar]
- Gilligan D. M., Satir B. H. Protein phosphorylation/dephosphorylation and stimulus-secretion coupling in wild type and mutant Paramecium. J Biol Chem. 1982 Dec 10;257(23):13903–13906. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Keryer G., Davis F. M., Rao P. N., Beisson J. Protein phosphorylation and dynamics of cytoskeletal structures associated with basal bodies in Paramecium. Cell Motil Cytoskeleton. 1987;8(1):44–54. doi: 10.1002/cm.970080107. [DOI] [PubMed] [Google Scholar]
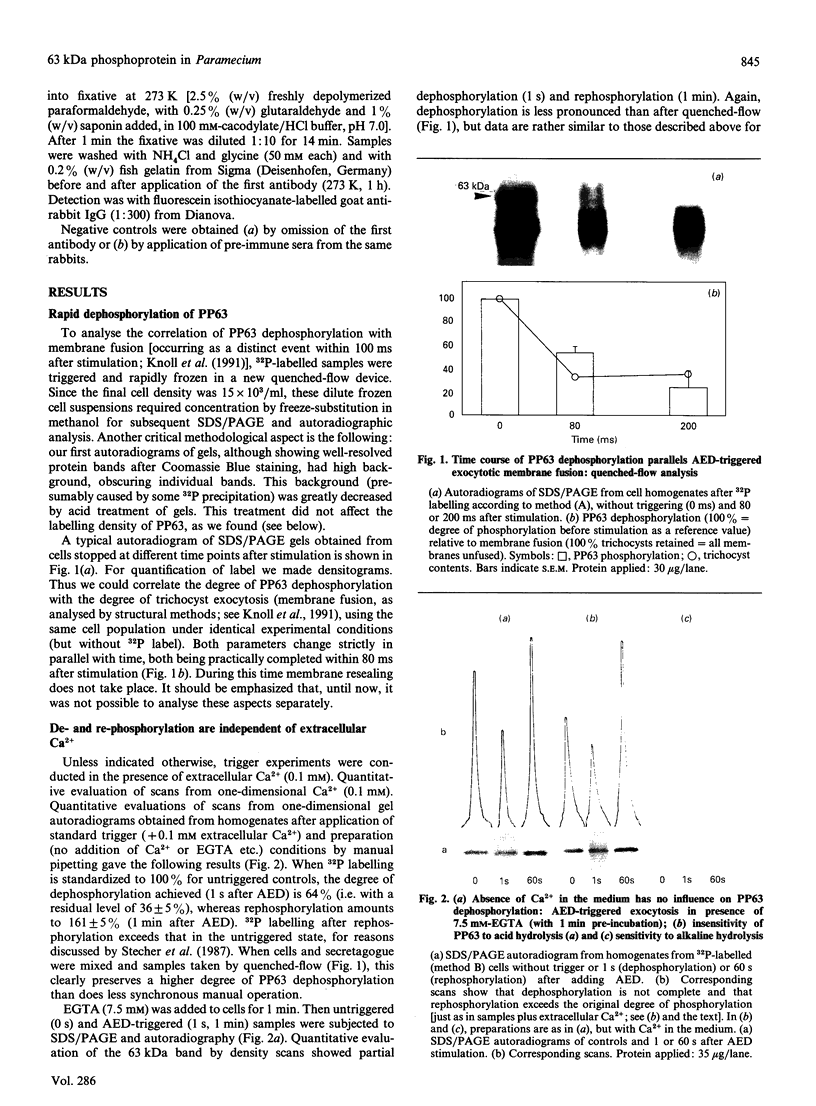
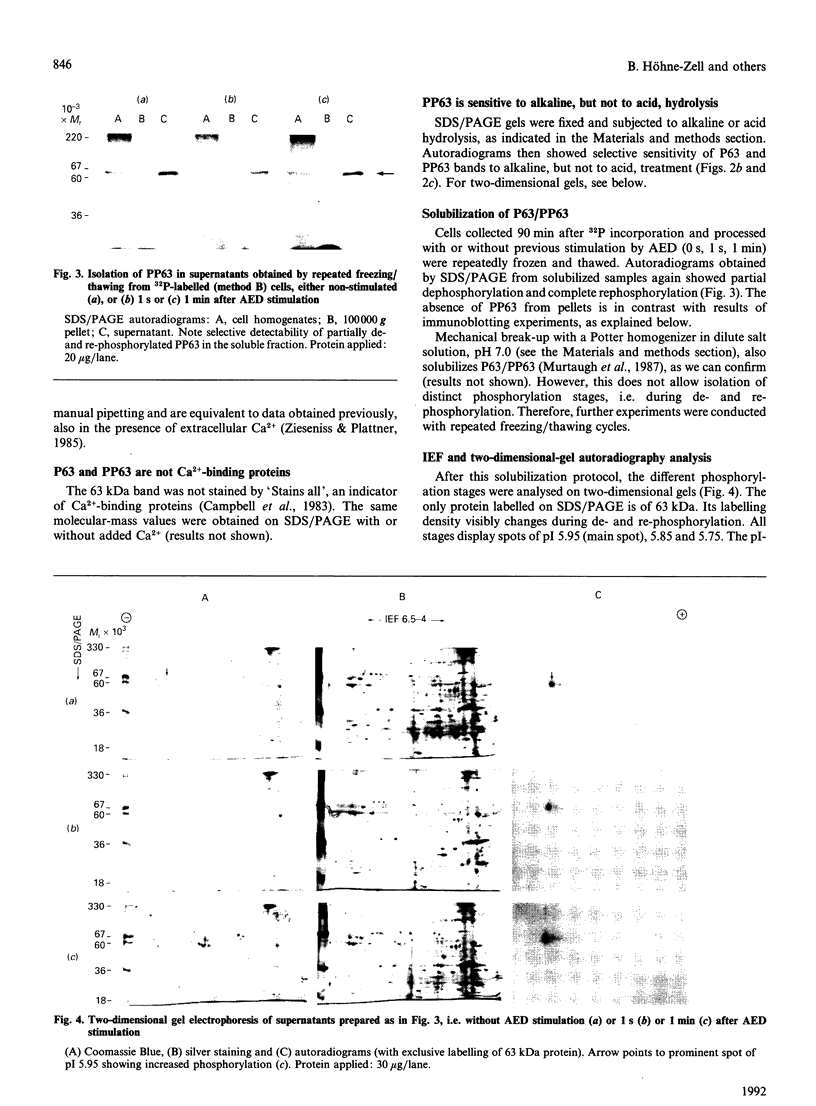
- Knoll G., Braun C., Plattner H. Quenched flow analysis of exocytosis in Paramecium cells: time course, changes in membrane structure, and calcium requirements revealed after rapid mixing and rapid freezing of intact cells. J Cell Biol. 1991 Jun;113(6):1295–1304. doi: 10.1083/jcb.113.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lumpert C. J., Kersken H., Plattner H. Cell surface complexes ('cortices') isolated from Paramecium tetraurelia cells as a model system for analysing exocytosis in vitro in conjunction with microinjection studies. Biochem J. 1990 Aug 1;269(3):639–645. doi: 10.1042/bj2690639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol. 1987 Jul;105(1):181–189. doi: 10.1083/jcb.105.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh T. J., Gilligan D. M., Satir B. H. Purification of and production of an antibody against a 63,000 Mr stimulus-sensitive phosphoprotein in Paramecium. J Biol Chem. 1987 Nov 15;262(32):15734–15739. [PubMed] [Google Scholar]
- Plattner H., Lumpert C. J., Knoll G., Kissmehl R., Höhne B., Momayezi M., Glas-Albrecht R. Stimulus-secretion coupling in Paramecium cells. Eur J Cell Biol. 1991 Jun;55(1):3–16. [PubMed] [Google Scholar]
- Plattner H., Miller F., Bachmann L. Membrane specializations in the form of regular membrane-to-membrane attachment sites in Paramecium. A correlated freeze-etching and ultrathin-sectioning analysis. J Cell Sci. 1973 Nov;13(3):687–719. doi: 10.1242/jcs.13.3.687. [DOI] [PubMed] [Google Scholar]
- Plattner H. Regulation of membrane fusion during exocytosis. Int Rev Cytol. 1989;119:197–286. doi: 10.1016/s0074-7696(08)60652-x. [DOI] [PubMed] [Google Scholar]
- Plattner H., Stürzl R., Matt H. Synchronous exocytosis in Paramecium cells. IV. Polyamino compounds as potent trigger agents for repeatable trigger-redocking cycles. Eur J Cell Biol. 1985 Jan;36(1):32–37. [PubMed] [Google Scholar]
- Pollard-Knight D., Read C. A., Downes M. J., Howard L. A., Leadbetter M. R., Pheby S. A., McNaughton E., Syms A., Brady M. A. Nonradioactive nucleic acid detection by enhanced chemiluminescence using probes directly labeled with horseradish peroxidase. Anal Biochem. 1990 Feb 15;185(1):84–89. doi: 10.1016/0003-2697(90)90259-c. [DOI] [PubMed] [Google Scholar]
- Roth J., Lentze M. J., Berger E. G. Immunocytochemical demonstration of ecto-galactosyltransferase in absorptive intestinal cells. J Cell Biol. 1985 Jan;100(1):118–125. doi: 10.1083/jcb.100.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H., Hamasaki T., Reichman M., Murtaugh T. J. Species distribution of a phosphoprotein (parafusin) involved in exocytosis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):930–932. doi: 10.1073/pnas.86.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H. Signal transduction events associated with exocytosis in ciliates. J Protozool. 1989 Jul-Aug;36(4):382–389. doi: 10.1111/j.1550-7408.1989.tb05531.x. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Srisomsap C., Reichman M., Marchase R. B. Parafusin, an exocytic-sensitive phosphoprotein, is the primary acceptor for the glucosylphosphotransferase in Paramecium tetraurelia and rat liver. J Cell Biol. 1990 Sep;111(3):901–907. doi: 10.1083/jcb.111.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B., Höhne B., Gras U., Momayezi M., Glas-Albrecht R., Plattner H. Involvement of a 65 kDa phosphoprotein in the regulation of membrane fusion during exocytosis in Paramecium cells. FEBS Lett. 1987 Oct 19;223(1):25–32. doi: 10.1016/0014-5793(87)80503-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Vilmart-Seuwen J., Kersken H., Stürzl R., Plattner H. ATP keeps exocytosis sites in a primed state but is not required for membrane fusion: an analysis with Paramecium cells in vivo and in vitro. J Cell Biol. 1986 Oct;103(4):1279–1288. doi: 10.1083/jcb.103.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilmart J., Plattner H. Membrane-integrated proteins at preformed exocytosis sites. J Histochem Cytochem. 1983 May;31(5):626–632. doi: 10.1177/31.5.6841968. [DOI] [PubMed] [Google Scholar]
- Whalley T., Crossley I., Whitaker M. Phosphoprotein inhibition of calcium-stimulated exocytosis in sea urchin eggs. J Cell Biol. 1991 May;113(4):769–778. doi: 10.1083/jcb.113.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieseniss E., Plattner H. Synchronous exocytosis in Paramecium cells involves very rapid (less than or equal to 1 s), reversible dephosphorylation of a 65-kD phosphoprotein in exocytosis-competent strains. J Cell Biol. 1985 Dec;101(6):2028–2035. doi: 10.1083/jcb.101.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]