Key Clinical Message
This case demonstrated the rare “shark fin” ECG pattern, an ST‐segment elevation typically seen in acute myocardial infarction. We reported a case of takotsubo cardiomyopathy secondary to influenza A infection with multiple organ failure, showing the shark fin sign and resulting in in‐patient mortality and various complication.
Keywords: cardiogenic shock, influenza A infection, shark fin ECG, Takotsubo cardiomyopathy
This case of takotsubo cardiomyopathy secondary to influenza A infection presented with a shark fin ECG, leading to multiple organ failure, various complications, and in‐patient mortality.
1. INTRODUCTION
The Shark fin electrocardiographic (ECG) pattern, characterized by a giant R wave with amplitude above 1 mV and QRS complex merging with the ST‐segment and T‐wave, is rare, occurring in approximately 1.4% of patients with ST‐segment elevation myocardial infarction (STEMI). 1 , 2 This distinct ECG pattern was found to be associated with a large ischemic burden and adverse in‐hospital outcomes, including ventricular arrhythmia, cardiogenic shock, and cardiac arrest. 3
Interestingly, recent data have highlighted the presence of the unique shark fin ECG features in patients with non‐ischemic conditions such as myopericarditis, severe hypokalemia, Brugada syndrome, and others. 4 , 5 , 6 However, the prognosis for each condition remains uncertain and varies on a case‐by‐case basis. 3 In our case report, we present a unique instance where a patient exhibited the Shark Fin sign in the context of Takotsubo cardiomyopathy (TTC) with profound shock and multiple organ failure due to influenza A infection.
2. CASE DESCRIPTION
An 80‐year‐old woman with end‐stage kidney disease (ESKD) on intermittent hemodialysis via arteriovenous fistula, type 2 diabetes, hypertension, and paroxysmal atrial fibrillation managed with Apixaban was brought to the hospital from a nursing home due to a 2‐day history of productive cough, dyspnea, and chest pain. Her caretaker noted that she had experienced increasing shortness of breath and chest pain over the past few days, which had restricted her usual activities. Typically independent, she had become bedridden with worsening symptoms and appeared more drowsy than usual. The chest pain was described as a constant, dull ache in the middle of the chest of which limited her daily activities. She denied having fever, dysuria, headache, diarrhea, abdominal pain, or other specific symptoms. Her medical history also includes hypertension, type 2 diabetes, class 1 obesity, and hyperlipidemia. Based on this presentation, she was brought in by emergency medical services for evaluation. Regarding her ESKD, patient received receives intermittent hemodialysis every Monday, Wednesday, and Friday. The patient has been on dialysis for 4 years due to hypertension and diabetes mellitus. The most recent HD session was 2 days prior to the current presentation, with no missed HD sessions reported. For her anemia, the patient received erythropoietin as recommended by nephrology during her dialysis sessions. She has not had any blood transfusions in the past year. Her colonoscopy cancer screenings are up‐to‐date, and results have been normal up until this presentation.
On examination, she was afebrile but tachycardic with a regular heart rate of 120 bpm, blood pressure of 100/60 mmHg, tachypneic with a respiratory rate of 25/min, and had an oxygen saturation of 90% on room air, improving to 97% with 4 L/min of oxygen nasal cannula. Cardiac examination revealed no jugular venous distension, normal S1 and S2 heart sounds, and no murmurs or S3 sound. Lung examination showed crackles at the bases of both lungs. Her throat was injected without tonsillar exudate, and there was no palpable lymphadenopathy. The abdominal examination was unremarkable. Her left arm AV fistula was intact, with a palpable thrill and no signs of infection. Neurological evaluation showed no signs of meningism, and motor and sensory function was grossly unremarkable.
3. METHODS
The initial laboratory evaluation in the emergency department (ED) revealed leukocytosis with white blood cell count (WBC) of 13.7 K/μL (normal range: 4.0–11.0 K/μL), normocytic anemia with hemoglobin level of 9.6 gm/dL at her baseline (normal range: 12.0–16.0 gm/dL), and normal platelets count. Creatinine was 4.38 mg/dL in the context of ESKD. The Nasopharyngeal respiratory viral panel was tested due to flu‐like symptoms of which was positive for influenza A infection.
An ECG performed upon arrival showed sinus tachycardia at a rate of 110 BPM without ischemic ST‐T changes (Figure 1). Initial troponin was elevated at 1.18 ng/mL (normal range: <0.03 ng/mL), prompting a cardiology consultation for elevated troponin in the context of chest pain influenza A infection. The differential diagnosis included acute myocardial infarction (AMI) type 1 and type 2, acute myocarditis, and pulmonary embolism (PE).
FIGURE 1.
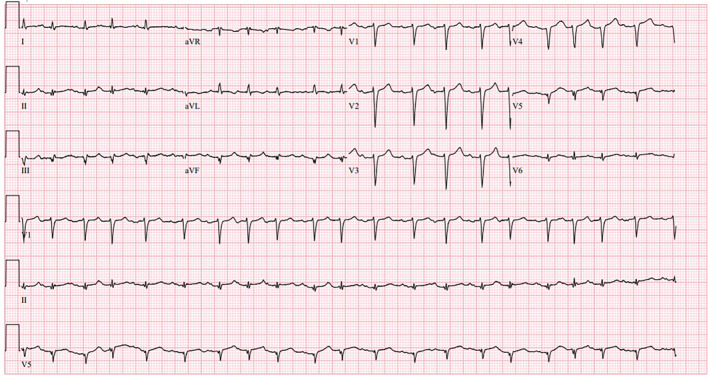
ECG on arrival of the patient.
To differentiate between these conditions, bedside transthoracic echocardiogram (TTE) was performed by emergency physician. The TTE revealed reduced left ventricular ejection fraction (LVEF), with no baseline cardiac function available for comparison. No regional wall motion abnormalities were found. The absence of regional wall motion abnormalities made acute myocardial infarction less likely.
Chest x‐ray showed no significant pulmonary infiltration and no cardiomegaly, which helped to rule out acute exacerbation of heart failure secondary to volume overload or severe infection‐related pulmonary involvement. A computed tomography (CT) pulmonary angiogram was performed and was negative for PE, ruling out PE as a cause of her symptoms and elevated troponin. Considering the positive influenza A test and the elevated troponin without clear evidence of acute coronary syndrome or PE, the differential diagnosis leaned towards acute myocarditis or stress‐induced cardiomyopathy (Takotsubo cardiomyopathy) related to the viral infection. The patient was admitted to the cardiac telemetry unit for the treatment of influenza A infection and management of acute HF. Oseltamivir, adjusted for renal function, was promptly started while the patient was in the ED due to the positive influenza A test and her flu‐like symptom with sign of respiratory distress. Given her reduced LVEF and elevated troponin in the context of a viral infection, close cardiac monitoring was warranted to manage potential complications and to observe her response to antiviral therapy and supportive care.
During her stay in the cardiac telemetry unit, subsequent troponin levels increased to 6.37 ng/mL within the next 12 h, accompanied by chest pain, shortness of breath, and hypoxic respiratory failure. These symptoms raised concerns for AMI necessitating further diagnostic evaluation. This led to an emergency coronary angiogram, which showed no epicardial coronary artery disease. Right heart catheterization revealed normal filling pressures with no signs of cardiogenic shock. The hemodynamics profiles were as follows: mean right atrial pressure was 1 mmHg, right ventricular pressure was 34/0 mmHg, mean pulmonary artery pressure was 25 mmHg, and mean pulmonary capillary wedge pressure was 10 mmHg. Pulmonary artery oxygen saturation was 67%, while systemic arterial oxygen saturation was 98%. The estimated cardiac output by the Fick method was 5.9 L/min, with a cardiac index of 3.5 L/min/m2. Repeated TTE was done by cardiologist on hospital admission Day 2 which showed LVEF of 25%–30%, associated with hypokinesis of the mid to apical LV segments and normal contractility of the basal segments, raising concerns for TTC (Video 1).
VIDEO 1.
Transthoracic echocardiogram (TTE) finding.
Given these findings, HF specialist was consulted for further evaluation and suggested adequate volume management via hemodialysis. Nephrologist was followed along to manage long‐term hemodialysis, ensuring optimal fluid and electrolyte balance. Guideline‐directed medical therapy (GDMT), including carvedilol and valsartan, was administered to optimize cardiac function. Carvedilol, beta‐adrenergic‐blocking drug with vasodilating properties, helps reduce myocardial oxygen demand and improve left ventricular function, while valsartan, an angiotensin II receptor blocker, helps reduce afterload and prevent cardiac remodeling. These medications are foundational in managing heart failure with reduced ejection fraction (HFrEF). Additionally, adequate oxygen support of 4 L/min nasal cannula was provided to alleviate hypoxia and improve oxygenation, addressing her hypoxic respiratory failure. This supportive measure was crucial in stabilizing her respiratory status while the underlying cardiac issues were addressed.
4. RESULTS (HOSPITAL COURSE)
On hospital admission Day 4, the patient experienced a worsening mental status, becoming difficult to arouse, with Kussmaul breathing and hypotension. The rapid response team was called, and the patient underwent endotracheal intubation for airway protection, acute hypoxic respiratory failure, and worsening metabolic acidosis. An ECG at the time of intubation showed significant ST‐T changes in the shark fin pattern, and repeated troponin levels were markedly elevated to 16 ng/mL (Figure 2). This prompted a repeated emergency coronary angiogram, which again showed no evidence of epicardial coronary artery disease. The thoracic CT was done which revealed new multifocal patchy consolidations in the right upper lobe, right lower lobe, and left lower lobe, likely indicating multifocal pneumonia. CT head without contrast showed no evidence of intracranial bleeding. The patient was transferred to the medical intensive care unit for further management of multifocal pneumonia from influenza A infection, with possible bacterial superinfection, TTC, hypoxic respiratory failure and profound shock requiring vasopressors. Despite receiving intravenous empirical antibiotics (Cefepime and Vancomycin), mechanical ventilation support, continuous renal replacement therapy, and advanced hemodynamic support in the ICU, she developed refractory shock. Unfortunately, on hospital admission Day 5, she went into cardiac arrest and could not be resuscitated.
FIGURE 2.
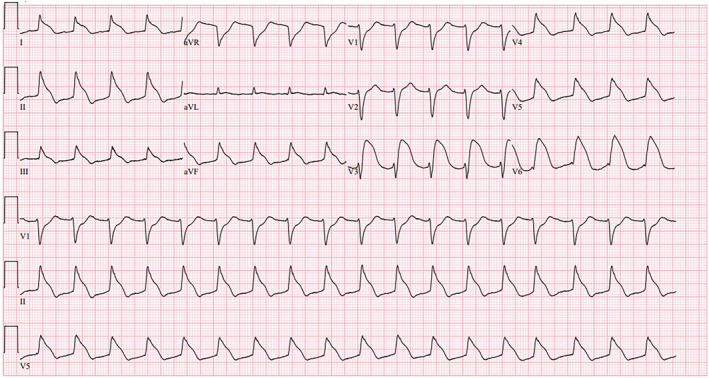
ECG on Day 4 of hospital admission.
The post‐mortem examination revealed cerebral edema with bilateral Uncal enlargement, downward herniation, and midbrain compression, along with hypertensive vascular changes. Cardiovascular examination showed patent great vessels, normal coronary arteries, no pericardial effusion, and no valvular vegetation. The leaflets of all four cardiac valves were thin and pliable, with normal commissures. Microscopic examination of cardiac tissues showed mild cardiac myocyte hypertrophy and minimal coronary artery atherosclerosis without evidence of myocarditis.
5. DISCUSSION
The shark fin sign is a rare ECG finding, identified in only 1.3%–1.9% of STEMI cases. 3 , 7 It is associated with a large ischemic burden, along with increasing the risk of severe complications such as cardiogenic shock, ventricular arrhythmia, and cardiac arrest. 2 However, in our case, the shark fin ECG was observed in a non‐ischemic scenario. Our patient had an influenza A infection complicated by acute hypoxic respiratory failure and new left ventricular (LV) dysfunction with a characteristic features of TTC based on TTE. Coronary angiogram was done twice, which showed no signs of coronary artery disease. Unfortunately, the patient's condition deteriorated, requiring intubation and mechanical ventilation. The ECG during decompensation showed the picture of shark fin with elevated troponin levels. Following this distinct type of ECG pattern, the patient rapidly declined and passed away within a day. Therefore, in our case, the shark fin ECG pattern was a poor prognostic marker linked to in‐hospital mortality.
Recent data have shown cases of the shark fin ECG pattern in non‐ischemic conditions such as TTC, myopericarditis, severe electrolyte imbalances, and severe vitamin deficiencies. For example, Chow et al. reported a 76‐year‐old female with severe hypokalemia and hypocalcemia post‐thyroidectomy who exhibited a shark fin ECG due to electrolyte derangement. 5 After correcting the imbalance, the patient's condition improved, and the ECG returned to baseline. Additionally, Hasibuan et al. described a 50‐year‐old male with a shark fin ECG pattern due to acute myopericarditis, which resolved completely with clinical improvement. 8 In light of TTC with shark fin ECG, Tarantino et al. studied 158 patients with TTC and found that only 3.2% exhibited the shark fin ECG pattern. 9 Among these TTC patients, the mortality rate was up to 40%, compared to 7% in those without the shark fin pattern. Additionally, TTC patients with the shark fin ECG pattern had higher rates of in‐hospital adverse events, including a greater likelihood of requiring mechanical ventilation. These findings highlight the importance of recognizing the distinct shark fin ECG pattern in patients with non‐ischemic conditions like TTC and emphasize the need for vigilant clinical monitoring during hospitalization and follow‐up.
While the mechanism of the shark fin ECG pattern in patients with TTC remains unclear, several explanations have been proposed. One suggested that a mismatch between endo‐epicardial wall tension and activation of stretch‐activated channels creates a transmural repolarization gradient, resulting in a coved‐type ST elevation similar to shark fin appearance. 9 Furthermore, Shimada et al. demonstrated in an animal model that regional myocardial stretch alters the action potential of cardiomyocytes, leading to mechanoelectrical feedback and ST‐segment elevation. 10 In patients with TTC, increased systolic wall tension at the apex may similarly affect action potentials, leading to this phenomenon. These ECG findings, also associated with adverse in‐hospital outcomes, may be attributed to a catecholamine surge from stress, causing myocardial injury, endothelial dysfunction, and disrupted cellular mechanisms. 11 This can lead to myocardial cell death and complications such as cardiogenic shock, ventricular arrhythmia, and other severe consequences similar to those in ischemic scenarios.
Management primarily involves conservative approaches and addressing the physical or emotional stressor, regardless of the presence of the shark fin ECG pattern. 12 There is limited evidence for GDMT in TTC patients with heart failure, and further studies are needed to demonstrate its long‐term benefits. Additional data is necessary to understand the predictive and clinical implications of features such as the shark fin ECG pattern and its association with mortality and adverse complication in TTC.
6. CONCLUSION
We presented a case of influenza A infection with TTC, complicated by acute hypoxic respiratory failure and profound shock. The patient exhibited a rare “shark fin” ECG pattern associated with rapid clinical deterioration and in‐hospital mortality. This highlights the importance of recognizing these distinct ECG patterns as potential predictors of adverse clinical outcomes, prompting clinicians to promptly manage and closely monitor patients' clinical status to prevent decompensation. Further research is needed to clarify the mechanism and link between this ECG pattern and adverse outcomes in various non‐ischemic scenarios.
AUTHOR CONTRIBUTIONS
Phuuwadith Wattanachayakul: Conceptualization; resources; validation; visualization; writing – original draft; writing – review and editing. Colton Jones: Writing – original draft; writing – review and editing. John Malin: Writing – original draft. Nandakumar Mohan: Writing – original draft. Jose Martinez Manzano: Writing – original draft; writing – review and editing. Emmanuel Akuna: Writing – original draft; writing – review and editing. Aman Amanullah: Supervision; validation; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
The authors did not receive financial support for this article's research, authorship, or publication.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Wattanachayakul P, Jones C, Malin J, et al. Shark fin sign: A rare EKG finding in a patient with Takotsubo cardiomyopathy from influenza A infection. Clin Case Rep. 2024;12:e9158. doi: 10.1002/ccr3.9158
DATA AVAILABILITY STATEMENT
All data underlying the results are included in the article; no additional source data are required.
REFERENCES
- 1. Rocha LG, Costa G, Gonçalves L. “Shark fin” electrocardiogram pattern in Takotsubo cardiomyopathy. Intern Emerg Med. 2024. doi: 10.1007/s11739-023-03525-z [DOI] [PubMed] [Google Scholar]
- 2. Zhang B, Yin Z‐W, Chen W. Shark fin electrocardiogram in the intensive care unit. Circulation. 2022;146:1099‐1102. doi: 10.1161/CIRCULATIONAHA.122.062034 [DOI] [PubMed] [Google Scholar]
- 3. Cipriani A, D'Amico G, Brunello G, et al. The electrocardiographic “triangular QRS‐ST‐T waveform” pattern in patients with ST‐segment elevation myocardial infarction: incidence, pathophysiology and clinical implications. J Electrocardiol. 2018;51:8‐14. doi: 10.1016/j.jelectrocard.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 4. Martins Carvalho M, Proença T, Alves Pinto R, Pinto R, Macedo F. Wernicke encephalopathy and beriberi disease presenting as STEMI‐equivalent. Monaldi arch chest dis. Arch Monaldi Mal Torace. 2023;93:93. doi: 10.4081/monaldi.2023.2513 [DOI] [PubMed] [Google Scholar]
- 5. Chow HB, Lim CT, Ho YH, et al. Pseudo‐infarction electrocardiographic changes in delayed onset hypoparathyroidism: a case report. Clin Case Reports. 2023;11:e7580. doi: 10.1002/ccr3.7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joki T, Nikus K, Laukkanen J. The electrocardiographic “triangular QRS‐ST‐T waveform” pattern: a marker of severe haemodynamic compromise in Takotsubo syndrome‐a case report. Eur Heart J Case Rep. 2020;4:1‐6. doi: 10.1093/ehjcr/ytaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alhatemi AQM, Hashim HT, Aziz EMH, Abdulhussain TK, Hashim AT. De winter syndrome in action: captured on defibrillator. Clin Case Reports. 2024;12:e8511. doi: 10.1002/ccr3.8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasibuan FS, Intan RE, Wilujeng HRT, et al. Triangular QRS‐ST‐T waveform electrocardiographic pattern in acute Myopericarditis: a case report from a limited‐resources hospital. Am J Case Rep. 2020;21:e926360. doi: 10.12659/AJCR.926360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tarantino N, Santoro F, Guastafierro F, et al. “Lambda‐wave” ST‐elevation is associated with severe prognosis in stress (takotsubo) cardiomyopathy. Ann Noninvasive Electrocardiol. 2018;23:e12581. doi: 10.1111/anec.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimada M, Nakamura Y, Asakura K, et al. Induction of ST‐segment elevation by regional myocardial stretch in normal canine hearts in vivo. Jpn Circ J. 1997;61:921‐926. doi: 10.1253/jcj.61.921 [DOI] [PubMed] [Google Scholar]
- 11. Pérez‐Riera AR, Abreu LC, Yanowitz F, et al. “Benign” early repolarization versus malignant early abnormalities: clinical‐electrocardiographic distinction and genetic basis. Cardiol J. 2012;19:337‐346. doi: 10.5603/cj.2012.0063 [DOI] [PubMed] [Google Scholar]
- 12. Matta AG, Carrié D. Epidemiology, pathophysiology, diagnosis, and principles of management of Takotsubo Cardiomyopathy: a review. Med Sci Monit: Int Med J Exp Clin Res. 2023;29:e939020. doi: 10.12659/MSM.939020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are included in the article; no additional source data are required.


