Abstract
Background
Chronic pain is frequent in persons living with spinal cord injury (SCI). Conventionally, the pain is treated pharmacologically, yet long‐term pain medication is often refractory and associated with side effects. Non‐pharmacological interventions are frequently advocated, although the benefit and harm profiles of these treatments are not well established, in part because of methodological weaknesses of available studies.
Objectives
To critically appraise and synthesise available research evidence on the effects of non‐pharmacological interventions for the treatment of chronic neuropathic and nociceptive pain in people living with SCI.
Search methods
The search was run on the 1st March 2011. We searched the Cochrane Injuries Group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), four other databases and clinical trials registers. In addition, we manually searched the proceedings of three major scientific conferences on SCI. We updated this search in November 2014 but these results have not yet been incorporated.
Selection criteria
Randomised controlled trials of any intervention not involving intake of medication or other active substances to treat chronic pain in people with SCI.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias in the included studies. The primary outcome was any measure of pain intensity or pain relief. Secondary outcomes included adverse events, anxiety, depression and quality of life. When possible, meta‐analyses were performed to calculate standardised mean differences for each type of intervention.
Main results
We identified 16 trials involving a total of 616 participants. Eight different types of interventions were studied. Eight trials investigated the effects of electrical brain stimulation (transcranial direct current stimulation (tDCS) and cranial electrotherapy stimulation (CES); five trials) or repetitive transcranial magnetic stimulation (rTMS; three trials). Interventions in the remaining studies included exercise programmes (three trials); acupuncture (two trials); self‐hypnosis (one trial); transcutaneous electrical nerve stimulation (TENS) (one trial); and a cognitive behavioural programme (one trial). None of the included trials were considered to have low overall risk of bias. Twelve studies had high overall risk of bias, and in four studies risk of bias was unclear. The overall quality of the included studies was weak. Their validity was impaired by methodological weaknesses such as inappropriate choice of control groups. An additional search in November 2014 identified more recent studies that will be included in an update of this review.
For tDCS the pooled mean difference between intervention and control groups in pain scores on an 11‐point visual analogue scale (VAS) (0‐10) was a reduction of ‐1.90 units (95% confidence interval (CI) ‐3.48 to ‐0.33; P value 0.02) in the short term and of ‐1.87 (95% CI ‐3.30 to ‐0.45; P value 0.01) in the mid term. Exercise programmes led to mean reductions in chronic shoulder pain of ‐1.9 score points for the Short Form (SF)‐36 item for pain experience (95% CI ‐3.4 to ‐0.4; P value 0.01) and ‐2.8 pain VAS units (95% CI ‐3.77 to ‐1.83; P value < 0.00001); this represented the largest observed treatment effects in the included studies. Trials using rTMS, CES, acupuncture, self‐hypnosis, TENS or a cognitive behavioural programme provided no evidence that these interventions reduce chronic pain. Ten trials examined study endpoints other than pain, including anxiety, depression and quality of life, but available data were too scarce for firm conclusions to be drawn. In four trials no side effects were reported with study interventions. Five trials reported transient mild side effects. Overall, a paucity of evidence was found on any serious or long‐lasting side effects of the interventions.
Authors' conclusions
Evidence is insufficient to suggest that non‐pharmacological treatments are effective in reducing chronic pain in people living with SCI. The benefits and harms of commonly used non‐pharmacological pain treatments should be investigated in randomised controlled trials with adequate sample size and study methodology.
Keywords: Humans, Acupuncture Therapy, Acupuncture Therapy/methods, Anxiety, Anxiety/therapy, Chronic Pain, Chronic Pain/psychology, Chronic Pain/therapy, Cognitive Behavioral Therapy, Cognitive Behavioral Therapy/methods, Depression, Depression/therapy, Electric Stimulation Therapy, Electric Stimulation Therapy/methods, Exercise Therapy, Exercise Therapy/methods, Hypnosis, Hypnosis/methods, Neuralgia, Neuralgia/psychology, Neuralgia/therapy, Nociceptive Pain, Nociceptive Pain/psychology, Nociceptive Pain/therapy, Pain Management, Pain Management/methods, Pain Measurement, Pain Measurement/methods, Quality of Life, Randomized Controlled Trials as Topic, Reproducibility of Results, Shoulder Pain, Shoulder Pain/therapy, Spinal Cord Injuries, Spinal Cord Injuries/complications, Transcranial Magnetic Stimulation, Transcranial Magnetic Stimulation/methods, Transcutaneous Electric Nerve Stimulation, Transcutaneous Electric Nerve Stimulation/methods
Plain language summary
Treatments other than medication for people with chronic pain after spinal cord injury
Many people living with spinal cord injury (SCI) have chronic pain. Besides pain medication, other treatment possibilities are commonly offered. This systematic review aims to summarise available evidence on the effectiveness and possible side effects of other forms of treatment.
We searched electronic databases until March 2011 and found 16 randomised controlled trials with a total of 616 participants. We grouped these studies by type of treatment into eight groups: Eight studies were on brain stimulation, of which five used electronic and three magnetic stimulation. Three studies were on exercise programmes, two on acupuncture and one each on self‐hypnosis, transcutaneous electrical nerve stimulation (TENS) and a cognitive behavioural programme. The included studies used a range of different methods to measure pain and other outcomes. Comparison groups also varied and included sham interventions, waiting lists and other pain treatments.
For any given type of intervention, only a few studies were found, and they included only small numbers of participants. Often the reported detail was insufficient. The overall quality of the studies was low. For instance, several studies used inappropriate comparison groups such as waiting lists. Consequently, the effectiveness of the treatments is uncertain. An additional search in November 2014 identified more recent studies that will be included in an update of this review.
For one type of treatment—transcranial direct current stimulation (tDCS)—results from two studies could be combined. The pooled results suggest that tDCS reduced pain in the short term and in the mid term. Also, exercise programmes for chronic shoulder pain provided pain relief. We found no evidence to suggest that repetitive transcranial magnetic stimulation (rTMS), cranial electrotherapy stimulation (CES), acupuncture, self‐hypnosis or TENS is better than the respective control interventions for reducing chronic pain. Regarding outcomes other than pain, such as anxiety, depression or quality of life, as well as long‐lasting side effects, no overall conclusions were possible, given that data were sparse. The included studies do not permit firm conclusions regarding whether treatments other than medication for chronic SCI pain are effective and safe. Trials with greater numbers of participants and improved study quality are needed to determine the effectiveness and safety of such treatments.
Background
Relevance
Chronic pain is a frequent and serious health problem for people living with spinal cord injury (SCI). Depending on the definition of pain and the study population, between 30% and 90% of people with SCI experience pain and describe it as a problem (Ballinger 2000; Salisbury 2003; Sie 1992). About one‐third of affected people experience pain as severe and debilitating (Siddall 2006). Pain has a significant impact on the physical, cognitive and emotional functioning of persons with SCI (Jensen 2007; Putzke 2002; Widerstrom‐Noga 2008). In addition, it substantially affects their quality of life and independence in activities of daily living (ADLs) and work. For instance, persons experiencing chronic shoulder pain frequently need substantially more physical assistance and incur greater risk of unemployment than those without such pain (Jensen 2005).
Classification
In able‐bodied individuals, chronic pain is defined as pain that persists past the normal time of healing and is present continuously or intermittently for at least three to six months (Dijkers 2009; Merskey 1994). To discriminate between acute and chronic pain, a cutoff of three months is often used in clinical practice, and a minimum duration of six months is preferable for research purposes. Unfortunately many published studies fail to provide an operational definition of chronic pain (Dijkers 2009).
Commonly used classification systems for SCI pain are based on aetiology (neuropathic/nociceptive pain), anatomical level of the injury or quality of pain (e.g. burning, dull). A literature review identified 28 different classification schemes used between 1949 and 2000 (Hicken 2002). In the absence of consensus on the classification of chronic SCI pain, previous pain studies have employed several different classifications. For the purpose of this systematic review, we employed the framework proposed by the Task Force for Pain Following Spinal Cord Injury of the International Association for the Study of Pain (Siddall 2000; Siddall 2001), which broadly distinguishes neuropathic and nociceptive pain.
Neuropathic pain is usually generated within the central nervous system, for instance following a spinal cord lesion or a pathological process, and is sometimes called ‘central pain.’ Less frequently, it has a peripheral origin, for instance, when a disease affects the nerve roots. Neuropathic pain typically continues long after elimination of the peripheral cause of acute pain and can be localised at the level of the neurological lesion or below. Generally, at‐level pain refers to pain occurring anywhere within the dermatome of the neurological injury and up to three levels below it. Below‐level pain occurs diffusely more than three dermatomes below the level of the neurological lesion. Pain that is at‐level or below‐level pain is usually described as burning, electrical or shooting. This pain may be unilateral or bilateral and may be associated with allodynia or hyperalgesia. Besides pain originating in the spinal cord lesion or from a spinal cord disease, other types of neuropathic pain may occur above or below the neurological level of injury, for example, as a symptom of diabetic neuropathy or compressive mononeuropathy (Widerstrom‐Noga 2008; Widerström‐Noga 2009).
Nociceptive pain is generally related to the musculoskeletal system and often is associated with movement. It is usually located at or above the neurological level of the SCI and is described as dull or aching. Nociceptive pain is frequently due to overuse of the shoulders or wrists. It may also originate in the visceral structures of the abdomen or thorax. Visceral nociceptive pain is frequently an indicator of an underlying pathology or dysfunction, such as infection or obstruction. Other types of nociceptive pain include headache and pain related to skin ulcers (Widerstrom‐Noga 2008; Widerström‐Noga 2009).
Interventions
For both neuropathic and nociceptive types of pain, pharmacological and non‐pharmacological interventions are available. Several studies have shown that chronic pain after SCI is often refractory to pharmacological treatment (SCIRE 2010). In addition, long‐term pain medication is associated with unwanted side effects such as constipation or toxicity and increased risk of addiction or abuse. Consequently, many people living with SCI and their attending healthcare professionals consider non‐pharmacological therapies as an alternative or adjunctive pain treatment (Cardenas 2006; Nayak 2001). However, the benefit and harm profile of these treatments remains unclear (Siddall 2006a). A paucity of well‐designed interventional studies have explored the effects of non‐pharmacological interventions for the treatment of chronic pain. A particular problem is the use of inappropriate control interventions such as waiting lists. It has been shown that in direct comparisons, placebo interventions tend to be superior to control interventions with no treatment or with waiting lists, in particular for pain outcomes (Hróbjartsson 2010). Currently, it is therefore difficult to make evidence‐based decisions on the use of non‐pharmacological treatments for chronic pain in persons with SCI.
Objectives
To critically appraise and synthesise available research evidence on the effects of non‐pharmacological interventions for the treatment of chronic neuropathic and nociceptive pain in people living with SCI.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) with and without blinding of participants and/or investigators.
Types of participants
We included trials conducted in people living with SCI irrespective of their age, gender and severity of disability. Both traumatic and non‐traumatic SCI aetiologies were considered. Studies were considered to focus on chronic pain if (1) the publication stated that the study was conducted to explore chronic pain, or (2) most participants with the pain problem lived in a community setting or (3) most participants suffered from pain longer than three months. Given that definitions for pain chronicity varied in the primary studies, we used the shorter minimal pain duration of three months (as compared with six months) to be inclusive.
Studies that included individuals with conditions other than SCI were included only if data from the SCI subgroup could be extracted separately by using the published data or by using additional data obtained from the study authors upon request.
Types of interventions
Experimental interventions
Non‐pharmacological study interventions were defined as experimental pain therapies that do not involve taking medicine or any other active substance. Eligible therapies included surgical interventions, exercise, acupuncture, massage, joint mobilisation, relaxation training, thermotherapy (warm or cold pack application), transcutaneous electrical nerve stimulation (TENS), static magnetic field therapy, brain stimulation and psychological or behavioural therapies (e.g. cognitive behavioural therapy, visual imaging, hypnotic therapy). Studies on other interventions, such as use of durable equipment, were considered only if the focus was on treatment of individuals with chronic pain.
Control interventions
Control interventions included active pharmacological or non‐pharmacological treatments, placebo and sham interventions or waiting list groups.
Types of outcome measures
Primary outcomes
Primary outcome measures consisted of any measure of pain on scales for pain intensity or pain relief. Included studies used various pain measures and measurement schemes during follow‐up, including numerical rating scales (NRSs), visual analogue scales (VASs) and pain questionnaires (e.g. McGill Pain Questionnaire, Wheelchair User's Shoulder Pain Index (WUSPI), Short Form (SF)‐36). Details on the observation period were extracted from the original reports. From studies that reported several outcome measures of pain, we extracted data for all measures and selected the most frequently reported measure across included studies for use in meta‐analysis. However, if we found a serious imbalance among baseline pain scores between intervention and control groups for the most common measure, we chose a pain measure without imbalance for further analysis.
Secondary outcomes
Secondary outcomes were measures of anxiety, depression or quality of life, as well as data on adverse events. The included studies used various instruments for measurement including NRSs or VASs for anxiety, and validated questionnaires for quality of life and depression, such as the Beck Depression Inventory (BDI), the Subjective Quality of Life Scale (SQOL) and the Perceived Quality of Life Scale (PQOL).
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co‐ordinator searched the following:
Cochrane Injuries Group Specialised Register (28 February 2011);
Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 1 of 12);
MEDLINE(OvidSP) (1948 to February week3 2011);
Embase (OvidSP) (1974 to 2011 Week 08);
PsycINFO (OvidSP) (1980 to 2011 week 7);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to February 2011);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to February 2011);
CINAHL Plus (EBSCO Host) (1939 to February 2011);
Physiotherapy Evidence Database (PEDro) (February 2011);
Allied and Complementary Medicine Database (AMED) (1996 to February 2011).
Search strategies are listed in Appendix 1.
We performed a further search in November 2014. Those results have been added to Studies awaiting classification and will be incorporated into the review at the next update.
Searching other resources
We screened the reference lists of included studies and other relevant papers (e.g. review articles) for additional eligible studies. We contacted two experts in the field and asked them to identify additional studies. We also manually searched the proceedings of three major scientific conferences on SCI: the meetings of the American Paraplegia Society (APS; 2001, 2004 to 2009), the American Spinal Injury Association (ASIA; 1998 to 2001, 2004 to 2010) and the International Spinal Cord Society (ISCOS; 2005 to 2010). We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (to 16 March 2011).
Data collection and analysis
The Cochrane Injuries Group Trials Search Co‐ordinator designed the search strategy, ran the searches described above and collated the search results in an Endnote database. Duplicates were removed before search results were sent to the review authors.
Selection of studies
Two review authors (EvE and IB) screened the titles and abstracts of studies identified in the literature search for eligibility. One review author (IB) made an initial selection of eligible references and excluded those that were clearly not eligible. Based on titles and abstracts, both review authors independently selected studies for further consideration. Differences in selection were discussed and decisions made by consensus. Full‐text versions of selected articles were retrieved from the library and again were rated independently by both review authors for inclusion or exclusion. Studies were included by joint decision. Studies with uncertain inclusion status at this stage were read by an additional review author (MWGB or IEH). The three review authors then discussed inclusion of this study until agreement could be reached. At both stages of study selection, initial disagreement was due to neglect of a selection criterion by one review author. If a study was excluded at this stage, we kept a copy of the article. References and reasons for exclusion are listed under Characteristics of excluded studies.
Data extraction and management
For each included trial, two review authors (IB and one of EvE, IEH or MWGB) independently extracted study data using a standardised data extraction form. Data extraction comprised the following.
Year of study conduct and year of publication, along with geographical location of the study.
Number, age and gender of participants and type of SCI.
Type of pain (neuropathic/nociceptive) targeted by the study intervention.
Type of study intervention and treatment duration.
Type of control intervention and treatment duration.
Duration of study recruitment and follow‐up.
Occurrence and types of adverse events.
Number of and reasons for withdrawals.
Whether the study was specifically designed to measure pain in SCI.
Relevant criteria for assessment of methodological study quality using the risk of bias tool of The Cochrane Collaboration.
Measures of treatment effect (outcome measures).
None of the included studies provided sufficient data for review authors to use the benchmarks of the IMMPACT (Initiative on Methods, Measurement and Pain Assessment in Clinical Trials) recommendations (Dworkin 2008) to interpret the clinical importance of pain relief.
Assessment of risk of bias in included studies
We used the risk of bias tool of The Cochrane Collaboration to assess risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) (Table 8.5.d) (Higgins 2011). Risk of bias was assessed by two review authors independently (IB and one of EvE, IEH or MWGB). We assessed the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. We deemed studies with high risk of bias in any single domain as having high overall risk of bias. For each domain, each investigator rated the risk of bias independently as 'low risk,' 'high risk' or ‘unclear risk’ (Higgins 2011). Criteria for study validity further included duration of the intervention and duration of follow‐up after the intervention (Moore 2010). We also looked for imbalance in baseline pain scores between intervention and control groups.
Measures of treatment effect
Data for measures of treatment effect were extracted for the time points of baseline measurement, last intervention and during follow‐up. To account for the effective time span of measurement after the last time of intervention in evaluating the durability of pain relief, we distinguished between short‐term, medium‐term and long‐term effects, if assessments covered the first week, week two to six or week seven or later, respectively. If outcome data were reported for several time points within one of these time periods, we extracted data for the last measurement time point in this time period.
The size of the treatment effect was expressed as the (standardised) mean difference of measures between intervention and control groups for each instrument used.
Unit of analysis issues
All studies used the individual participant as the unit of analysis. When appropriate, we aimed to combine data from cross‐over trials with those from other trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16.4.5) (Higgins 2011) (i.e. cross‐over trials would have been included in any meta‐analyses if the trial report indicated low risk of carry‐over effects and complete retention of participants during the intervention periods). In the end, none of the three cross‐over trials was included in any meta‐analyses because of large differences in study methods.
Dealing with missing data
When data were missing, we contacted the lead authors of the trial by email and asked them for needed data. If we were not able to obtain the missing data, the trial was not included in the meta‐analysis.
Assessment of heterogeneity
Clinical heterogeneity was assessed on the basis of study design, population and intervention. We initially aimed to distinguish comparisons of active interventions versus placebo or sham interventions versus those with no treatment groups. However, because of the paucity of studies for each type of intervention, this analysis could not be carried out. When applicable, we assessed statistical heterogeneity among studies before meta‐analysis using the I² statistic, with values of 50% or greater considered as showing substantial heterogeneity (Higgins 2002; Higgins 2003), and the Chi2 test, with a threshold P value < 0.1. When significant heterogeneity was found, we aimed to perform subgroup analyses to further investigate sources of heterogeneity.
Assessment of reporting biases
We initially planned to use graphical or formal statistical assessments to detect reporting bias but refrained from doing this because in all eight groups of trials by type of intervention, fewer than 10 trials were included.
Data synthesis
Each type of intervention (e.g. acupuncture, exercise programme) was analysed separately. All analyses were done using Review Manager 5.1 software. When possible, meta‐analysis was conducted using the random‐effects model. The fixed‐effect model was used to combine in two meta‐analyses data from pairs of similar study groups from two factorial trials (Doctor 1996; Soler 2010). All included studies were used in the primary analysis regardless of their risk of bias. For the purpose of meta‐analysis, ordinal outcome data were treated as continuous variables. Forest plots were created when data from two or more included studies could be combined.
Subgroup analysis and investigation of heterogeneity
Ancillary subgroup analyses for adult versus paediatric studies were not feasible because trials in children with SCI could not be found. Another pre‐specified subgroup analysis by SCI lesion level (paraplegia/tetraplegia) has not been carried out because the included trial reports did not contain sufficient data on the level of SCI. Only one trial provided data separately for tetraplegic and paraplegic participants (Curtis 1999).
Sensitivity analysis
In a planned sensitivity analysis, we explored whether the methodological quality of the included studies had any effect on combined estimates. For this purpose, studies with high overall risk of bias were excluded.
Results
Description of studies
For a detailed description of studies, see the sections Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
This review fully incorporates the results of searches conducted up to March 2011. A further 485 potentially eligible reports were identified by a search update conducted in November 2014, but these studies have not yet been incorporated into the results and will be addressed in the next update. See the Characteristics of studies awaiting classification section for details.
We identified 1602 potentially eligible references through electronic literature searches (Figure 1). After duplicate references were removed, 1301 unique references were left. We excluded 1140 references after screening titles and abstracts. Of the remaining 161 references, an additional 125 references were excluded after consultation with a second review author (EvE or IEH); 36 references were assessed for eligibility and read in full. A total of 20 publications met the inclusion criteria. Three articles reported on the same trial (Hicks 2003). We identified Hicks 2003 as the principal paper providing all relevant data and disregarded the other two publications.
1.
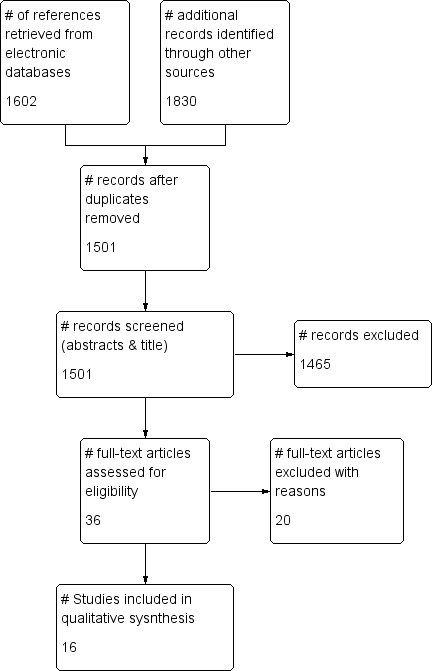
Study flow diagram for search run in March 2011.
Three articles reported on a series of investigations conducted by the same research group (Lefaucheur 2007) in study populations with various health conditions including SCI. Investigators provided us with individual data for the participants with SCI. Outcome data on some participants were used in two of the three publications. We regarded this as one set of data for each individual and considered the data as resulting from a single trial with a total of 19 participants. We refer to the combined dataset as Lefaucheur 2007. Consequently, 16 individual trials involving 616 people were included in the review.
Included studies
Country of origin, setting and language of publication
The included studies were conducted in Brazil, Canada, France, Israel, the Netherlands, UK, USA, Spain and South Korea. Interventions took place at rehabilitation centres, inpatient or outpatient clinics of general hospitals and research institutions. All study reports were written in English.
Type of interventions
Types of interventions and controls are listed in Figure 2. Overall, interventions used in the included trials were diverse and ranged from experimental brain stimulation techniques to exercise programmes and self‐hypnosis. As a result of this clinical heterogeneity in the included studies, we regarded pooling of all outcome data in one meta‐analysis as inappropriate and decided to combine outcome data by type of intervention only.
2.
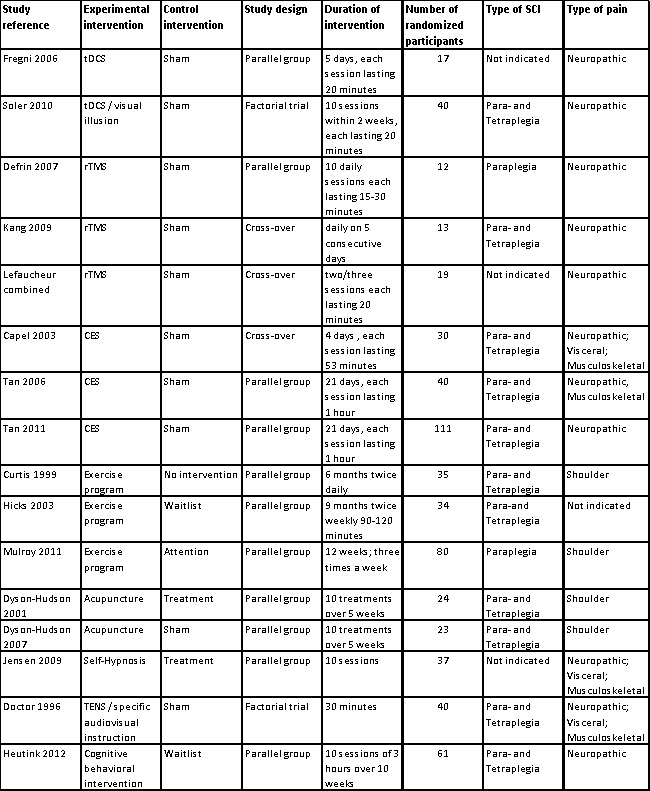
Main characteristics of included studies.
The effects of transcranial direct current stimulation (tDCS) were investigated in two trials (Fregni 2006; Soler 2010). This therapy uses constant, low current delivered directly to the brain area of interest via small electrodes.
Three trials investigated the effects of repetitive transcranial magnetic stimulation (rTMS) (Defrin 2007; Kang 2009; Lefaucheur 2007). This technique uses a magnet instead of an electrical current to activate the brain; a device is used to generate short pulses of magnetic fields to stimulate areas of the brain thought to be associated with pain.
Three studies looked at cranial electrotherapy stimulation (CES), also called transcranial electrotherapy (TCET), which uses a small, pulsed electrical current applied across a patient's head (Capel 2003; Tan 2006; Tan 2011).
Three studies investigated the effects of exercise programmes on chronic pain (Curtis 1999; Hicks 2003; Mulroy 2011). Two studies investigated the effects of acupuncture (Dyson‐Hudson 2001; Dyson‐Hudson 2007) and one the effect of self‐hypnosis (Jensen 2009). One study examined the effects of transcutaneous electrical nerve stimulation (TENS), which uses electrodes applied to the skin to deliver intermittent stimulation to surface nerves, thus blocking the transmission of pain signals (Doctor 1996). Finally, one study used an intervention programme comprising educational, cognitive and behavioural elements targeted at coping with chronic pain (Heutink 2012).
Type of controls
Ten of the included studies used sham interventions in the control group (Capel 2003; Defrin 2007; Doctor 1996; Dyson‐Hudson 2007; Fregni 2006; Kang 2009; Lefaucheur 2007; Soler 2010; Tan 2006; Tan 2011). Two studies used a waiting list (Heutink 2012; Hicks 2003), one had a 'no treatment' group (Curtis 1999) and another a control group with a minimal intervention that can be considered 'no treatment' (Mulroy 2011). An additional two studies provided active control interventions: Dyson‐Hudson 2001 used Trager integration treatment, and Jensen 2009 used electromyography (EMG) biofeedback. Three studies were cross‐over studies (Capel 2003; Kang 2009; Lefaucheur 2007).
Sample size and participants
Overall 616 participants with SCI were enrolled in the included studies. Total sample size varied from 12 to 111 participants (Figure 2). The two tDCS studies included a total of 57 participants—17 persons in the first and 40 in the second study. The three rTMS trials included 44 participants; study sample size ranged from 12 to 19. The CES studies included 181 participants; study sample size ranged from 30 to 111. The exercise studies included 149 participants; study size ranged from 34 to 80 participants. The two acupuncture studies included 47 randomly assigned participants—23 persons in the first and 24 in the second study. The self‐hypnosis trial included 37, the TENS trial 40 and the trial using a behavioural program 61 participants.
In all but one trial, the participants were of both sexes. In the study by Tan et al, only male veterans were included (Tan 2006). Most studies enrolled more male than female participants; the distribution was 289 male (77.5%) to 84 female participants (22.5%) in the 13 studies that provided data on gender distribution. For three studies (Capel 2003; Curtis 1999; Tan 2011), gender distribution of study participants was not available. The mean age of participants ranged from 35 to 56 years.
Eleven studies included persons with paraplegia or tetraplegia (Figure 2). Two studies included participants with paraplegia only (Defrin 2007; Mulroy 2011). For three studies (Fregni 2006; Jensen 2009; Lefaucheur 2007), the SCI level of participants was not indicated in the publications. Three studies (Defrin 2007; Fregni 2006; Hicks 2003) included solely participants with traumatic SCI. All other studies did not restrict eligibility by the cause of SCI. In one trial, injuries of all participants were classified as American Spinal Injury Association (ASIA) group A or B, although this was not an inclusion criterion (Soler 2010). Two studies provided insufficient information about the type of SCI (Jensen 2009; Lefaucheur 2007). However, Jensen 2009 referred to another publication (Jensen 2005) in which injury levels of the same study population were C1 to S4.
Two trials consisted of mixed study populations that also included participants with conditions other than SCI (Curtis 1999; Lefaucheur 2007). For analysis, only data on the SCI subpopulation were used.
The included studies targeted a variety of chronic pain conditions: Seven studies included solely participants with neuropathic pain (Defrin 2007; Fregni 2006; Heutink 2012; Kang 2009; Lefaucheur 2007; Soler 2010; Tan 2011). Of those, Defrin 2007 defined the pain as central pain and Lefaucheur 2007 as neurogenic. Four studies included participants with neuropathic or other pain and especially mentioned visceral or musculoskeletal pain (Capel 2003; Doctor 1996; Jensen 2009; Tan 2006). Four studies focused on shoulder pain, of which two stated explicitly that the pain was of musculoskeletal origin (Dyson‐Hudson 2001; Dyson‐Hudson 2007), whereas the other two did not specify pain type (Curtis 1999; Mulroy 2011). No information about the type of pain was provided for one study (Hicks 2003). In six studies, the minimal pain duration was six months (Defrin 2007; Heutink 2012; Kang 2009; Lefaucheur 2007; Soler 2010; Tan 2011), and in four trials it was three months (Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Tan 2006). For six trials, no clear definition of chronicity was provided in the publications, or pain duration was not used as an inclusion criterion (Capel 2003; Curtis 1999; Doctor 1996; Hicks 2003; Jensen 2009; Mulroy 2011).
Duration of intervention and follow‐up
The duration of study interventions varied from one single point in time to a period of nine months with twice‐weekly interventions. All studies assessed the short‐term effects of the interventions (i.e. study outcomes were measured immediately after the last intervention or in the ensuing 24 hours). None of the studies collected data for the time period between 24 hours and the first week after the intervention. Eight studies (Defrin 2007; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Heutink 2012; Kang 2009; Mulroy 2011; Soler 2010) investigated mid‐term effects (i.e. between one and six weeks after the last intervention). Only four studies assessed long‐term effects (i.e. longer than six weeks after the last intervention) (Heutink 2012; Jensen 2009; Kang 2009; Soler 2010).
Outcomes
Primary outcomes
Across trials, pain was assessed with the use of various self‐reported measurement scales and at various time points. Fourteen studies (Capel 2003; Curtis 1999; Defrin 2007; Doctor 1996; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Heutink 2012; Jensen 2009; Kang 2009; Mulroy 2011; Soler 2010; Tan 2006; Tan 2011) defined pain as a primary outcome. Seven studies measured pain by NRS ranging from zero to ten (Dyson‐Hudson 2001; Dyson‐Hudson 2007; Heutink 2012; Jensen 2009; Kang 2009; Soler 2010; Tan 2006). In these studies, the definition of the maximum scale value was “most intense pain imaginable” (Kang 2009; Jensen 2009), “pain as bad as you can imagine” (Tan 2006; Heutink 2012), “worst pain ever experienced” (Dyson‐Hudson 2001; Dyson‐Hudson 2007) and “unbearable pain” (Soler 2010). Also, the questions on pain experience were operationalised in different ways. For instance, participants were asked for “average pain intensity during the preceding 24 hours” (Kang 2009; Soler 2010) or “current pain intensity” (Jensen 2009). Three trials used VAS ranging from zero to ten. Of those, two defined minimum and maximum anchor values as “no pain” and “worst pain possible” (Fregni 2006; Mulroy 2011); one trial (Lefaucheur 2007) failed to provide a scale description when measuring pain as one of several (secondary) outcomes. Four studies used the Brief Pain Inventory (BPI) (Kang 2009; Soler 2010; Tan 2006; Jensen 2009), and two studies (Hicks 2003; Mulroy 2011) used pain items of the Short Form Health Survey (SF‐36) as secondary outcome measures. Four studies used the Wheelchair User's Shoulder Pain Index (WUSPI) (Curtis 1999; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Mulroy 2011). Four additional instruments were used by only one study each: the McGill Pain Questionnaire (SF‐MPQ) (Capel 2003), the Verbal Rating Scale (VRS) (Dyson‐Hudson 2001), the Descriptor Differential Scale (DDS) for pain intensity and pain unpleasantness (Doctor 1996) and the Chronic Pain Grade (CPG) questionnaire (Heutink 2012). In the statistical analyses of all included studies, pain outcome data derived from ordinal scales were treated as continuous outcomes and were expressed as mean values (with standard deviations) or relative change.
Secondary outcomes
For nine studies, information about occurrence of adverse effects was available (Capel 2003; Curtis 1999; Doctor 1996; Dyson‐Hudson 2001; Fregni 2006; Lefaucheur 2007, Mulroy 2011; Soler 2010; Tan 2011). Ten studies measured anxiety, depression or quality of life. However, large heterogeneity was noted in the instruments used. Six studies measured anxiety, with each using a different instrument: the Spielberger State and Trait Anxiety Inventory (STAI) (in three different versions), VASs or NRSs for anxiety and the Hospital Anxiety and Depression Scale (HADS) (Capel 2003; Doctor 1996; Fregni 2006; Heutink 2012; Soler 2010; Tan 2011). Seven studies measured depression using three different scales: the Beck Depression Index (BDI), the Centre for Epidemiologic Studies Depression Scale (CES‐D) and the HADS (Capel 2003; Defrin 2007; Fregni 2006; Heutink 2012; Hicks 2003; Jensen 2009; Tan 2011). Four studies measured quality of life, each using a different instrument: the Subjective Quality of Life Scale (SQOL) on a seven‐point Likert scale (Mulroy 2011), the Perceived Quality of Life Scale (PQOL) on a seven‐point scale (Hicks 2003), the Life Satisfaction Questionnaire (Lisat‐9) on a six‐point scale (Heutink 2012) and the physical and mental component summaries of the 12‐item Short Form Health Survey (PCS and MCS of the SF‐12) (Tan 2011). None of the included studies provided data on the cost of treatments under study.
Excluded studies
For detailed descriptions, see Characteristics of excluded studies.
We excluded 12 studies after reading the whole article. Of those, nine were not (properly) randomly assigned (Coşkun Çelik 2005; Ditor 2003; Dyson‐Hudson 2007b; Norrbrink 2006; Norrbrink 2009; Perry 2010; Saitoh 2007; Wardell 2006; Xing 2010). Two trials did not examine chronic pain (Crowe 2000; Lefaucheur 2010), and one study included only one participant with SCI in the control group (Hughes 2006). Four studies await classification because randomisation remained unclear (Hirayama 2006; Livshits 2002; Saitoh 2006; Saitoh 2007a) despite our attempts to contact the study authors.
Risk of bias in included studies
For a summary of the risk of bias assessment, see Figure 3 and Figure 4. Four studies were of low or unclear risk of bias in all domains (Fregni 2006; Kang 2009; Soler 2010; Tan 2006).
3.
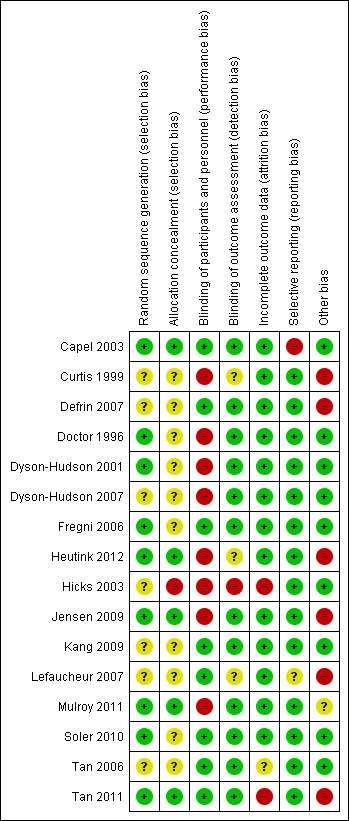
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
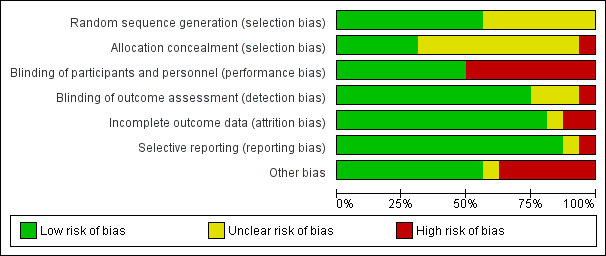
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Sequence generation (selection bias)
Nine studies were judged as having low risk of selection bias due to sequence generation (Capel 2003; Doctor 1996; Dyson‐Hudson 2001; Fregni 2006; Heutink 2012; Jensen 2009; Mulroy 2011; Soler 2010; Tan 2011). Seven studies with no information about the randomisation method used were rated as having unclear risk of bias (Curtis 1999; Defrin 2007; Dyson‐Hudson 2007; Hicks 2003; Kang 2009; Lefaucheur 2007; Tan 2006).
Allocation
Five studies were judged as having low risk of selection bias due to allocation to treatment groups (Capel 2003; Heutink 2012; Jensen 2009; Mulroy 2011; Tan 2011). For 10 studies, no information about concealment was provided; consequently they were rated as having unclear risk of bias (Curtis 1999; Defrin 2007; Doctor 1996; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Kang 2009; Lefaucheur 2007; Soler 2010; Tan 2006). One study (Hicks 2003) was considered to have high risk of selection bias because allocation of treatment was not concealed.
Blinding
In eight trials, participants and study personnel were blinded; consequently, they were rated as having low risk of performance or detection bias (Capel 2003; Defrin 2007; Fregni 2006; Kang 2009, Lefaucheur 2007; Soler 2010; Tan 2006; Tan 2011). Eight studies that provided no information about blinding were rated as having high risk of bias. Six of these studies (Curtis 1999; Dyson‐Hudson 2001; Heutink 2012; Hicks 2003, Jensen 2009, Mulroy 2011) indicated that blinding was not possible because of the nature of the study intervention. This group included all three studies on exercise interventions. In the study of Dyson‐Hudson 2007, the acupuncturist was not blinded, and in the study of Doctor 1996, blinding of the therapist and of participants was reported to be only partially successful.
The publications of 12 studies stated that outcome assessors were blinded (Capel 2003; Defrin 2007; Doctor 1996; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Jensen 2009, Kang 2009, Mulroy 2011; Soler 2010; Tan 2006; Tan 2011). Three study reports did not specify whether outcome assessors were blinded (Curtis 1999; Heutink 2012; Lefaucheur 2007). The authors of one study (Hicks 2003) declared that the outcome assessor was not blinded; consequently, it was rated as having high risk of bias for this domain.
Incomplete outcome data
Thirteen study reports included outcome data for all included participants. We considered these studies to have low risk of attrition bias (Capel 2003; Curtis 1999; Defrin 2007; Doctor 1996; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Heutink 2012; Jensen 2009; Kang 2009; Lefaucheur 2007; Mulroy 2011; Soler 2010). We rated one study (Tan 2006), which provided only incomplete explanations about missing outcome data, as having unclear risk. Two studies that did not include all pre‐specified outcome data were rated as having high risk of bias (Hicks 2003; Tan 2011).
Selective reporting
Fourteen trials were judged as having low risk of reporting bias; information provided in the publications alone (Curtis 1999; Defrin 2007; Doctor 1996; Dyson‐Hudson 2001; Dyson‐Hudson 2007; Fregni 2006; Hicks 2003; Kang 2009; Soler 2010; Tan 2006; Tan 2011) or in both the study protocol and the publication (Jensen 2009; Heutink 2012; Mulroy 2011) did not suggest that relevant outcomes remained unpublished. For five studies (Fregni 2006; Jensen 2009; Heutink 2012; Mulroy 2011; Tan 2011), information on planned study outcomes was available in trial registries; comparison with the publications did not indicate that relevant outcomes were left out selectively. One study was rated as having unclear risk of bias (Lefaucheur 2007). The remaining study (Capel 2003) was considered to have high risk of selective reporting because it did not provide pain score values for all time points.
Other potential sources of bias
For nine studies we identified no other potential source of bias; their risk of other biases was considered low. Mulroy 2011 was rated as having unclear risk of bias because participants in the intervention group were free to continue exercise during the follow‐up period. Six studies were rated as having high risk of other biases: In the trial series of Lefaucheur 2007, outcome data on the same participants were likely used in multiple comparisons. In four studies (Curtis 1999; Defrin 2007; Jensen 2009; Tan 2011), baseline pain measures in the intervention and control groups were substantially imbalanced. In three of these studies, no other pain outcome measure was used. In Tan 2011, the BPI pain intensity subscale and the PQAS were used, in addition to three pain outcomes with imbalance at baseline, but the validity of these data remained unclear. Statistical methods used to adjust for baseline imbalance included analysis of variance (ANOVA) in three studies (Curtis 1999; Defrin 2007; Jensen 2009) and non‐parametric analysis of variance (Kruskal‐Wallis test) in the fourth (Tan 2011).
Effects of interventions
Transcranial direct current stimulation (tDCS) versus sham tDCS
Short‐term outcomes
Two trials (of low or unclear risk of bias) assessed the effects of tDCS versus sham tDCS on chronic pain in the short term using a VAS or NRS (Fregni 2006; Soler 2010). The pooled mean difference between tDCS and sham tDCS was ‐1.9 scale units (95% CI ‐3.48 to ‐0.33; P value 0.02) in favour of tDCS (Analysis 1.1). The study of Soler 2010 was a factorial trial combining two interventions (i.e. tDCS and visual illusion of walking). We combined the data from both groups with active tDCS, irrespective of the visual illusion intervention, because effect modification by the latter was not indicated.
1.1. Analysis.
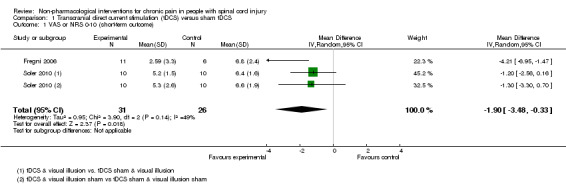
Comparison 1 Transcranial direct current stimulation (tDCS) versus sham tDCS, Outcome 1 VAS or NRS 0‐10 (short‐term outcome).
Mid‐term and long‐term outcomes
In both trials, tDCS reduced pain also during the mid‐term follow‐up of 16 to 38 days post‐intervention (Fregni 2006; Soler 2010). The pooled mean difference was ‐1.87 scale units (95% CI ‐3.30 to ‐0.45; P value 0.01) in favour of tDCS (Analysis 1.3).
1.3. Analysis.
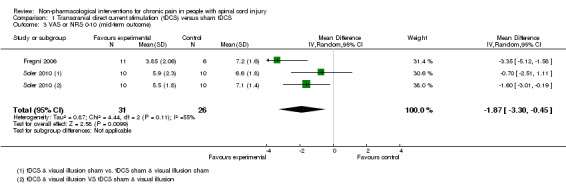
Comparison 1 Transcranial direct current stimulation (tDCS) versus sham tDCS, Outcome 3 VAS or NRS 0‐10 (mid‐term outcome).
The study by Soler 2010 included long‐term follow‐up until 12 weeks. The mean difference between active tDCS and sham tDCS was ‐0.73 scale units (95% CI ‐1.82 to 0.35; P value 0.18) in favour of tDCS (Analysis 1.5).
1.5. Analysis.
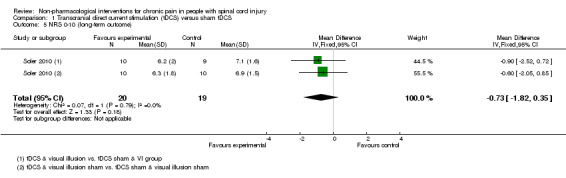
Comparison 1 Transcranial direct current stimulation (tDCS) versus sham tDCS, Outcome 5 NRS 0‐10 (long‐term outcome).
Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS
Short‐term outcomes
Estimates from three studies on rTMS are displayed in Analysis 2.1 but were not combined, given the important differences in study design and the unavailability of detailed data needed for meta‐analysis. Point estimates for mean difference in NRS favoured rTMS in one study (Lefaucheur 2007) and favoured the sham intervention in the other two studies (Defrin 2007; Kang 2009). However, in two studies (Kang 2009; Lefaucheur 2007), the confidence intervals were large, including the null effect. In the sensitivity analysis excluding two studies with high overall risk of bias (Defrin 2007; Lefaucheur 2007), only the study of Kang 2009 remained. In this study, the estimated mean reduction in pain NRS was 0.73 (95% CI ‐0.68 to 2.14, P value 0.31).
2.1. Analysis.
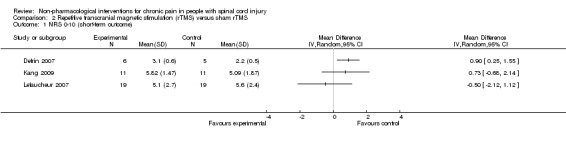
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 1 NRS 0‐10 (short‐term outcome).
Mid‐term and long‐term outcomes
One rTMS study included data from mid‐term and long‐term follow‐up (Kang 2009). In the mid term, the mean reduction in pain NRS with rTMS was ‐1.09 points (95% CI ‐2.77 to 0.59; P value 0.20), and in the long term it was ‐0.21 points (95% CI ‐1.95 to 1.53; P value 0.81).
Cranial electrotherapy stimulation (CES) versus sham CES
Short‐term outcomes
In three studies, the short‐term effect of CES on pain was investigated (Capel 2003; Tan 2006; Tan 2011). The study by Capel 2003 was planned as a cross‐over study. Because the SF‐MPQ pain scores of participants receiving CES treatment during the first study period improved as compared with the scores of those receiving sham treatment, the second study period was abandoned and active CES was used in both study groups thereafter. Consequently, only comparative data from the first period are available. They were reported only as percentage changes in pain score from baseline—not as absolute values.
Two studies provided absolute estimates for the short‐term effect of CES on pain reduction (Tan 2006; Tan 2011). As different measurement instruments were used, we combined estimates for daily pain using NRS and for pain intensity using the BPI subscale by calculating the standardised mean difference (SMD). The combined estimate suggested no short‐term effects of CES on pain (Analysis 3.1).
3.1. Analysis.
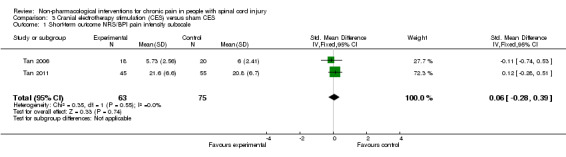
Comparison 3 Cranial electrotherapy stimulation (CES) versus sham CES, Outcome 1 Short‐term outcome NRS/BPI pain intensity subscale.
In the sensitivity analysis excluding studies with high overall risk of bias, only one study (Tan 2006) remained. It reported a mean pain reduction of 5.73 NRS points with CES and of 6.00 NRS points with sham CES. The mean difference was ‐0.27 NRS points (95% CI ‐1.86 to 1.32) or ‐0.11 SMD units (95% CI ‐0.74 to 0.53) (Analysis 3.1).
Exercise programme versus wait list control or no intervention
Short‐term outcomes
Three studies assessed exercise programmes for pain reduction: In the trial by Curtis et al, exercise and control groups differed in WUSPI pain scores at baseline (22.6 (standard deviation (SD) 21.8) units vs 11.05 (SD 11.9) units) (Curtis 1999). The mean change in WUSPI score between baseline and end of treatment after six months was ‐7.77 units (SD 19.01) in the exercise group and 2.8 (SD 12.74) in the control group, respectively. For the tetraplegic subgroup, corresponding mean difference values were 0.086 (SD 15.06) and 2.57 (SD 17.85), and for the paraplegic subgroup 14.64 (SD 20.32) and 3.0 (SD 7.69). Whether pain scores differed between experimental and control groups over time was not evaluated.
In another study (Hicks 2003), the mean difference in scores on the SF‐36 item for pain experience was ‐1.9 (95% CI ‐3.4 to ‐0.4; P value 0.01) in favour of the exercise group.
A third study (Mulroy 2011) tested exercise in combination with a standard movement optimisation intervention versus an 'attention only' control intervention (i.e. viewing of a one‐hour educational video). Exercise as compared with the control intervention led to a mean difference of ‐2.8 units on the pain VAS (95% CI ‐3.77 to ‐1.83; P value < 0.00001) and ‐30.70 units in the WUSPI score (95 % CI ‐43.31 to ‐18.09; P value 0.00001).
Estimates from the latter two trials were converted to standardised mean differences and are displayed in Analysis 4.10 and Analysis 4.11, but they were not combined, given the large differences between study and control interventions.
4.10. Analysis.
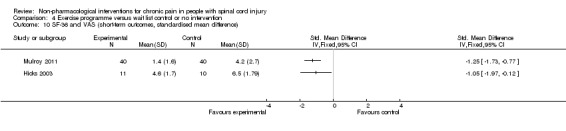
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 10 SF‐36 and VAS (short‐term outcomes, standardised mean difference).
4.11. Analysis.
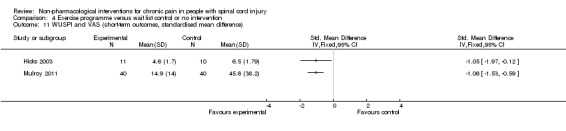
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 11 WUSPI and VAS (short‐term outcomes, standardised mean difference).
Mid‐term outcomes
One study (Mulroy 2011) measured pain reduction after a mid‐term follow‐up of four weeks. The mean difference in pain VAS was ‐2.50 (95% CI ‐3.48 to ‐1.52; P value < 0.00001) and in WUSPI score ‐26.40 (95% CI ‐37.62 to ‐15.18; P value < 0.00001) in favour of the exercise group.
All three studies were fraught with high overall risk of bias. In particular, the comparison with 'no treatment' or waiting lists as control interventions likely leads to an overestimation of the effectiveness of the exercise programmes provided in these studies. Consequently, no conclusion on their effectiveness can be drawn.
Self‐hypnosis versus electromyography (EMG) biofeedback
One study (Jensen 2009) compared self‐hypnosis with EMG biofeedback. Considerable imbalance in baseline measurements was noted among three pain outcomes: Average daily NRS values for pain were 5.99 (SD 1.83) in the self‐hypnosis group and 3.44 (SD 1.48) in the EMG biofeedback group. The NRS for pain unpleasantness was 6.83 (SD 1.69) and 3.91 (SD 2.07), respectively. The BPI score was 5.23 (SD 2.49) and 2.33 (SD 1.68), respectively.
Short‐term outcomes
After the treatment period, average NRS values for pain were 5.09 (SD 1.92) in the self‐hypnosis group and 3.36 (SD 1.28) in the biofeedback group. The NRS for pain unpleasantness was 5.1 (SD 12.12) and 2.88 (SD 1.02), respectively. The BPI score was 4.58 (SD 2.57) and 3.05 (SD 2.06), respectively.
Long‐term outcomes
Jensen et al also compared pain outcomes after three months. The mean NRS for daily pain was 4.90 (SD 2.13) in the self‐hypnosis group and 3.13 (SD 1.42) in the EMG biofeedback group. Mean NRS values for pain unpleasantness were 5.13 (SD 2.71) and 2.80 (SD 1.59), respectively, and mean BPI scores were 4.54 (SD 2.4) and 2.87 (SD 2.33), respectively.
This study was fraught with high overall risk of bias; consequently, no conclusion can be drawn regarding the effectiveness of self‐hypnosis as compared with EMG biofeedback.
Acupuncture versus sham acupuncture or Trager treatment
Short‐term and mid‐term outcomes
One study investigated the effects of acupuncture versus sham acupuncture on short‐term and mid‐term pain outcomes (Dyson‐Hudson 2007). The mean difference in NRS values was ‐1.10 (95% CI ‐3.42 to 1.22; P value 0.35) in the short term and ‐0.5 (95% CI ‐3.13 to 2.13; P value 0.71) in the mid term (i.e. after five weeks). The mean difference in WUSPI score was ‐3.30 (95% CI ‐18.77 to 12.17; P value 0.68) in the short term and ‐4.6 (95% CI ‐24.24 to 15.04; P value 0.65) in the mid term.
An earlier study by the same group compared the effects of acupuncture versus Trager treatment on short‐term and mid‐term outcomes (Dyson‐Hudson 2001). The mean difference in WUSPI scores was 1.70 (95% CI ‐21.91 to 25.31; P value 0.89) in the short term and 16.0 (95% CI ‐9.03 to 41.03; P value 0.21) in the mid term (i.e. after five weeks).
Estimates from both trials are displayed in Analysis 5.1 for the short‐term outcome and in Analysis 5.2 for the mid‐term outcome, but these values were not combined because of differences in study design. Both studies were fraught with high overall risk of bias; consequently, no conclusion can be drawn on the effectiveness of acupuncture as compared with sham acupuncture or Trager treatment.
5.1. Analysis.
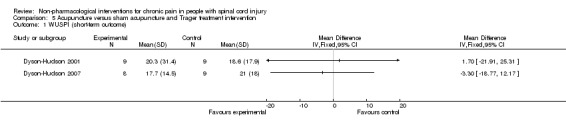
Comparison 5 Acupuncture versus sham acupuncture and Trager treatment intervention, Outcome 1 WUSPI (short‐term outcome).
5.2. Analysis.
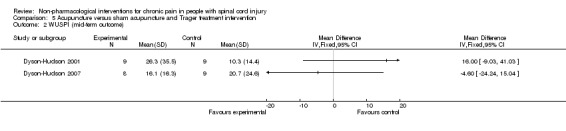
Comparison 5 Acupuncture versus sham acupuncture and Trager treatment intervention, Outcome 2 WUSPI (mid‐term outcome).
Transcutaneous electrical nerve stimulation (TENS) versus sham TENS
Short‐term outcomes
In one study (Doctor 1996), a factorial design was used to study whether pain (measured by DDS) can be reduced in the short term by two interventions: TENS (vs sham TENS) and an experimental manipulation to create a positive (vs neutral) treatment expectation. Data were analysed and reported separately for participants with neuropathic and musculoskeletal pain. In both of these subgroups, a substantial difference in the effects of TENS was noted between those with positive and neutral expectations (Analysis 6.1; Analysis 6.2). In participants with neuropathic pain and neutral expectations, the mean difference in DDS was ‐0.30 units (95% CI ‐5.56 to 4.96; P value 0.91), and in those with positive expectations ‐5.10 units (95% CI ‐8.41 to ‐1.79; P value 0.003). In participants with musculoskeletal pain and neutral expectations, the mean difference in DDS was 1.30 units (95% CI ‐0.19 to 2.79; P value 0.09), and in those with positive expectations ‐3.00 units (95% CI ‐6.41 to 0.41; P value 0.09).
6.1. Analysis.
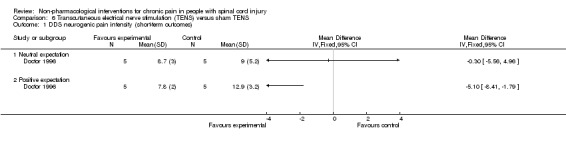
Comparison 6 Transcutaneous electrical nerve stimulation (TENS) versus sham TENS, Outcome 1 DDS neurogenic pain intensity (short‐term outcomes).
6.2. Analysis.
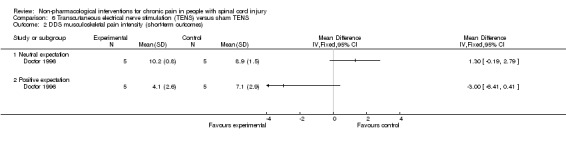
Comparison 6 Transcutaneous electrical nerve stimulation (TENS) versus sham TENS, Outcome 2 DDS musculoskeletal pain intensity (short‐term outcomes).
The study was fraught with high overall risk of bias; consequently, no conclusion can be drawn on the effectiveness of TENS as compared with a sham intervention.
Multi‐disciplinary cognitive behavioural programme versus wait list control
Short‐term and long‐term outcomes
One study investigated the effects of a multi‐disciplinary cognitive behavioural programme (Heutink 2012) on short‐term pain reduction. The mean difference in CPG pain intensity scores between experimental and waiting list control group was ‐2.0 (95% CI ‐9.26 to 5.26; P value 0.59). After three months (long‐term outcomes), the mean difference in CPG pain intensity scores was 0.40 (95% CI ‐7.3 to 8.1; P value 0.92).
The study was fraught with high overall risk of bias. In particular, the comparison with a waiting list as the control intervention likely leads to overestimation of the effectiveness of the experimental intervention. Consequently, no conclusion can be drawn on the effectiveness of this multi‐disciplinary cognitive behavioural programme.
Secondary outcome measures
The included studies provided insufficient data to permit reliable conclusions on the effects of the interventions on outcomes other than pain. Ten studies measured the effects of the interventions on anxiety, depression or quality of life (Figure 5). Six studies measured anxiety (Capel 2003; Doctor 1996; Fregni 2006; Heutink 2012; Soler 2010; Tan 2011), seven measured depression (Capel 2003; Defrin 2007; Fregni 2006; Heutink 2012; Hicks 2003; Jensen 2009; Tan 2011) and four studies measured quality of life (Heutink 2012; Hicks 2003; Mulroy 2011; Tan 2011). Of the ten studies, solely six provided outcome data (Defrin 2007; Heutink 2012; Hicks 2003; Jensen 2009; Mulroy 2011; Tan 2011). As with pain outcomes, imbalance at baseline between intervention and control groups was found for secondary outcomes in several studies (Defrin 2007; Heutink 2012; Jensen 2009; Tan 2011).
5.
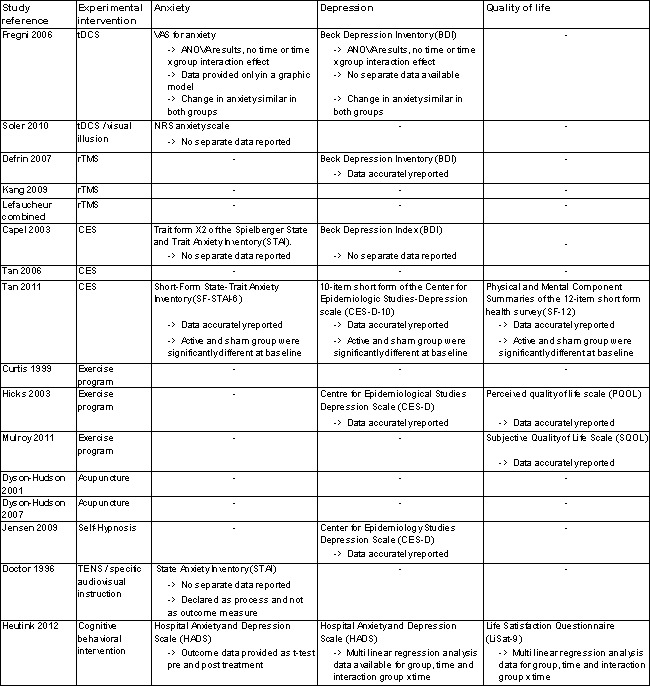
Secondary outcome measures.
Heutink 2012 measured the effects of a multi‐disciplinary cognitive behavioural programme on anxiety in short‐term and mid‐term outcomes. Mean differences in HADS anxiety scores were ‐0.10 (95% CI ‐1.86 to 1.66; P value 0.91) and 0.30 (95% CI ‐1.51 to 2.11; P value 0.74), respectively, for short‐term and long‐term outcomes. The study authors concluded: “The anxiety score of the intervention group showed a significant decrease between t1 and t2, and between t1 and t3, while the anxiety score of the control group showed no significant change.”
The study by Defrin 2007 measured the effects of rTMS on depression in the short term and the mid term. The mean difference in BDI depression scores was 7.0 (95 % CI ‐6.48 to 20.48; P value 0.31) and 4.0 (95 % CI ‐7.66 to 15.66; P value 0.50) for short‐term and mid‐term outcomes, respectively. The study authors concluded that "both real and sham groups exhibited a significant reduction in BDI values at the end of the treatment series compared with pre‐treatment levels.” Hicks 2003 measured the effects of an exercise programme on depression in the short term. The mean difference in CES‐D scores was ‐6.0 (95% CI ‐15.87 to 3.87; P value 0.23).
The studies of Hicks 2003 and Mulroy 2011 measured the effects of an exercise programme on quality of life. Hicks 2003 used the PQOL scale, and the mean difference was 10.8 (95% CI ‐4.2 to 25.8; P value 0. 16) for the short term. Mulroy 2011 used the SQOL scale for short‐term and mid‐term outcomes, and mean differences were 0.3 (95% CI ‐0.22 to 0.82; P value 0.25) and 0.5 (95% CI ‐0.03 to 1.03; P value 0.07), respectively.
Adverse effects
Four publications mentioned explicitly that no adverse effects occurred (Capel 2003; Doctor 1996; Dyson‐Hudson 2007; Lefaucheur 2004). Five publications described adverse effects (Curtis 1999; Fregni 2006; Mulroy 2011; Soler 2010; Tan 2011). In the tDCS trials (Fregni 2006; Soler 2010), seven participants had mild headache, and five mentioned itching under the electrodes. In the study by Soler 2010, three participants in the visual illusion group felt tired after the “walking.” In the CES trial by Tan 2011, adverse effects occurred in 41 participants in the active group and in 56 participants in the sham group. Most commonly mentioned were “ears pulse, tingle, sting, itch, small electric feeling or ear clips too tight,” “feeling drowsy, sleepy, fell asleep, relaxing” and “feeling dizzy, lightheaded or crooked.” In the exercise studies (Curtis 1999; Mulroy 2011), neck, shoulder and elbow injuries occurred in five participants from the intervention group. Overall, a paucity of evidence was available on serious or long‐lasting side effects of the interventions. Seven study reports did not mention whether or not adverse effects occurred (Defrin 2007; Dyson‐Hudson 2001; Heutink 2012; Hicks 2003; Jensen 2009; Kang 2009; Tan 2011).
Discussion
Summary of main results
We reviewed and critically appraised research evidence on the effectiveness and safety of non‐pharmacological interventions for the treatment of chronic pain in people living with SCI. We identified 16 randomised trials with a total of 616 participants with SCI and a variety of non‐pharmacological treatment approaches. Given the multiple methodological problems of these studies, an overall conclusion about their effectiveness and safety is not possible.
Transcranial direct current stimulation (tDCS) was superior to a sham intervention when the estimates of two studies were combined. However, overall evidence for the effectiveness of tDCS in reducing chronic pain in SCI was scarce and inconclusive. Data available from randomised studies on repetitive transcranial magnetic stimulation (rTMS) did not support its effectiveness in reducing chronic pain in persons living with SCI. One study including several related experiments suggested its effectiveness in a mixed study population but not in the SCI subgroup (Lefaucheur 2007). A second study (Defrin 2007) did not find a beneficial effect of active rTMS over a sham intervention, and a third study (Kang 2009) was inconclusive with regard to pain reduction.
Two studies on cranial electrotherapy stimulation (CES) had methodological weaknesses including high risk of selective reporting (Capel 2003) and baseline imbalance (Tan 2011), and a third study (Tan 2006) was inconclusive with regard to pain reduction.
Three trials reported a beneficial effect of an exercise programme on chronic pain (Mulroy 2011; Curtis 1999; Hicks 2003), but all had methodological shortcomings including lack of blinding and proper randomisation. In addition, the use of 'no treatment' or wait list control interventions in pain studies is problematic because it might yield different pain outcomes even when compared with placebo interventions (Hróbjartsson 2010).
In two trials acupuncture was not superior to sham acupuncture or Trager treatment in reducing pain (Dyson‐Hudson 2001; Dyson‐Hudson 2007).
Self‐hypnosis was compared with EMG biofeedback in one trial (Jensen 2009). Although the study authors concluded that both interventions decreased pain, the data are difficult to interpret as no sham control group was included and significant imbalance in baseline pain scores was evident between groups.
Only one trial focused on transcutaneous electrical nerve stimulation (TENS) (Doctor 1996). Results of this study suggest that treatment expectations and type of pain (neuropathic or musculoskeletal) are effect modifiers. However, available data do not allow firm conclusions.
A multi‐disciplinary cognitive behavioural programme was compared with no treatment (waiting list) in one trial (Heutink 2012). Although this type of control group likely favoured the experimental intervention, it revealed no short‐term or long‐term benefits of the programme.
Overall completeness and applicability of evidence
Completeness of the evidence
Overall, each type of intervention group included only a few studies; the maximum number of separate studies included in a meta‐analysis was two. In these meta‐analyses, statistical heterogeneity was low to moderate, with I2 values ranging from 0% to 55%. Both tDCS trials (Fregni 2006; Soler 2010) were included in a meta‐analysis, and upon request one study author provided additional data. Evidence on the effectiveness of rTMS, CES and exercise programmes was poor because two of three rTMS trials, two of three CES trials and all three exercise programme trials had high overall risk of bias. These studies were excluded from the sensitivity analysis. With only one trial for self‐hypnosis (Jensen 2009), one for TENS (Doctor 1996) and one for behavioural therapy (Heutink 2012), evidence for these three types of interventions was scarce and, again, was fraught with methodological problems. Two trials investigated the effects of acupuncture. However, one study compared acupuncture versus a sham intervention (Dyson‐Hudson 2007) and another versus Trager treatment (Dyson‐Hudson 2001); consequently, we refrained from pooling the data.
We initially planned to conduct subgroup analyses for trials in patients with different types of SCI or pain. However, the small number of included studies made this impossible. Further, we did not perform a planned sensitivity analysis for studies in which persons with SCI were only a subgroup because both trials with mixed populations had major methodological shortcomings related to baseline imbalance (Curtis 1999) and outcome reporting (Lefaucheur 2007). Nine of the 16 included trials provided information about the safety of the intervention or mentioned adverse effects. Thus, evidence on the safety of non‐pharmacological trials was incomplete.
Regarding representation of both sexes, the study populations of included trials reflect the adult SCI population, with about 70% of persons being male (van den Berg 2010). The mean age of the trial populations ranged from 35 to 56 years, and wide age ranges were noted in the individual studies. It is important to note that none of the trials included participants younger than 18 years of age, suggesting that non‐pharmacological treatments for chronic pain in children with SCI have not been studied in randomised trials.
Applicability
Among the range of non‐pharmacological pain treatments currently used in routine clinical practice, only some have been investigated in the included trials. TENS, physical training and acupuncture are commonly used for pain reduction (Cardenas 2006; Heutink 2011; Norrbrink 2004). When asked about pain treatments used, persons with SCI mentioned massage, heat therapy and other physiotherapy as the most commonly used treatments for chronic pain (Cardenas 2006; Norrbrink 2004; Widerstrom‐Noga 2003). Other frequently applied therapies include ice therapy, mental relaxation and range‐of‐motion training, psychological treatment and neurosurgery. We identified solely one randomised study on psychological treatment (Heutink 2012) but none on the other interventions. In particular, none of the neurosurgical interventions proposed for pain treatment have been evaluated in randomised controlled trials.
Most of the studied interventions are potentially applicable in any person with SCI pain. Nevertheless, some specific interventions have certain contraindications that limit their applicability. For instance, brain stimulation therapies are contraindicated in persons with a family or personal history of epilepsy, serious psychological or psychiatric disorders or metal implants. Acupuncture is not indicated in persons with bleeding disorders.
Quality of the evidence
For any of the eight intervention types, the maximum number of trials included in the meta‐analysis was two. All but two studies (Mulroy 2011; Tan 2011) had sample sizes of 40 or fewer participants. With more than 100 participants, the recent study by Tan 2011 illustrates that in this particular population, larger multi‐centre trials are feasible. Given that SCI is infrequent in many countries, the number of participants who are eligible for a clinical trial is low. To obtain study populations of adequate size, multi‐centre studies and larger research collaborations are crucially needed. In meta‐analyses of osteoarthritis trials with pain outcomes, it could be shown that studies with small sample size overestimated the benefit of therapeutic interventions as compared with larger studies (Nüesch 2010).
None of the included trials were considered to have low risk of bias. Twelve studies were judged as being at high risk of bias in at least one of the seven domains of the risk of bias tool of The Cochrane Collaboration and were excluded in the sensitivity analysis. The most predominant weaknesses were lack of blinding of participants and personnel and selection of potentially invalid control interventions, potentially invalidating any conclusions regarding the effectiveness of interventions. In eight trials, blinding was precluded by the nature of the study intervention or was not successful. Blinding of participants and personnel is a major challenge in RCTs. In particular with non‐pharmacological treatments, the development of adequate control interventions is challenging. One way to attempt to reduce bias involves using 'attention control groups' with similar frequency and duration of contact with health care professionals as those in the treatment group but with no active intervention. However, several included studies used only waiting list or 'no treatment' control groups. Despite randomisation, several trials were fraught with an imbalance between study groups in baseline pain scores. This was the second most common potential source of bias. In eight trials, participants had both neuropathic and non‐neuropathic pain conditions. Given that an intervention might work better or worse with one particular type of pain, the case mix may hamper correct interpretation of the outcome data. Published aggregated data did not allow stratified analyses by pain type.
We included only trials that addressed chronic pain. However, these trials included participants at different stages of chronic pain, and outcome measures were taken at different time points relative to SCI onset and time of intervention. This represents an important source of heterogeneity. The data do not allow conclusions on the optimal time point for starting chronic pain treatment after SCI.
The trials included in our review used a broad range of measures for pain, which reflects the absence of commonly accepted standards for this outcome. In the included publications, most instruments used were described as having good validity and reliability. However, the International Spinal Cord Pain Data Set (Widerstrom‐Noga 2008) and the Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury (NIDRR SCI) Measures meeting (Bryce 2007) specifically recommended that a numerical rating scale should be used to measure pain intensity. The latter also recommended use of the SF‐36 pain item and MPI or BPI to measure pain interference (Bryce 2007). Further, the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT) recommended several outcome measures for different pain domains, although these recommendations were not SCI‐specific (Dworkin 2008; IMMPACT Recommendations 2005). To date, such collaborative efforts have not led to a unified framework for studies on chronic SCI pain.
Potential biases in the review process
Our search strategy comprised extensive literature searches of several major electronic databases, trial registers and conference proceedings as well as contact with experts in the field. Despite these searches, we may have missed eligible studies, in particular if they were not published in indexed peer‐reviewed journals. Our search update yielded several potentially eligible studies (listed under Characteristics of studies awaiting classification) that will be considered in an update of this review. Because of the small number of trials identified for each of the intervention types, we refrained from performing formal statistical tests to detect reporting bias.
To provide a critical discussion of the current state of interventional research on non‐pharmacological SCI pain treatments, we chose to include studies with methodological weaknesses such as unblinded participants, waiting list controls or obvious imbalances in pain measurements at baseline. We stress that these studies are potentially biased towards and may overestimate the effectiveness of experimental treatments. We further emphasise that inclusion of a trial in this review should not be interpreted as accepting it as internally valid and trust that our own conclusions are sufficiently cautious.
In the statistical analyses of all included studies, the data derived from ordinal scales were treated as continuous outcomes and expressed as mean, standard deviation or relative change. These choices made by the trialists also determined our approach and, consequently, represent one of the methodological limitations of our review. It has been argued that ordinal scales do not have the same mathematical properties as continuous variables (Merbitz 1989), and that this may lead to incorrect inference from the data (Stucki 1996). One solution is to convert outcome data to the cardinal metric through use of the Rasch model and to perform an individual patient data meta‐analysis (Newby 2009). However, limited available aggregated data did not allow this approach in this review.
Agreements and disagreements with other studies or reviews
In 2010, the Spinal Cord Injury Rehabilitation Evidence (SCIRE) project published a review on pain treatment following spinal cord injury (SCIRE 2010), which also covered non‐pharmacological approaches and surgical interventions. Although our current review was restricted deliberately to randomised controlled trials, the SCIRE review also included non‐randomised studies (e.g. those using before‐after comparisons). This explains why, for some types of interventions, the conclusions of the SCIRE review differ from ours. In the SCIRE review, tDCS and CES trials were found to be effective. Our findings are consistent with the findings of the SCIRE project for tDCS but do not confirm the effectiveness of CES. For rTMS, the SCIRE review identified solely the trial of Defrin 2007 and concluded that repetitive transcranial magnetic stimulation reduces post‐SCI pain, in contrast to the findings of our review. For exercise programmes, the SCIRE authors made a distinction between exercise for post‐SCI pain and for shoulder pain and identified additional studies comparing baseline and post‐treatment measures (pre‐post trial). They concluded that exercise reduces both post‐SCI pain and shoulder pain, which is in line with our findings. Also for acupuncture, their conclusions were consistent with ours: Acupuncture may reduce post‐SCI pain but is no more effective than Trager treatment or sham acupuncture. Further, the SCIRE review identified a pre‐post study on hypnotic suggestion that was not included in our review. It concluded that hypnosis may reduce pain intensity post‐SCI. Based on two controlled trials, the SCIRE review concluded that cognitive behavioural pain management alone does not alter post‐SCI pain. Furthermore, SCIRE identified two non‐randomised trials on cognitive behavioural therapy. These review authors concluded that cognitive behavioural therapy alone does not change post‐SCI pain intensity; however in combination with pharmacological treatment, it might be effective. We identified solely one randomised controlled trial with 61 participants. Results of this study are insufficient to permit firm conclusions about the effects of the behavioural program on pain.
A Cochrane review on non‐invasive brain stimulation for chronic pain covered several interventions (tDCS, rTMS and CES) that were within the scope of our review (O'Connell 2010). In addition to seven studies (Capel 2003; Defrin 2007; Kang 2009; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Tan 2006) with a focus on SCI, this review included pain trials conducted in patients with health conditions other than SCI. The review authors stated that low‐frequency rTMS was not effective in the treatment of chronic pain. For CES and tDCS, they found the evidence insufficient to allow conclusions on their effectiveness. Given that the scope of this review differed from the scope of ours, the findings are not fully comparable. Further, some of the included trials were evaluated differently: First, O'Connell 2010 included all three publications of Lefaucheur et al (Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008) as separate trials, whereas we combined the data after clarification with the trialists. Second, the study of Defrin 2007 was included in their meta‐analysis, whereas we excluded it from our sensitivity analysis because of the substantial imbalance in pain scores seen at baseline and the resulting risk of bias.
We came across several studies on non‐pharmacological pain treatments that used study designs other than randomised trials (e.g. surveys). In a recent study, people with SCI were asked which non‐pharmacological pain treatments they perceived as most effective (Heutink 2011). Massage and relaxation therapy were mentioned most commonly. Acupuncture, physiotherapy and exercise, massage and relaxation and psychological treatments were found to be effective. In a survey in a Swedish SCI population, the participants rated TENS as minimally effective and physical training as more effective in reducing pain (Norrbrink 2004). Only half of the participants rated acupuncture as effective. In yet another survey of 120 people living with SCI pain, 47.5% of respondents reported a considerable pain reduction with physical therapy and 61.5% a slight reduction with psychological treatment (Widerstrom‐Noga 2003). Such cross‐sectional data might inform about the common use and perceived effects of non‐pharmacological pain treatments but cannot be compared directly with the results of trials designed to determine treatment effects.
Authors' conclusions
Implications for practice.
Evidence is insufficient to show that non‐pharmacological treatments are effective in reducing chronic pain in people living with SCI. Available evidence on the effectiveness and safety of several specific pain interventions is scarce and is fraught with methodological weaknesses: Most interventions were investigated only in small samples or in series of trials conducted by a single research group, sometimes including only one randomised study of acceptable methodological quality. For instance, in one recent trial (Mulroy 2011), an intervention combining exercise and standard movement optimisation was associated with a statistically and clinically significant reduction in shoulder pain, but this finding needs to be confirmed in other studies, ideally by studies using more valid control interventions and conducted by independent groups. In everyday practice, a variety of other non‐pharmacological interventions are applied. Several recent trials on the interventions already considered here as well as other types of treatment such as massage (e.g. Chase 2013) were identified in the search update and will be considered in an updated version of this review.
Implications for research.
Given that existing evidence has been derived from small trials with low methodological quality, adequately powered trials with improved design and methods (e.g. with regard to choice of control interventions) are needed. More recent studies included in our review are encouraging in that investigators addressed some of the weaknesses of earlier studies with regard to methodology and reporting quality (Soler 2010; Tan 2011). Furthermore, the current evidence is very heterogeneous in terms of study design, study population, outcome measures and interventions. New multi‐centre trials should focus primarily on interventions that are commonly used in daily practice. Study outcomes would preferably be chosen on the basis of current guidelines such as the IMMPACT and NIRRD SCI recommendations (Bryce 2007; Dworkin 2008; IMMPACT Recommendations 2005). These recommendations favour NRS for measuring pain intensity and BPI or MPI for measuring physical functioning and pain interference after SCI. In line with these recommendations, the methodology of future studies on chronic SCI pain could be improved by measuring pain immediately after experimental treatment periods as well as in the mid term or long term. In 2008, an expert group of clinicians specialising in SCI care developed the International Spinal Cord Injury Pain Data Set, which is available as both a basic version and a self‐report version (Widerstrom‐Noga 2008). This guide recommends that pain measures be used in SCI research with the aim of achieving comparability of results between studies.
We acknowledge that it might be impossible to blind participants and personnel for certain interventions. However, when possible, blinding should be done, and it may be possible to blind outcome assessors at a minimum. In intervention and control groups, similar protocols should be used to reduce the risk of attention bias and to appropriately determine the net effects of the experimental intervention. Waiting list and no treatment groups are discouraged as they may lead to overestimation of the effectiveness of experimental interventions (Hróbjartsson 2010). Most of the included study reports contained little information about adverse effects. Future studies should address the safety aspects of non‐pharmacological treatments more thoroughly and should report potential harm in greater detail. Of note, we found no study focusing on SCI pain treatment in children and adolescents. This may reflect the general paucity of randomised evidence for interventions in children. However, we suggest that non‐pharmacological treatments to reduce or cope with chronic pain in children and adolescents with SCI may differ in their effectiveness as compared with that in adults, and that targeted research in this age group is warranted.
Acknowledgements
We thank the trial search co‐ordinators of the Cochrane Injuries Group, Ms Karen Blackwell and Ms Deirdre Beecher, for help provided in searching literature databases and the librarians of Swiss Paraplegic Research, Nottwil, Switzerland for support provided in retrieving literature.
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group Specialised Register 1.(spine or spinal or vertebrae) AND (fracture* or wound* or trauma* or injur* or damag*) 2.(“spinal cord”) AND (contusion or laceration or transaction or trauma or ischemia) 3.(“central cord” OR “central spinal cord”) AND syndrome 4.paraplegi* or quadriplegi* or tetraplegi* 5.1 or 2 or 3 or 4 6.Pain or neuropath* or nocicept* 7.5 and 6 Cochrane Central Register of Controlled Trials #1MeSH descriptor Spinal Cord Injuries explode all trees #2MeSH descriptor Spinal Cord Ischemia explode all trees #3MeSH descriptor Central Cord Syndrome explode all trees #4myelopathy near3 (traumatic or post‐traumatic) #5(spine or spinal or vertebrae) near3 (fracture* or wound* or trauma* or injur* or damag*) #6(spinal cord) near3 (contusion or laceration or transaction or trauma or ischemia) #7central cord injury syndrome #8central spinal cord syndrome #9MeSH descriptor Spinal Cord explode all trees #10SCI:ti #11SCI:ab #12paraplegi* or quadriplegi* or tetraplegi* #13(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14Pain or neuropath* or nocicept* #15(#13 AND #14) MEDLINE (OvidSP) 1.exp Spinal Cord Injuries/ 2.exp Spinal Cord Ischemia/ 3.exp Central Cord Syndrome/ 4.(myelopathy adj3 (traumatic or post‐traumatic)).ab,ti. 5.((spine or spinal or vertebrae) adj3 (fracture* or wound* or trauma* or injur* or damag*)).ab,ti. 6.(spinal cord adj3 (contusion or laceration or transaction or trauma or ischemia)).ab,ti. 7.central cord injury syndrome.ab,ti. 8.central spinal cord syndrome.ab,ti. 9.exp Cervical Vertebrae/in [Injuries] 10.exp Spinal Cord/in [Injuries] 11.SCI.ab,ti. 12.exp Paraplegia/ 13.exp Quadriplegia/ 8.(paraplegi* or quadriplegi* or tetraplegi*).ab,ti. 14.or/1‐14 15.exp Pain/ 16.exp Pain, Intractable/ 17.exp Pain Measurement/ 18.exp Pain Threshold/ 19.exp Nociceptors/ 20.(pain adj5 (refer* or refractory or intractable or receptor* or nocicept* or muskuloskeletal or chronic or intens* or threshold* or shoulder* or abdominal* or back or neuropath*)).ab,ti. 21.((nocicept* adj3 neuron*) or pain*).ti. 22.16 or 17 or 18 or 19 or 20 or 21 or 22 23.15 and 23 24.randomi?ed.ab,ti. 25.randomized controlled trial.pt. 26.controlled clinical trial.pt. 27.placebo.ab. 28.clinical trials as topic.sh. 29.randomly.ab. 30.trial.ti. 31.25 or 26 or 27 or 28 or 29 or 30 or 31 32.(animals not (humans and animals)).sh. 33.32 not 33 34.24 and 34
Embase (OvidSP) 1.exp spinal cord injury/ 2.exp central cord syndrome/ 3.exp cervical spine injury/ 4.exp cervical spinal cord injury/ 5.exp Spinal Cord Ischemia/ 6.(myelopathy adj3 (traumatic or post‐traumatic)).ab,ti. 7.((spine or spinal or vertebrae) adj3 (fracture* or wound* or trauma* or injur* or damag*)).ab,ti. 8.(spinal cord adj3 (contusion or laceration or transaction or trauma or ischemia)).ab,ti. 9.central cord injury syndrome.ab,ti. 10.central spinal cord syndrome.ab,ti. 11.exp Spinal Cord/ 12.exp INJURY/ 13.11 and 12 14.SCI.ab,ti. 15.exp Paraplegia/ 16.exp SPASTIC PARAPLEGIA/ 17.exp Quadriplegia/ 18.(paraplegi* or quadriplegi* or tetraplegi*).ab,ti. 19.1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 13 or 14 or 15 or 16 or 17 or 18 20.exp Pain/ 21.exp intractable pain/ 22.exp pain assessment/ 23.exp pain threshold/ 24.exp nociceptive receptor/ 25.exp neuropathic pain/ 26.(pain adj5 (refer* or refractory or intractable or receptor* or nocicept* or muskuloskeletal or chronic or intens* or threshold* or shoulder* or abdominal* or back or neuropath*)).ab,ti. 27.((nocicept* adj3 neuron*) or pain*).ti. 28.20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29.19 and 28 30.exp Randomized Controlled Trial/ 31.exp controlled clinical trial/ 32.randomi?ed.ab,ti. 33.placebo.ab. 34.*Clinical Trial/ 35.randomly.ab. 36.trial.ti. 37.30 or 31 or 32 or 33 or 34 or 35 or 36 38.exp animal/ not (exp human/ and exp animal/) 39.37 not 38 40.29 and 39 ISI Web of Science: Science Citation Index‐Expanded; Social Sciences Citation Index; Conference Proceedings Citation Index‐Science; Conference Proceedings Citation Index‐Social Sciences & Humanities #1Topic=((spine or spinal or vertebrae) same (fracture* or wound* or trauma* or injur* or damag*)) OR Topic=((spinal cord) same (contusion or laceration or transaction or trauma or ischemia)) OR Topic=((central cord OR central spinal cord) same (syndrome)) OR Topic=(paraplegi* or quadriplegi* or tetraplegi*) #2Topic=(Pain or neuropath* or nocicept*) #3#1 and #2 #4Topic=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) #5Topic=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) OR Topic=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) #6#4 and #5 #7Topic=(human*) #8#6 and #7 #9#3 and #8
CINAHL Plus (EBSCO) 1.Spinal cord 2.SCI 3.Paraplegia 4.Tetraplegia 5.Quadriplegia 6.(paraplegi* or quadriplegi* or tetraplegi*) 7.spinal cord and laceration 8.spinal cord and transaction 9.spinal cord and trauma 10.spinal cord trauma 11.spinal cord and ischemia 12.Spinal Cord Ischemia 13.spinal cord injur 14.Central Cord Syndrome 15.((spine or spinal) and (fracture or wound or trauma or injur or damage or lesion)) 16.(myelopathy and (traumatic or post‐traumatic)) 17.(spinal cord) and (contusion or laceration or transaction or trauma or ischemia)) 18.myelopathy and traumatic 19.traumatic myelopathy 20.post‐traumatic myelopathy 21.myelopathy and post‐traumatic 22.spine or spinal and fracture 23.spinal fracture 24.spine or spinal and wound 25.spine or spinal and trauma 26.spine or spinal and injur 27.spine or spinal and damage 28.spine or spinal and lesion 29.spinal cord and contusion 30.spinal cord and laceration 31.central cord injury syndrome 32.central spinal cord syndrome 33.Cervical Vertebrae Injur 34.S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 35.Pain 36. Pain and Intractable 37.Pain Measurement 38.Pain Threshold 39.nocicept* pain 40.(pain and (refer* or refractory or intractable or receptor* or nocicept* or muskuloskeletal or chronic or intens* or threshold* or shoulder* or abdominal* or back or neuropath*)) 41.nocicept* and neuron* or pain 42.Nociceptors 43.S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 44.34 and 43 45. Search limited using Randomized Controlled Trial OR CLINICAL TRIAL WHO International Clinical Trials Registry Platform (ICTRP) Spinal cord injury = 209 Spinal cord inj* pain = 18 Spinal cord inj* and pain = 28 Parapleg* pain =0 Tetrapleg* pain = 0 Tetraplegia pain =0 Spinal cord inj* and RCT =0 Spinal cord injury and randomized control trial =0 Spinal cord injury and pain = 39 AMED (((FT=spinal cord injur? AND FT=pain ) OR TI=spinal cord injur? "AND" pain ) OR (CT D "pain AND spinal cord injur"? OR UT="pain AND spinal cord injur"? OR IT="pain AND spinal cord injur"? OR SH="pain AND spinal cord injur"?)) AND pps=human)))
Data and analyses
Comparison 1. Transcranial direct current stimulation (tDCS) versus sham tDCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS or NRS 0‐10 (short‐term outcome) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.48, ‐0.33] |
| 2 VAS anxiety 0‐10 (short‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 VAS or NRS 0‐10 (mid‐term outcome) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.30, ‐0.45] |
| 4 VAS anxiety 0‐10 (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 NRS 0‐10 (long‐term outcome) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.82, 0.35] |
1.2. Analysis.
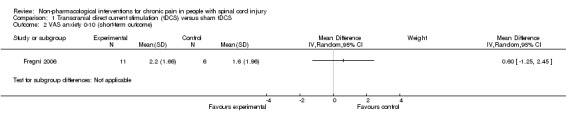
Comparison 1 Transcranial direct current stimulation (tDCS) versus sham tDCS, Outcome 2 VAS anxiety 0‐10 (short‐term outcome).
1.4. Analysis.
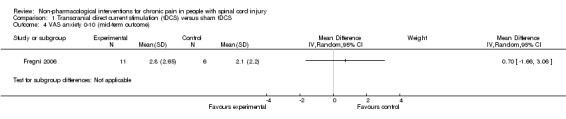
Comparison 1 Transcranial direct current stimulation (tDCS) versus sham tDCS, Outcome 4 VAS anxiety 0‐10 (mid‐term outcome).
Comparison 2. Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NRS 0‐10 (short‐term outcome) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 BPI (short‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 BDI (short‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 NRS 0‐10 (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 BPI (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 BDI (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 NRS 0‐10 (long‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 BPI (long‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
2.2. Analysis.
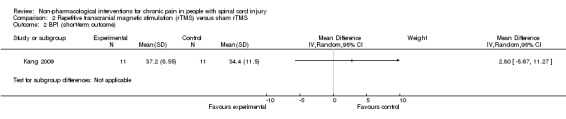
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 2 BPI (short‐term outcome).
2.3. Analysis.
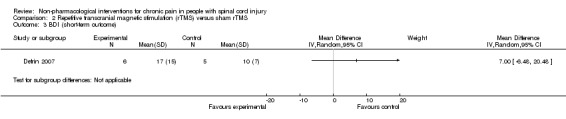
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 3 BDI (short‐term outcome).
2.4. Analysis.
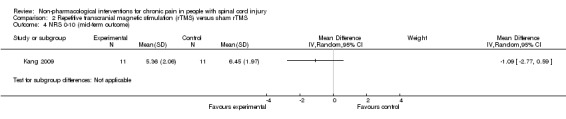
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 4 NRS 0‐10 (mid‐term outcome).
2.5. Analysis.
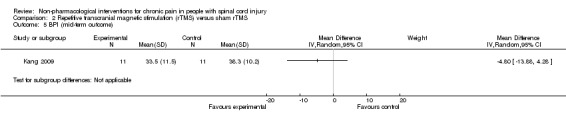
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 5 BPI (mid‐term outcome).
2.6. Analysis.

Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 6 BDI (mid‐term outcome).
2.7. Analysis.
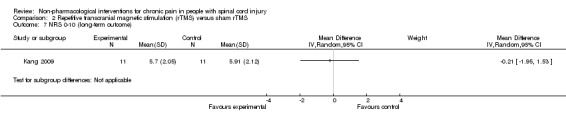
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 7 NRS 0‐10 (long‐term outcome).
2.8. Analysis.
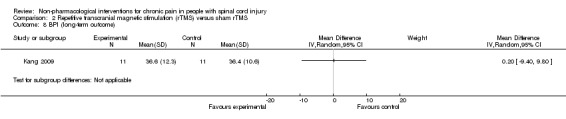
Comparison 2 Repetitive transcranial magnetic stimulation (rTMS) versus sham rTMS, Outcome 8 BPI (long‐term outcome).
Comparison 3. Cranial electrotherapy stimulation (CES) versus sham CES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term outcome NRS/BPI pain intensity subscale | 2 | 138 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.28, 0.39] |
| 2 Sensitivity analysis NRS 0‐10 (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
3.2. Analysis.
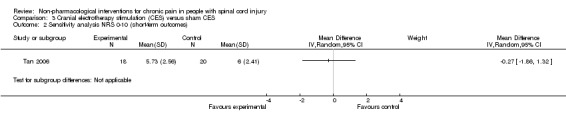
Comparison 3 Cranial electrotherapy stimulation (CES) versus sham CES, Outcome 2 Sensitivity analysis NRS 0‐10 (short‐term outcomes).
Comparison 4. Exercise programme versus wait list control or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 WUSPI (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 VAS 0‐10 (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 SF‐36 (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 CES‐D (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 SQOL (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 PQOL (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 WUSPI (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 VAS 0‐10 (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 SQOL (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10 SF‐36 and VAS (short‐term outcomes, standardised mean difference) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 WUSPI and VAS (short‐term outcomes, standardised mean difference) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
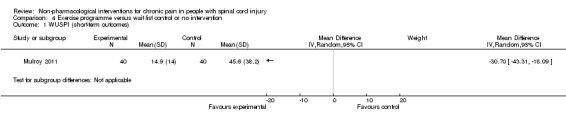
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 1 WUSPI (short‐term outcomes).
4.2. Analysis.
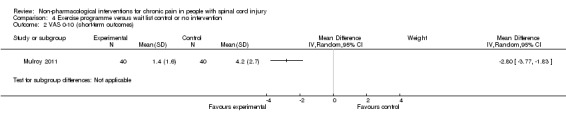
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 2 VAS 0‐10 (short‐term outcomes).
4.3. Analysis.
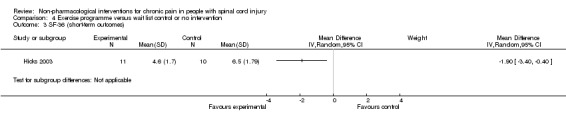
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 3 SF‐36 (short‐term outcomes).
4.4. Analysis.
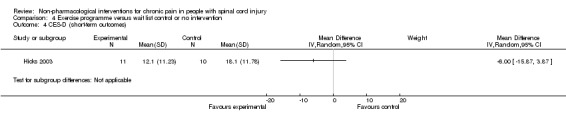
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 4 CES‐D (short‐term outcomes).
4.5. Analysis.
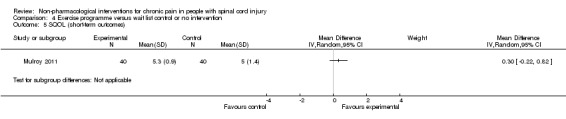
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 5 SQOL (short‐term outcomes).
4.6. Analysis.
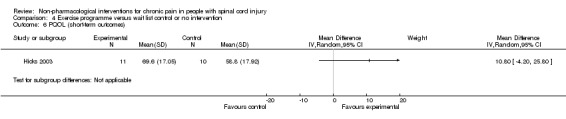
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 6 PQOL (short‐term outcomes).
4.7. Analysis.
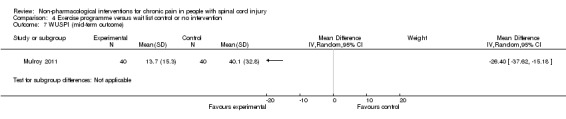
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 7 WUSPI (mid‐term outcome).
4.8. Analysis.
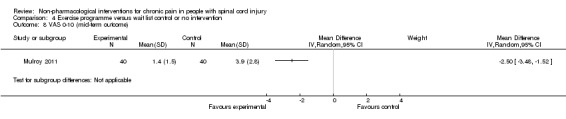
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 8 VAS 0‐10 (mid‐term outcome).
4.9. Analysis.
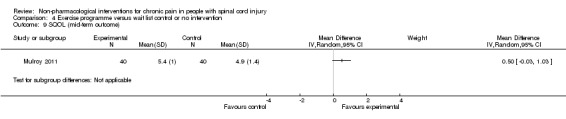
Comparison 4 Exercise programme versus wait list control or no intervention, Outcome 9 SQOL (mid‐term outcome).
Comparison 5. Acupuncture versus sham acupuncture and Trager treatment intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 WUSPI (short‐term outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 WUSPI (mid‐term outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 NRS 0‐10 (short‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 NRS 0‐10 (mid‐term outcome) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
5.3. Analysis.
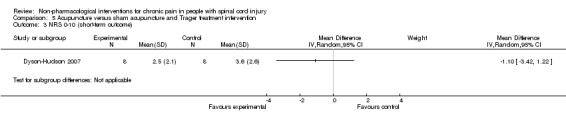
Comparison 5 Acupuncture versus sham acupuncture and Trager treatment intervention, Outcome 3 NRS 0‐10 (short‐term outcome).
5.4. Analysis.
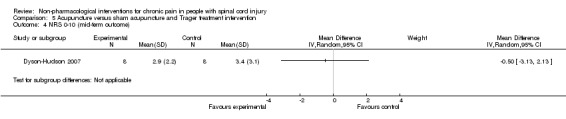
Comparison 5 Acupuncture versus sham acupuncture and Trager treatment intervention, Outcome 4 NRS 0‐10 (mid‐term outcome).
Comparison 6. Transcutaneous electrical nerve stimulation (TENS) versus sham TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DDS neurogenic pain intensity (short‐term outcomes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Neutral expectation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Positive expectation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 DDS musculoskeletal pain intensity (short‐term outcomes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Neutral expectation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Positive expectation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Multi‐disciplinary cognitive‐behavioural programme versus wait list control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CPG, pain Intensity (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 CPG, pain‐related disability (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 HADS, anxiety (short‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 CPG, pain intensity (long‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 CPG, pain‐related disability (long‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 HADS, anxiety (long‐term outcomes) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
7.1. Analysis.
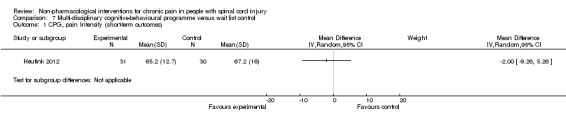
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 1 CPG, pain Intensity (short‐term outcomes).
7.2. Analysis.
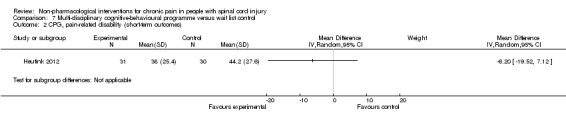
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 2 CPG, pain‐related disability (short‐term outcomes).
7.3. Analysis.
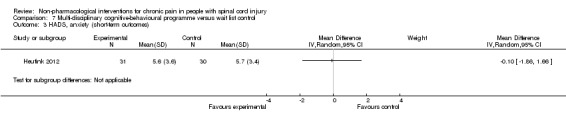
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 3 HADS, anxiety (short‐term outcomes).
7.4. Analysis.
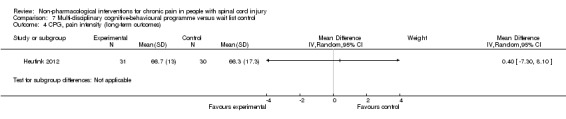
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 4 CPG, pain intensity (long‐term outcomes).
7.5. Analysis.
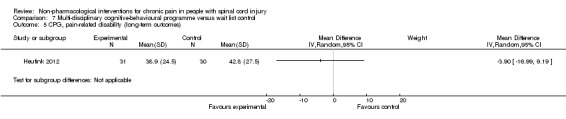
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 5 CPG, pain‐related disability (long‐term outcomes).
7.6. Analysis.
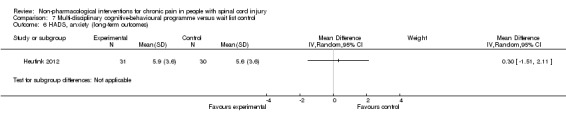
Comparison 7 Multi‐disciplinary cognitive‐behavioural programme versus wait list control, Outcome 6 HADS, anxiety (long‐term outcomes).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Capel 2003.
| Methods | Cross‐over trial | |
| Participants | N (intervention/control): 30 (15/15) Type of SCI: paraplegic and tetraplegic. Lesion level was cervical in 9 participants, thoracic in 16 and lumbar in 5 Type of pain: chronic neuropathic, nerve root entrapment, visceral, musculoskeletal pain or pain of unknown origin (no definition of chronicity) Additional pain treatment: reduction and (if possible) cease of other analgesic, anxiolytic or antidepressive medication Age (years) mean/SD (range): not indicated Gender (male/female): not indicated Country of study: UK Setting: residential educational centre |
|
| Interventions | Experimental group: non‐invasive transcranial electrostimulation (tCET) Control group: sham tCET Duration of intervention: 53 minutes, twice daily on 4 successive days |
|
| Outcomes | Pain: McGill Pain Questionnaire (SF‐MPQ) present pain index subscale, based on 6‐point VAS scale (range 0‐5) with 10 cm width (no anchors stated) Anxiety: State Trait Anxiety Inventory (STAI) Depression: Beck Depression Index (BDI) Measurement time points: before first intervention and after each session Follow‐up measures: none |
|
| Notes | During the second cross‐over intervention period, it was decided that all participants should receive active treatment. Data from this study period were disregarded. Data from the first study period were included as if originating from a parallel‐group trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants could choose their devices, which had an identical look; this was considered equivalent to drawing lots from an urn |
| Allocation concealment (selection bias) | Low risk | Was assumed because of randomisation methods used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The devices were numbered for identification but neither the administrators nor the recipients of the treatment could distinguish between the devices" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The questionnaires were collated and forwarded to professional statisticians, Priority Search, Sheffield, UK, who analyzed the data after the first and second arm[s] of the study, unaware as to which group received tCET and which received sham treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants dropped out for reasons not related to study participation |
| Selective reporting (reporting bias) | High risk | Pain score values were not provided for all measurement time points |
| Other bias | Low risk | No other bias was observed |
Curtis 1999.
| Methods | Parallel‐group randomised controlled trial, referred to as pilot study | |
| Participants | N (intervention/control): 42, of those 35 with SCI (16/19) Type of SCI: tetraplegic or paraplegic with ASIA criteria C6 or lower Type of pain: shoulder pain (no definition of chronicity) Additional pain treatment: not indicated Age (years) mean/SD (range): 35/8 (range not indicated) Gender (male/female): 35/7 Country of study: USA Setting: outpatient clinic |
|
| Interventions | Experimental group: home‐based exercise programme including 2 static stretching exercises and 3 strengthening exercises for posterior shoulder muscles, twice‐daily stretching, once‐daily strengthening. Control group: no treatment Duration of intervention: 6 months |
|
| Outcomes | Pain: Wheelchair User's Shoulder Pain Index (WUSPI), measures pain intensity during performance of daily living with 15 items, using VAS 10 cm with anchors "no pain" to "worst pain ever experienced" (score range 0‐150) Measurement time points: before intervention and every 2 months during 6‐month period Follow‐up measures: none |
|
| Notes | Study author provided data on the SCI subgroup upon request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible because of the nature of the experimental intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants dropped out for reasons not related to the study intervention |
| Selective reporting (reporting bias) | Low risk | Study author provided data on the SCI subgroup upon request |
| Other bias | High risk | Important baseline difference in pain scores between intervention and control groups |
Defrin 2007.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control): 12 randomly assigned, 11 analysed (6/5) Type of SCI: traumatic SCI; 2 participants with complete, 9 with incomplete lesions, lesion level between T4 and T12 Type of pain: chronic central pain, minimum duration of 12 months Additional pain treatment: Participants were instructed not to change usual dosage throughout the experiment and follow‐up periods Age (years) mean/SD (range): 54/6 (44‐60) Gender (male/female): 7/4 Country of study: Israel Setting: outpatient clinic of rehabilitation centre at general hospital |
|
| Interventions | Experimental group: repetitive transcranial magnetic stimulation (rTMS) of the motor cortex. 500 trains were delivered at a frequency of 5 Hz for 10 seconds with 30 second intertrain interval Control group: sham rTMS Duration of intervention: 10 daily sessions, each lasting 15‐30 minutes |
|
| Outcomes | Chronic pain intensity: VAS with 11‐point scale (range 0‐10), anchors "no pain" to "the most intense pain sensation imaginable" Chronic pain experience: McGill Pain Questionnaire (MPQ) Depression: Beck Depression Inventory (BDI) Measurement time points: VAS: before each session, then every 5 minutes during session and immediately thereafter Pain threshold: before first and after 5th and 10th sessions MPQ: before first session, then after 5th and 10th sessions and between 2 and 6 weeks after last intervention BDI: before first and after 10th session and between 2 and 6 weeks after last intervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients as well as the person conducting the outcome measurements were blind to the type of treatment received" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients as well as the person conducting the outcome measurements were blind to the type of treatment received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data indicated, one dropout due to reason not related to study |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | High risk | Important baseline difference in pain scores between intervention and control groups |
Doctor 1996.
| Methods | Factorial randomised controlled trial | |
| Participants | N (intervention/control): 40 (20 neuropathic pain/20 musculoskeletal pain) Type of SCI: C4 and below (range C4‐L3, most common was C5). 42% of participants had complete sensory loss, 35% complete motor and sensory loss Type of pain: neuropathic and musculoskeletal pain (average duration in participants was 9.35 years, SD 9) Additional pain treatment: no Age (years) mean/SD (range): 46/14.7 (range not indicated) Gender (male/female): 35/5 Country of study: USA Setting: inpatient and outpatient spinal cord unit |
|
| Interventions | Participants were allocated to 1 of 4 groups:
TENS was a continuous square wave pulse stimulation delivered at 100 pulses per second and individual adaption for each participant such that a painless paraesthesia was produced. A positive/neutral treatment expectation was created by showing a short instructional video to participants with a script of positive/neutral content Duration of intervention: 1 session of 30 minutes |
|
| Outcomes | Pain: Descriptor Differentiation Scale (DDS) of pain intensity and pain unpleasantness Measurement time points: baseline, during intervention after 10 and 20 minutes, immediately after intervention (baseline + 30 minutes) and 30 minutes after treatment (baseline + 1 hour) Follow‐up measures: 30 minutes after treatment |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated (personal communication by author) |
| Allocation concealment (selection bias) | Unclear risk | Not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double blinding was not successful according to the trial report |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The research assistant, who had the most contact with each subject, also could not predict TENS or expectancy condition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data indicated |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | Low risk | No other bias was observed |
Dyson‐Hudson 2001.
| Methods | Parallel‐group randomised clinical trial | |
| Participants | N (intervention/control): 24 enrolled; 18 completed study and data were analysed (9/9) Type of SCI: 8 with tetraplegia and 16 with paraplegia, lesion level between C6 and T12 Type of pain: chronic musculoskeletal shoulder pain for longer than 3 months Additional pain treatment: no additional medication prescribed during study duration, weekly log for usual intake of medication Age (years) mean/SD (range): 43.5/11.1 (28‐69) (for final sample of 18 participants: 45.1/11.4 (no range stated)) Gender (male/female): 18/6; for final sample: 14/4 Country of study: USA Setting: outpatient clinic of rehabilitation hospital |
|
| Interventions | Experimental group: acupuncture with 6 local and 2 distal points in sessions lasting 20‐30 minutes Second intervention group: Trager treatment (specific type of manual therapy) with sessions lasting approximately 45 minutes Duration of intervention: 10 sessions over 5 weeks |
|
| Outcomes | Pain: Wheelchair User's Shoulder Pain Index (WUSPI),15‐item self‐report questionnaire, total index score 0‐150 NRS for average, most severe and least severe shoulder pain (11‐point scale ranging from 0‐10 with anchors "no pain" to "worst pain ever experienced"); 6‐point verbal rating scale (VRS) with anchors "much worse" to "cured/pain free" ("no change" in middle of scale) for change in pain (not included in meta‐analysis) Weekly log of activity, medication intake and pain Measurement time points: WUSPI and NRS weekly, VRS at baseline and after follow‐up period Follow‐up measures: 5 weeks after end of intervention |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All subjects completing the baseline were randomised consecutively by the investigators by using blocked randomisation into either an acupuncture or a Trager treatment group. Subjects with a prior history of acupuncture or Trager were randomised separately by means of a coin toss" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because of the type of intervention. Quote: "Neither subjects nor practitioners were blinded to the treatments they received or performed, respectively" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Only evaluators were blinded to treatment group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four subjects withdrew during the first week of the baseline period, before treatment group randomization (1 for medical reasons; 3 for personal reasons). Two additional subjects withdrew during the treatment period because of unrelated medical conditions" |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | Low risk | No other bias was observed |
Dyson‐Hudson 2007.
| Methods | Parallel‐group randomised clinical trial | |
| Participants | N (intervention/control): 23 included; 17 analysed (8/9) Type of SCI: 8 with tetraplegia, 15 with paraplegia Type of pain: chronic musculoskeletal shoulder pain for longer than 3 months Additional pain treatment: Participants were instructed to document intake in weekly diaries Age (years) mean/SD (range): 39.9/10.3 (21‐65) Gender (male/female): 18/5 Country of study: USA Setting: outpatient clinic at a clinical research centre |
|
| Interventions | Experimental group: acupuncture Control group: sham acupuncture Duration of intervention: 10 sessions over 5 weeks (range 5‐8 weeks) |
|
| Outcomes | Pain: Wheelchair User's Shoulder Pain Index (WUSPI) Numerical rating scale (NRS) measuring average shoulder pain intensity on a 11‐point scale (range 0‐10) with anchors "no pain" to "worst pain ever experienced" Measurement time points: weekly assessment during baseline period (4 weeks), treatment period (5 weeks) and follow‐up period (5 weeks) Follow‐up measures: during 5 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how block randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded but risk of bias due to impossibility of blinding acupuncturist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All participants and the principal investigator were blinded as to group assignment. In an effort to maintain the blind, participants were asked to not discuss details about their treatment experience with other participants or the principal investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 1 (NRS) of 2 pain scores were missing for only 1 participant. Risk of resulting bias was deemed low |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | Low risk | No other bias was observed |
Fregni 2006.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control): 17 (11/6) Type of SCI: traumatic SCI, Frankel score A‐D Type of pain: stable chronic neuropathic pain for at least 3 preceding months Additional pain treatment: standard medication (any changes were recorded) Age (years) mean/SD (range): 35.7/13.3 (range not indicated) Gender (male/female): 14/3 Country of study: Brazil Setting: pain clinic |
|
| Interventions | Experimental group: transcranial direct current stimulation (tDCS) with a constant current of 2 mA intensity (subthreshold intensity) Control group: sham tDCS Duration of intervention: 20 minutes during 5 consecutive days |
|
| Outcomes | Pain: 11‐point (0‐10) VAS for pain during preceding 24 hours with anchors "no pain" to "worst pain possible" Depression: Beck Depression Index (BDI) Anxiety: VAS with range 0‐10 and anchors "no anxiety" to "worst anxiety ever" Measurement time points: at baseline, before and after each session and once during follow‐up period Follow‐up measures: 16 days |
|
| Notes | Block randomisation with ratio of 2:1 (intervention to control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of study entrance and block randomisation using previous computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was labelled as double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded evaluator rated the pain using the visual analogue scale for pain, Clinician Global Impression and Patient Global Assessment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors performed ITT analysis |
| Selective reporting (reporting bias) | Low risk | Study author provided additional data upon request |
| Other bias | Low risk | No other bias was observed |
Heutink 2012.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control): 61 (31/30) Type of SCI: tetraplegia and paraplegia of traumatic and non‐traumatic aetiology, with complete and incomplete lesions Type of pain: chronic neuropathic pain for at least 6 months Additional pain treatment: standard medication Age (years) mean/SD (range): 58.8/11.4 (range not stated) Gender (male/female): 39/22 Country of study: Netherlands Setting: 4 SCI rehabilitation centres |
|
| Interventions | Experimental group: programme comprising educational, cognitive and behavioural elements targeted at coping with chronic pain Control group: waiting list Duration of intervention: 10 sessions of 3 hours over 10 weeks, 'comeback session' after 3 weeks |
|
| Outcomes | Pain: Chronic Pain Grade (CPG) questionnaire and intensity of average pain, worst pain and current pain on 11‐point NRS, no anchors defined Depression and anxiety: Hospital Anxiety and Depression Scale (HADS): a 14‐item self‐report measure with 2 scales for anxiety and depression, each scoring 0‐21, anchors not stated Quality of life: Life Satisfaction Questionnaire (LiSat‐9) Measurement time points: at baseline, at end of programme (3 months) and at 6 months Follow‐up measures: 3 months after last session of programme |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Website for randomisation (personal communication by author) |
| Allocation concealment (selection bias) | Low risk | Website for randomisation (personal communication by author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed according to ITT principle |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to study protocol |
| Other bias | High risk | Despite proper randomisation, important baseline difference in scores for participation in activities |
Hicks 2003.
| Methods | Parallel‐group randomised controlled trial with matched design (2 exercise: 1 control) | |
| Participants | N (intervention/control): 34 (21/13) Type of SCI: tetraplegia and paraplegia with ASIA classification A‐D, 1‐24 years after trauma Type of pain: not indicated Additional pain treatment: no Age (years) mean/SD (range): only range reported: 19‐65 years Gender (male/female): 23/11 Country of study: Canada Setting: outpatient centre for health promotion and rehabilitation |
|
| Interventions | Experimental group: exercise training (and bimonthly education session) Control group: waiting list Duration of intervention: twice‐weekly training for 90‐120 minutes during 9 months |
|
| Outcomes | Pain: 2 SF‐36 items on pain experience and interference with normal work in the past 4 weeks, with 6 response options from "non/not at all" to "very severe pain/extremely" Stress: Perceived Stress Scale (PSS) with 14 items and 6‐point frequency scale with anchors "all of the time" and "none of the time" Depression: Centre of Epidemiological Studies Depression Scale (CES‐D) with 4‐point Likert scale, score range 0‐60 Quality of life: 11‐item perceived quality of life scale (PQOL) with 4 additional items Measurement time points: baseline and after 3, 6 and 9 months Follow‐up measures: none |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not indicated |
| Allocation concealment (selection bias) | High risk | Participants were matched before randomisation (ratio 2 active: 1 control) according to age, years post‐SCI and mortality risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because of type of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors (personal communication from authors) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses did not account for dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | Low risk | No other bias was observed |
Jensen 2009.
| Methods | Parallel‐group randomised controlled trial (ratio 2 exercise:1 control) | |
| Participants | N (intervention/control): 37 (23/14) Type of SCI: spinal cord injury for 6 months or longer Type of pain: daily bothersome chronic pain (no definition of chronicity); 17 participants had neuropathic and 20 had non‐neuropathic pain Additional pain treatment: regular medication Age (years) mean/SD (range): 49.5/not indicated (19‐70) Gender (male/female): 28/9 Country of study: USA Setting: not indicated |
|
| Interventions | Experimental group: self‐hypnosis Control group: electromyography biofeedback relaxation training Duration of intervention: 10 sessions with variable frequency from daily to weekly (depending on preferences of participants) |
|
| Outcomes | Pain: NRS for average daily pain and current pain intensity using 11‐point scale (score 0‐10) anchored "no pain" to "most intense pain sensation imaginable"; modified Brief Pain Inventory (BPI) Depression: Center for Epidemiology Studies Depression Scale (CES‐D) Measurement time points: pretreatment and post‐treatment and at end of follow‐up Follow‐up measures: 3 months |
|
| Notes | Participants had been recruited earlier for another prior pain study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed (personal communication by authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because of types of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded (personal communication by study authors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data reported |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical and match study protocol |
| Other bias | High risk | Important baseline differences in pain scores between intervention and control groups |
Kang 2009.
| Methods | Cross‐over trial | |
| Participants | N (intervention/control): 13; of those, 11 analysed; each participant received both rTMS and sham rTMS Type of SCI: 6 with paraplegia and 5 with tetraplegia; 6 with motor incomplete and 5 with motor complete SCI Type of pain: chronic neuropathic pain for at least 15 months Additional pain treatment: Participants were instructed not to change their usual pain medication Age (years) mean/SD (range): 54.8/13.7 years (33 to 75) Gender (male/female): 6/5 Country of study: South Korea Setting: outpatient clinic of university hospital |
|
| Interventions | Experimental group: rTMS (20 trains of 10 Hz stimulation during 5 seconds with 55 second interval) Control group: sham rTMS Duration of intervention: daily on 5 consecutive days, real and sham rTMS were separated by a 12‐week "wash‐out period" |
|
| Outcomes | Pain: 11‐point NRS (range 0‐10) for average and worst pain during the preceding 24 hours, anchored "no pain sensation" to "most intense pain sensation imaginable" Brief Pain Inventory (BPI) (not included in meta‐analysis) Measurement time points: before, immediately after 3rd and 5th sessions; then 1, 3, 5 and 7 weeks after last intervention Follow‐up measures: 7 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation code mentioned but no additional information about randomisation method Quote: "The real and sham rTMS stimulations were separated by 12 weeks and performed in a random order according to the prepared allocation code" Comment: less critical in cross‐over design |
| Allocation concealment (selection bias) | Unclear risk | Not indicated but deemed less critical in cross‐over study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "(...) the investigator who applied the stimulation coil to the participants was not involved in the recruitment or evaluation of the participants" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To ensure that the study was performed double‐blind, 1 researcher applied the magnetic stimulation and a different researcher collected the clinical data; the latter researcher was not aware of the type of rTMS (real or sham) that had been used for each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts during intervention period |
| Selective reporting (reporting bias) | Low risk | Data are reported comprehensively |
| Other bias | Low risk | No other bias was observed |
Lefaucheur 2007.
| Methods | Cross‐over trial | |
| Participants | N (intervention/control): 19. Each participant received both repetitive transcranial magnetic cortical stimulation (rTMS) and sham rTMS Type of SCI: cervical level, aetiology: ischaemic, traumatic, surgical, post‐traumatic cervical syringomyelia. Information was provided in only 1 of 3 publications (Lefaucheur 2006) Type of pain: chronic intractable unilateral neurogenic pain for 1‐7 years Additional pain treatment: standard individual treatment Age (years) mean/SD (range): mean/SD not indicated (range 34‐72) Gender (male/female): 15/4 Country of study: France Setting: not indicated |
|
| Interventions | Experimental group: rTMS, 20 trains of 6 seconds with 54 second interval at 10 Hz or a single train of 20 minutes at 1 Hz Control group: sham rTMS Duration of intervention: 2 or 3 sessions of 20 minutes each; sessions were separated by at least 3 weeks |
|
| Outcomes | Pain level: VAS with range 0‐10, relative change in pain level before versus after session Measurement time points: pre‐treatment and post‐treatment Follow‐up measures: none |
|
| Notes | Data on participants from 3 studies by same group were combined (Lefaucheur 2004,Lefaucheur 2006; Lefaucheur 2008). For detailed information about individual studies, see Characteristics of studies awaiting classification (Lefaucheur 2004, 2006 and 2008) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “one of the following two protocols was applied in a random order” Comment: The method of randomisation is not specified but was deemed less critical in a cross‐over study |
| Allocation concealment (selection bias) | Unclear risk | Not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not informed that a sham intervention was being provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not indicated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information about dropouts. Likelihood of dropout during 2 intervention periods was deemed low |
| Selective reporting (reporting bias) | Unclear risk | Outcome data were reported clearly and comprehensively. 2 of the 3 publications did not include information about adverse events |
| Other bias | High risk | Multiple publications of some outcome data are not made transparent in publications but were disclosed upon request |
Mulroy 2011.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control)= 80 (40/40) Type of SCI: all with paraplegia, 64 had motor complete lesions (ASIA A or B) Type of pain: chronic shoulder pain. Average pain duration in participants was 66 months Additional pain treatment: not indicated Age (years) mean/SD: 45.0/11.2 (range not indicated) Gender male/female: 57/23 Country of study: USA Setting: outpatient clinic of rehabilitation centre |
|
| Interventions | Experimental group: home‐based exercise programme to optimise movement, consisting of shoulder strengthening and stretching exercises, along with recommendations on how to optimise the movement technique of transfers, raises and wheelchair propulsions Control group: viewing of a 1‐hour educational video (as minimal intervention) Duration of intervention: exercise programme 3 times a week over 12 weeks |
|
| Outcomes | Pain: VAS for shoulder pain (0‐10 cm), anchors not indicated; Wheelchair User's Shoulder Pain Index (WUSPI) total score 150
Quality of life: Subjective Quality of Life Scale (SQOL) and SF‐36 with 8 subscales Measurement time points: All participants were evaluated before and after the 12‐week intervention, and 4 weeks after the end of the intervention Follow‐up measures: 4 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed until the time of intervention assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because of type of study intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants were assessed by a blinded evaluator before and after the 12‐week intervention and 4 weeks after the end of the intervention (week 16) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete case analysis and intention‐to‐treat (ITT) analysis for all randomly assigned participants |
| Selective reporting (reporting bias) | Low risk | VAS scores for change between post‐intervention and follow‐up were not reported (for ITT analysis, all data were used) |
| Other bias | Unclear risk | Participants were free to continue exercises during follow‐up phase (possible source of bias) |
Soler 2010.
| Methods | Factorial randomised controlled trial | |
| Participants | N (intervention/control): 40, of those 39 analysed in 4 groups (N = 10, 10, 9, 10) Type of SCI: lesion level C4‐T12, ASIA classification A and B, 1‐31 years post‐SCI Type of pain: chronic neuropathic pain with pain intensity of 4 or higher on NRS 0‐10 for at least 6 months Additional pain treatment: Publication lists individual pain medication for each participant Age (years) mean/SD (range): 45/15.5 (21‐66) Gender (male/female): 30/9 Country of study: Spain Setting: rehabilitation centre |
|
| Interventions | 4 study groups:
Duration of intervention: 10 sessions of 20 minutes each during 2 weeks |
|
| Outcomes | Pain: NRS (0‐10) for average pain intensity in previous 24 hours with anchors "no pain" to "unbearable pain" Brief Pain Inventory (BPI) interference subscale, score range 0‐10 with anchors "no interference" to "complete interference" (not included in meta‐analysis) Anxiety: NRS anxiety scale range 0‐10 with anchors "no anxiety" to "worst anxiety" Adverse effects: open‐ended question Measurement time points: baseline; days 14, 24 and 38; and after 12 weeks Follow‐up measures: 12 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer‐generated list as randomization strategy" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "A second researcher, who applied the interventions, remained blind to the findings of the clinical evaluation. Assignment of the patients to the treatment interventions was random, and patients remained blinded to their treatment condition and the specific hypotheses of the study" "At the end of the treatment sessions none of the patients could tell whether they had undergone real or sham transcranial DCS, even when explicitly asked" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The same researcher, who was blind to the treatment interventions, performed all clinical evaluations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts unrelated to study |
| Selective reporting (reporting bias) | Low risk | Study author provided missing data upon request |
| Other bias | Low risk | No other bias was observed |
Tan 2006.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control): 40 enrolled, 38 analysed (18/20) Type of SCI: ASIA A‐C classification: 16 with paraplegia (6 active/10 sham) and 8 with tetraplegia (4 active/4 sham); ASIA D classification: 14 (8 active/6 sham); 33 with traumatic aetiology (15 active/18 sham) and 5 non‐traumatic (3 active/2 sham). Delay since SCI between 6 months and 60 years Type of pain: chronic musculoskeletal or neuropathic pain (12 in active/11 in sham group), musculoskeletal pain (6 in active/9 in sham group), duration of at least 3 months Additional pain treatment: not indicated Age (years) mean/SD (range): experimental group: 56.0/8.3; control group: 56.6/10.9; overall range: 38‐82 years (not indicated for study groups) Gender (male/female): 40/0 Country of study: USA Setting: medical centre, SCI care line |
|
| Interventions | Experimental group: cranial electrotherapy stimulation (CES) with 100 µA (subthreshold) Control group: sham CES Duration of intervention: 1 hour during consecutive 21 days |
|
| Outcomes | Pain: NRS (scale 0‐10) for daily pain with anchors "no pain" to "pain as bad as you can imagine" Brief Pain Inventory (BPI) modified for persons with disabilities, pain intensity subscale (0‐10) with anchors "no pain" to "pain as bad as you can imagine," participants rated pain "at its worst in the past 24 hours", "at its least in the past 24 hours", "on average" and "right now" (not included in meta‐analysis) Interference of pain with QOL: modified BPI scale 0‐10 with anchors "does not interfere" to "interferes completely" Measurement time points: daily pre‐intervention and post‐intervention Follow‐up measures: not indicated |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not indicated |
| Allocation concealment (selection bias) | Unclear risk | Not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "Participants were unable to determine whether they were receiving active or sham CES, since the amount of electrical stimulation was set at a subthreshold level and could not be changed by the participants." "The investigators, research assistant (RA), and participants were blinded to treatment type until the end of the initial phase" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded until the end of the initial phase |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about 2 dropouts. Likelihood that data on dropouts would change results was deemed low |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | Low risk | No other bias was observed |
Tan 2011.
| Methods | Parallel‐group randomised controlled trial | |
| Participants | N (intervention/control): 105 (46/59) Type of SCI: complete and incomplete tetraplegia or paraplegia Type of pain: chronic neuropathic pain at or below lesion level for at least 6 months with intensity of 5 or higher on 10‐point NRS Additional pain treatment: not indicated Age (years) mean/SD (range): active group: 52.1/10.5 (27‐79), sham group: 52.5/11.7 (26‐80) Gender (male/female): 90/15 (intervention 38, control 52 male) Country of study: USA Setting: outpatient clinics at 4 rehabilitation centres; device was applied at home |
|
| Interventions | Experimental group: cranial electrotherapy stimulation (CES) of 100 microamperes subsensation Control group: sham CES Duration of intervention: 1 hour daily during 21 days |
|
| Outcomes | Pain: Pain Intensity and Pain Interference Subscales of Brief Pain Inventory (BPI) adapted for persons with disability Pain subscale of SF‐36; Paroxysmal, Deep, and Surface Pain subscales of the Pain Quality Assessment Scale (PQAS); pain beliefs and pain coping (Maladaptive and Adaptive Coping) subscales of the Two‐Item Measures of Pain Beliefs and Coping Strategies (not included in meta‐analysis) Quality of life: Short Form Health Survey (SF‐12) Anxiety: Short‐Form State‐Trait Anxiety Inventory (SF‐STAI‐6) Depression: 10‐item short form of the Center for Epidemiologic Studies‐Depression Scale (CES‐D‐10) Measurement time points: pre‐intervention and post‐intervention Follow‐up measures: none |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by selecting a device from a box initially containing equal numbers of active and sham devices" |
| Allocation concealment (selection bias) | Low risk | Concealment ensured by allocation method |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The equipment was set up for a double‐blind study by the manufacturer such that the participants could not differentiate active from sham CES devices" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Research staff members who interacted with the participants (e.g. recruited and trained participants, administered questionnaires, followed up by telephone) did not know which devices were sham and which were active" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses did not account for dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in Results and Methods sections are identical |
| Other bias | High risk | Baseline imbalance in 3 of 4 pain outcome measures (BPI pain interference subscale, 36‐item short‐form health survey (SF‐36) pain subscale, 2‐item measures of pain beliefs and coping strategies maladaptive coping subscale)) and in 3 outcome measures on anxiety and depression (SF‐12 mental component summary, CES‐D‐10 and SF‐STAI‐6) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Coşkun Çelik 2005 | No proper randomisation (personal communication by study author) |
| Crowe 2000 | Study on pain in acute phase after SCI |
| Ditor 2003 | Follow‐up publication of study by Hicks et al (2003). Study report provides data from participants who agreed to continue with the study intervention as well as additional follow‐up measurements and no additional randomisation |
| Dyson‐Hudson 2007b | Quasi‐experimental study |
| Hughes 2006 | Included only 1 participant with SCI |
| Lefaucheur 2010 | Did not focus on treatment for chronic pain |
| Norrbrink 2006 | No proper randomisation. Quote: "Due to the low number of volunteers, it was not possible to carry out 2 parallel groups of treatment regimens, making randomisation impossible" |
| Norrbrink 2009 | No proper randomisation. Quote: "Every other patient enrolled was assigned to start with HF TENS" |
| Perry 2010 | Controlled study without randomisation |
| Saitoh 2007 | Retrospective case series |
| Wardell 2006 | No proper randomisation (treatment allocation by geographical location of residence) |
| Xing 2010 | No proper randomisation (treatment allocation according to admission time) |
Characteristics of studies awaiting assessment [ordered by study ID]
Alcobendas‐Maestro 2012.
| Methods | Randomised controlled trial |
| Participants | N (intervention/control): 40/40 Type of SCI: C2‐T12 spinal cord injury, classified as AIS grades C and D Type of pain: not indicated |
| Interventions | Experimental group: Lokomat Control group: overground therapy for walking Duration of intervention: 40 sessions during 8 weeks |
| Outcomes | Pain: visual analogue scale (VAS) (0‐10) |
| Notes | Will be considered in next update |
Arienti 2011.
| Methods | Experimental study |
| Participants | N (intervention/control): 13/17/16 Type of SCI: traumatic SCI, 33 participants with complete SCI (ASIA level A), 14 with incomplete SCI (ASIA level B, C, D) Type of pain: 21 with pure neuropathic pain and 26 with both nociceptive and neuropathic pain Additional pain treatment: All participants were treated with antidepressants Gender (male/female): 39/7 Country of study: Italy Setting: inpatient spinal cord unit |
| Interventions | 3 experimental groups: osteopathic treatment, pharmacological treatment and pharmacological and osteopathic treatment |
| Outcomes | Pain: 11‐point verbal numerical scale (VNS), range 0‐10, anchors "absence of pain" to "worst possible pain experienced" |
| Notes | Will be considered in next update |
Chase 2013.
| Methods | Pilot single‐centre study, randomised cross‐over trial |
| Participants | N (intervention/control): 20/20 Type of SCI: 33 participants with tetraplegia, 7 with paraplegia Type of pain: any level of pain Age (years) mean/SD (range): 40.24/13.8 Gender (male/female): 33/7 Country of study: USA Setting: free‐standing, not‐for‐profit, comprehensive rehabilitation centre specialising in SCI rehabilitation |
| Interventions | Experimental group: broad compression massage (BCM) Control group: light contact touch (LCT) Duration of intervention: six 20‐minute treatment sessions over 2 weeks |
| Outcomes | Pain: Brief Pain Inventory Short Form (BPI‐SF), modified to assess symptoms in the last 24 hours |
| Notes | Will be considered in next update |
Hirayama 2006.
| Methods | Experimental study with randomly assigned sham interventions |
| Participants | Among others, 2 people with spinal cord lesion |
| Interventions | Repetitive transcranial magnetic stimulation (rTMS) |
| Outcomes | Visual analogue scale (VAS) and short form of McGill Pain Questionnaire (SF‐MPQ) |
| Notes | Unclear whether randomly assigned (study authors did not respond to our requests), possible overlap with Saitoh 2007a |
Jette 2013.
| Methods | Randomised cross‐over study |
| Participants | N (intervention/control): 16 participants Type of SCI: complete or incomplete motor SCI, 12 paraplegia, 4 tetraplegia Type of pain: chronic neuropathic pain (for at least 3 months) |
| Interventions | Experimental group: repetitive transcranial magnetic cortical stimulation (rTMS), 10 Hz (total of 2000 stimuli) in random order over the hand or leg Control group: sham rTMS |
| Outcomes | Pain: 11‐point NRS (range 0‐10) with anchors 0 = no pain sensation to 10 = the worst possible pain |
| Notes | Will be considered with next update |
Lefaucheur 2004.
| Methods | Cross‐over trial |
| Participants | N (intervention/control): total study size 60, of those 12 with SCI Type of SCI: not indicated Type of pain: chronic intractable unilateral lower limb pain of neurogenic aetiology (no definition of chronicity) Additional pain treatment: not indicated Age (years) mean/SD (range): 53.5/12.0 (34‐72) Gender (male/female): 9/3 Country of study: France Setting: not indicated |
| Interventions | Experimental group: repetitive transcranial magnetic cortical stimulation (rTMS), 10 Hz over hand motor cortex (20 trains over 5 seconds with 55 second intertrain interval) Control group: sham rTMS Duration of intervention: 1 session of 20 minutes per week, interventions separated by at least 3 weeks |
| Outcomes | Pain: 11‐point VAS (range 0‐10), relative change in pain level pre/post each session (no anchors stated) Measurement time points: pre‐treatment and post‐treatment Follow‐up measures: no |
| Notes | Study author provided individual data for SCI subgroup upon request |
Lefaucheur 2006.
| Methods | Cross‐over trial |
| Participants | N (intervention/control): total study size 22, of those 4 with SCI Type of SCI: cervical level, aetiology: ischaemic, traumatic, surgical, post‐traumatic cervical syringomyelia Type of pain: chronic intractable unilateral lower limb pain of neurogenic aetiology for 4‐7 years Additional pain treatment: individual standard treatment Age (years) mean/SD (range): 63/7.8 (range 48‐72) Gender (male/female): 2/2 Country of study: France Setting: not indicated |
| Interventions | Experimental group: repetitive transcranial magnetic cortical stimulation (rTMS), 10 Hz (20 trains during 6 seconds with 54 second intertrain interval) or a single train during 20 minutes at 1 Hz and 90% of rTMS using an active coil (1200 pulses) Control group: sham rTMS Duration of intervention: 3 sessions of 20 minutes separated by at least 3 weeks |
| Outcomes | Pain: 11‐point VAS (range 0‐10), relative change in pain level pre/post each session Measurement time points: pre‐treatment and post‐treatment Follow‐up measures: no |
| Notes | Data on 3 of the 4 SCI participants were already used in previous publication (Lefaucheur 2004) |
Lefaucheur 2008.
| Methods | Cross‐over trial |
| Participants | N (intervention/control): total study size 46, of those 10 with SCI Type of SCI: not indicated Type of pain: chronic drug‐resistant unilateral neuropathic pain in at least the hand for at least 1 year Additional pain treatment: not indicated Age (years) mean/SD (range): 54/11.8 (range 34‐72) Gender (male/female): 9/1 Country of study: France Setting: not indicated |
| Interventions | Experimental group: repetitive transcranial magnetic cortical stimulation (rTMS), 10 Hz (20 trains during 6 seconds with 54 second intertrain interval) or a single train of 20 minutes in duration at 1 Hz and 90% of rTMS over the motor cortex using an active coil (1200 pulses) Control group: sham rTMS Duration of intervention: 3 sessions of 20 minutes separated by at least 3 weeks |
| Outcomes | Pain: 11‐point VAS 0‐10, relative change in pain level pre/post each session Measurement time points: pre‐treatment and post‐treatment Follow‐up measures: no |
| Notes | Data on 4 of the 10 SCI participants were already used in previous publication (Lefaucheur 2004) |
Litchke 2012.
| Methods | Pilot randomised study |
| Participants | Wheelchair rugby athletes |
| Interventions | 2 types of respiratory resistance training |
| Outcomes | SF‐36 v2 item 'Bodily pain,' health‐related QOL was a secondary study outcome |
| Notes | Will be considered with next update |
Livshits 2002.
| Methods | Experimental study |
| Participants | N (intervention/control): 20/20 Type of SCI: paraplegia (spinal cord lesions at thoracic level) Type of pain: severe painful spasticity of lower extremities Age (years) mean/SD (range): 27.6/7.8 in group with Pourpre technique and 27.1/8.3 with Bischof II technique Gender (male/female): 29/11 Country of study: Israel and Russia Setting: in‐hospital spinal care unit |
| Interventions | 2 experimental groups using different surgical treatments for painful spasticity (longitudinal T‐myelotomy by Bischof II technique or Pourpre technique) |
| Outcomes | Pain: McGill Short Questionnaire Spasticity: muscle tone and muscle spasm according to Ashworth and spasm frequency scales |
| Notes | Unclear whether randomised (study authors did not respond to our requests) |
Middaugh 2013.
| Methods | Randomised controlled trial |
| Participants | N (intervention/control): 8/7 Type of SCI: level C6 or lower; 2 or more years' duration Type of pain: musculoskeletal pain in shoulder girdle region Age (years) mean/SD (range): 38.1/not indicated (23‐56) Gender (male/female): 12/3 Country of study: USA Setting: university hospital |
| Interventions | Experimental group: EMG biofeedback and exercise Control group: exercise |
| Outcomes | Pain: WUSPI |
| Notes | Will be considered in next update |
Muller 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be considered in next update |
Saitoh 2006.
| Methods | Experimental study |
| Participants | Patients with intractable deafferentation pain; 2 with spinal cord lesions |
| Interventions | Repetitive transcranial magnetic stimulation (rTMS) |
| Outcomes | Visual analogue scale (VAS) and short form of McGill Pain Questionnaire (SF‐MPQ) |
| Notes | Unclear whether randomised (authors did not respond to our requests), possible overlap with Saitoh 2007a |
Saitoh 2007a.
| Methods | Experimental study with randomly assigned sham interventions |
| Participants | Patients with intractable deafferentation pain; 2 with spinal cord lesions |
| Interventions | Repetitive transcranial magnetic stimulation (rTMS) |
| Outcomes | Pain measured on a visual analogue scale (VAS) |
| Notes | Unclear whether randomised (study authors did not respond to our requests), possible overlap with Hirayama 2006 |
Wrigley 2013.
| Methods | Randomised cross‐over design |
| Participants | N (intervention/control): 10 Type of SCI: thoracic SCI Type of pain: neuropathic pain for longer than 6 months Age (years) mean/SD (range): 56.1/14.9 Gender (male/female): 8/2 Country of study: Australia |
| Interventions | Experimental group: transcranial direct current stimulation (tDCS) with a constant current of 2 mA intensity Control group: sham tDCS Duration of intervention: 20 minutes over 5 consecutive days |
| Outcomes | Pain: Neuropathic Pain Scale (NPS) (item 9) using an 11‐point numerical rating scale (0 = not unpleasant; 10 = the most unpleasant sensation imaginable, ‘‘intolerable’’) Depression: Beck Depression Index (BDI) |
| Notes | Will be considered with next update |
Yilmaz 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be considered in next update |
Yoon 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be considered in the next update |
Characteristics of ongoing studies [ordered by study ID]
NCT00523016.
| Trial name or title | Acupuncture Treatment to Reduce Burning Pain in Spinal Cord Injury (APSCI) |
| Methods | Randomised controlled trial |
| Participants | People with traumatic SCI (adult onset) and burning pain below SCI level |
| Interventions | Acupuncture with electrical stimulation (electroacupuncture); sham acupuncture |
| Outcomes | Primary: improvement in burning pain; secondary: improvement in quality of life measurements |
| Starting date | September 2007 |
| Contact information | Linda M Rapson, Toronto Rehabilitation Institute, Lyndhurst Centre, Canada |
| Notes |
NCT00663663.
| Trial name or title | Efficacy of Telephone‐Delivered Cognitive Behavioral Therapy for Chronic Pain |
| Methods | Randomised controlled trial |
| Participants | People with AMP, MS or SCI with pain of at least 6 months' duration, average pain intensity in the past month greater than 3 on a 0‐10 numerical rating scale |
| Interventions | Behavioural intervention: telephone‐delivered intervention |
| Outcomes | Primary: average pain intensity in the past week |
| Starting date | September 2009 |
| Contact information | Dawn M. Ehde, University of Washington, USA |
| Notes |
NCT00678548.
| Trial name or title | Effect of Positive Guided Imagery on Patients With in or Below‐Level Chronic Pain Related to Spinal Cord Injury |
| Methods | Randomised controlled trial |
| Participants | People with traumatic and non‐traumatic SCI with chronic pain in or below the level of injury |
| Interventions | Intervention 1: behavioural: pleasant guided imagery Intervention 2: behavioural: pain diary |
| Outcomes | Primary: pain intensity, as measured by NRS and BPIQ Secondary: BDI, BAI and SF‐36 |
| Starting date | October 2008 |
| Contact information | Gunnar Leivseth, Norwegian University of Science and Technology, Trondheim, Norway |
| Notes |
NCT01087918.
| Trial name or title | Effects of Automated Treadmill Training and Lower Extremity Strength Training on Walking‐related and Other Outcomes in Subjects With Chronic Incomplete Spinal Cord Injury |
| Methods | Randomised controlled trial |
| Participants | People with chronic incomplete spinal cord injury |
| Interventions | Device: Lokomat (driven gait orthosis) training sessions of 45 minutes, 4 times/week, during 4 weeks Other: strength training sesions of 45 minutes, 4 times/week, during 4 weeks |
| Outcomes | Secondary outcome: VAS pain |
| Starting date | July 2009 |
| Contact information | H. van Hedel, Rehabilitation Center Affoltern am Albis, University Children's Hospital Zurich, Switzerland |
| Notes |
NCT01112774.
| Trial name or title | Investigation of the Mechanisms of Transcranial Direct Current Stimulation of Motor Cortex for the Treatment of Chronic Pain in Spinal Cord Injury |
| Methods | Randomised controlled trial |
| Participants | People with traumatic spinal cord injury (complete or incomplete) and stable chronic pain for at least the 3 preceding months with score higher or equal to 4 cm (0 cm = 'no pain' and 10 cm = 'worst possible pain') on the visual analogue scale (VAS) |
| Interventions | 10 sessions of active or sham tDCS |
| Outcomes | Changes in pain between baseline, 2 weeks of treatment and 2 weeks of follow‐up |
| Starting date | April 2010 |
| Contact information | Felipe Fregni, Spaulding Rehabilitation Hospital, Boston, USA |
| Notes |
NCT01236976.
| Trial name or title | SCIPA Full‐On: Intensive Exercise Program After Spinal Cord Injury |
| Methods | Randomised controlled trial |
| Participants | People with complete or incomplete spinal cord injury between C6 and T12 |
| Interventions | Combination of body weight–supported treadmill training (BWSTT), cycling assisted with functional electrical stimulation (FES) and exercise of trunk and upper and lower extremities |
| Outcomes | Not specified. Quote: "To determine the relative effectiveness of a comprehensive exercise program compared to a generic upper body strength and fitness training program on neurological improvement" |
| Starting date | December 2010 |
| Contact information | Mary Galea, University of Melbourne, Australia |
| Notes |
Differences between protocol and review
We had planned to conduct subgroup analyses for trials in patients with different types of SCI or pain and with different types of control interventions. However, the small number of included studies precluded these analyses. For the same reason, we did not perform planned sensitivity analyses for studies with mixed populations including a subgroup with SCI and for trials of different methodological quality (e.g. allocation concealment, blinding, intention‐to‐treat analysis), and we did not perform imputation of missing values.
We had intended to analyse continuous outcomes as mean change from baseline; however, few studies reported mean change from baseline. Comparisons were consequently based on differences between mean values from last measurement.
In the section Unit of analysis issues, we now explain that none of the three included cross‐over trials was included in any meta‐analysis because of large differences in study methods.
In the section Data synthesis, we now explain that meta‐analysis was conducted using the random‐effects model and that the fixed‐effect model was used only to combine data from pairs of similar study groups from two factorial trials in two meta‐analyses. Further, we specify that, for the purpose of meta‐analysis, ordinal outcome data were treated as continuous variables.
Contributions of authors
IB wrote the review protocol and drafted the final manuscript, searched electronic databases and conference proceedings, screened identified references, selected trials for inclusion, extracted trial data and liaised with trialists, assessed methodological quality, performed statistical analyses and interpreted results. IEH extracted trial data, assessed methodological quality and provided input on draft versions of the protocol and review. MWGB extracted trial data, assessed methodological quality, provided statistical advice and provided input on draft versions of the protocol and review. RdB and DJ provided input on draft versions of the protocol and review. EvE wrote the review protocol and drafted the final manuscript, selected trials for inclusion, extracted trial data, assessed methodological quality and guided statistical analyses and interpretation of results. All review authors approved the final version of this review.
Sources of support
Internal sources
Swiss Paraplegic Research, Nottwil, Switzerland.
External sources
No sources of support supplied
Declarations of interest
IB: None known.
IEH: None known.
MWGB: None known.
RdB: None known.
DJ: None known.
EvE: None known.
New
References
References to studies included in this review
Capel 2003 {published data only}
- Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord 2003;41(2):109‐17. [DOI] [PubMed] [Google Scholar]
Curtis 1999 {published and unpublished data}
- Curtis KA, Tyner TM, Zachary L, Lentell G, Brink D, Didyk T, et al. Effect of a standard exercise protocol on shoulder pain in long‐term wheelchair users. Spinal Cord 1999;37(6):421‐9. [DOI] [PubMed] [Google Scholar]
Defrin 2007 {published data only}
- Defrin R, Grunhaus L, Zamir D, Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1574‐80. [DOI] [PubMed] [Google Scholar]
Doctor 1996 {published and unpublished data}
- Doctor JN. An evaluation of transcutaneous electrical nerve stimulation (TENS) for the treatment of pain related to a spinal cord injury. University of California, San Diego and San Diego State University 1996:310.
Dyson‐Hudson 2001 {published data only}
- Dyson‐Hudson TA, Shiflett SC, Kirshblum SC, Bowen JE, Druin EL. Acupuncture and Trager psychophysical integration in the treatment of wheelchair user's shoulder pain in individuals with spinal cord injury. Archives of Physical Medicine and Rehabilitation 2001;82(8):1038‐46. [PUBMED: 11494182] [DOI] [PubMed] [Google Scholar]
Dyson‐Hudson 2007 {published data only}
- Dyson‐Hudson TA, Kadar P, LaFountaine M, Emmons R, Kirshblum SC, Tulsky D, et al. Acupuncture for chronic shoulder pain in persons with spinal cord injury: a small‐scale clinical trial. Archives of Physical Medicine and Rehabilitation 2007;88(10):1276‐83. [DOI] [PubMed] [Google Scholar]
Fregni 2006 {published data only}
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham‐controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006;122(1‐2):197‐209. [DOI] [PubMed] [Google Scholar]
Heutink 2012 {published and unpublished data}
- Heutink M, Post MW, Bongers‐Janssen HM, Dijkstra CA, Snoek GJ, Spijkerman DC, et al. The CONECSI trial: results of a randomized controlled trial of a multidisciplinary cognitive behavioral program for coping with chronic neuropathic pain after spinal cord injury. Pain 2012; Vol. 153, issue 1:120‐8. [DOI] [PubMed]
Hicks 2003 {published data only}
- Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long‐term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well‐being. Spinal Cord 2003;41(1):34‐43. [DOI] [PubMed] [Google Scholar]
- Latimer AE, Ginis KA, Hicks AL, McCartney N. An examination of the mechanisms of exercise‐induced change in psychological well‐being among people with spinal cord injury. Journal of Rehabilitation Research and Development 2004;41(5):643‐52. [DOI] [PubMed] [Google Scholar]
- Martin Ginis KA, Latimer AE, McKechnie K, Ditor DS, McCartney N, Hicks AL. Using exercise to enhance subjective well‐being among people with spinal cord injury: the mediating influences of stress and pain. Rehabilitation Psychology 2003;48(3):157‐64. [Google Scholar]
Jensen 2009 {published and unpublished data}
- Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, et al. Effects of self‐hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal‐cord injury. The International Journal of Clinical and Experimental Hypnosis 2009;57(3):239‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2009 {published data only}
- Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Archives of Physical Medicine and Rehabilitation 2009;90(10):1766‐71. [DOI] [PubMed] [Google Scholar]
Lefaucheur 2007 {published and unpublished data}
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(9):1044‐9. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67(9):1568‐74. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. Journal of Neurology, Neurosurgery, and Psychiatry 2004;75(4):612‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulroy 2011 {published data only}
- Mulroy SJ, Thompson L, Kemp B, Hatchett PP, Newsam CJ, Lupold DG, et al. Strengthening and optimal movements for painful shoulders (STOMPS) in chronic spinal cord injury: a randomized controlled trial. Physical Therapy 2011;91(3):305‐24. [DOI] [PubMed] [Google Scholar]
Soler 2010 {published data only}
- Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 2010;133(9):2565‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2006 {published data only}
- Tan G, Rintala DH, Thornby JI, Yang J, Wade W, Vasilev C. Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. Journal of Rehabilitation Research and Development 2006;43(4):461‐74. [DOI] [PubMed] [Google Scholar]
Tan 2011 {published and unpublished data}
- Tan G, Rintala DH, Jensen MP, Richards JS, Holmes S, Parachuri R, et al. Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi‐site randomized controlled trial with a secondary 6‐month open‐label phase. The Journal of Spinal Cord Medicine 2011;34(2):285‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Coşkun Çelik 2005 {published and unpublished data}
- Celik EC, Erhan B, Gunduz B, Lakse E. The effect of low‐frequency TENS in the treatment of neuropathic pain in patients with spinal cord injury. [Medulla spinalis yaralanmali hastalardakinöropatik ağriya akupunktur benzeritens’in etkisi]. Spinal Cord 2013;51(4):334‐7. [DOI] [PubMed] [Google Scholar]
Crowe 2000 {published data only}
- Crowe J, MacKay‐Lyons M, Morris H. A multi‐centre, randomized controlled trial of the effectiveness of positioning on quadriplegic shoulder pain. Physiotherapy Canada 2000;52(4):266‐73. [Google Scholar]
Ditor 2003 {published data only}
- Ditor DS, Latimer AE, Ginis KA, Arbour KP, McCartney N, Hicks AL. Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord 2003;41(8):446‐50. [DOI] [PubMed] [Google Scholar]
Dyson‐Hudson 2007b {published data only}
- Dyson‐Hudson TA, Sisto SA, Bond Q, Emmons R, Kirshblum SC. Arm crank ergometry and shoulder pain in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1727‐9. [DOI] [PubMed] [Google Scholar]
Hughes 2006 {published data only}
- Hughes RB, Robinson‐Whelen S, Taylor HB, Hall JW. Stress self‐management: an intervention for women with physical disabilities. Women's Health Issues 2006;16(6):389‐99. [DOI] [PubMed] [Google Scholar]
Lefaucheur 2010 {published data only}
- Lefaucheur JP, Jarry G, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS reduces acute pain provoked by laser stimulation in patients with chronic neuropathic pain. Clinical Neurophysiology 2010;121(6):895‐901. [DOI] [PubMed] [Google Scholar]
Norrbrink 2006 {published data only}
- Norrbrink Budh C, Kowalski J, Lundeberg T. A comprehensive pain management programme comprising educational, cognitive and behavioural interventions for neuropathic pain following spinal cord injury. Journal of Rehabilitation Medicine 2006;38(3):172‐80. [DOI] [PubMed] [Google Scholar]
Norrbrink 2009 {published data only}
- Norrbrink C. Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. Journal of Rehabilitation Research and Development 2009;46(1):85‐93. [PubMed] [Google Scholar]
Perry 2010 {published data only}
- Perry KN, Nicholas MK, Middleton JW. Comparison of a pain management program with usual care in a pain management center for people with spinal cord injury‐related chronic pain. The Clinical Journal of Pain 2010;26(3):206‐16. [DOI] [PubMed] [Google Scholar]
Saitoh 2007 {published data only}
- Saitoh Y, Yoshimine T. Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochirurgica Suppl 2007;97(Pt 2):51‐6. [DOI] [PubMed] [Google Scholar]
Wardell 2006 {published data only}
- Wardell DW, Rintala DH, Duan Z, Tan G. A pilot study of healing touch and progressive relaxation for chronic neuropathic pain in persons with spinal cord injury. Journal of Holistic Nursing 2006;24(4):231‐40; discussion 241‐4. [DOI] [PubMed] [Google Scholar]
Xing 2010 {published data only}
- Xing ST, Wang D, Wen XH, Wu ZQ, Sun Q, Zhang DW, et al. Clinical research of electroacupuncture combined with acupoint‐injection of botulinum toxin A in treating the muscle spasticity by spinal cord injury. Zhongguo Gu Shang 2010;23(5):350‐3. [PubMed] [Google Scholar]
References to studies awaiting assessment
Alcobendas‐Maestro 2012 {published data only}
- Alcobendas‐Maestro M, Esclarin‐Ruz A, Casado‐Lopez RM, Munoz‐Gonzalez A, Perez‐Mateos G, Gonzalez‐Valdizan E, et al. Lokomat robotic‐assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabilitation and Neural Repair 2012;26(9):1058‐63. [DOI] [PubMed] [Google Scholar]
Arienti 2011 {published data only}
- Arienti C, Dacco S, Piccolo I, Redaelli T. Osteopathic manipulative treatment is effective on pain control associated to spinal cord injury. Spinal Cord 2011;49(4):515‐9. [DOI] [PubMed] [Google Scholar]
Chase 2013 {published data only}
- Chase T, Jha A, Brooks CA, Allshouse A. A pilot feasibility study of massage to reduce pain in people with spinal cord injury during acute rehabilitation. Spinal Cord 2013;51(11):847‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirayama 2006 {published data only}
- Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation‐guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 2006;122(1‐2):22‐7. [DOI] [PubMed] [Google Scholar]
Jette 2013 {published data only}
- Jette F, Cote I, Meziane HB, Mercier C. Effect of single‐session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabilitation and Neural Repair 2013;27(7):636‐43. [DOI] [PubMed] [Google Scholar]
Lefaucheur 2004 {published and unpublished data}
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. Journal of Neurology, Neurosurgery, and Psychiatry 2004;75(4):612‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefaucheur 2006 {published data only}
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67(9):1568‐74. [DOI] [PubMed] [Google Scholar]
Lefaucheur 2008 {published data only}
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(9):1044‐9. [DOI] [PubMed] [Google Scholar]
Litchke 2012 {published data only}
- Litchke L, Lloyd L, Schmidt E, Russian C, Reardon R. Effects of concurrent respiratory resistance training on health‐related quality of life in wheelchair rugby athletes: a pilot study. Topics in Spinal Cord Injury Rehabilitation 2012;18(3):264‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livshits 2002 {published data only}
- Livshits A, Rappaport ZH, Livshits V, Gepstein R. Surgical treatment of painful spasticity after spinal cord injury. Spinal Cord 2002;40(4):161‐6. [DOI] [PubMed] [Google Scholar]
Middaugh 2013 {published data only}
- Middaugh S, Thomas KJ, Smith AR, McFall TL, Klingmueller J. EMG biofeedback and exercise for treatment of cervical and shoulder pain in individuals with a spinal cord injury: a pilot study. Topics in Spinal Cord Injury Rehabilitation 2013;19(4):311‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2014 {published data only}
- Mu ller R, Gertz K, Molton I, Terrill A, Bombardier C, Ehde DM, et al. Pilot testing a positive psychology intervention in individuals with chronic, disability‐related pain. Archives of physical medicine and rehabilitation Conference. American Congress of Rehabilitation Medicine Annual Conference, ACRM. 2014 Oct 07‐11;Toronto, ON Canada. 2014:e9‐e10.
Saitoh 2006 {published data only}
- Saitoh Y, Hirayama A, Kishima H, Oshino S, Hirata M, Kato A, et al. Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochirurgica Suppl 2006;99:57‐9. [DOI] [PubMed] [Google Scholar]
Saitoh 2007a {published data only}
- Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high‐frequency repetitive transcranial magnetic stimulation of the primary motor cortex. Journal of Neurosurgery 2007;107(3):555‐9. [DOI] [PubMed] [Google Scholar]
Wrigley 2013 {published data only}
- Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain 2013;154(10):2178‐84. [DOI] [PubMed] [Google Scholar]
Yilmaz 2014 {published data only}
- Yilmaz B, Kesikburun S, Yasar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. Journal of Spinal Cord Medicine 2014;37:397‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2014 {published data only}
- Yoon EJ, Kim YK, Kim H‐R, Kim SE, Lee Y, Shin HI. Transcranial Direct Current Stimulation to Lessen Neuropathic Pain After Spinal Cord Injury: A Mechanistic PET Study. Neurorehabilitation & Neural Repair 2014;28:250‐259. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00523016 {published data only}
- Acupuncture Treatment to Reduce Burning Pain in Spinal Cord Injury (APSCI). Ongoing study September 2007.
NCT00663663 {published data only}
- Efficacy of Telephone‐Delivered Cognitive Behavioral Therapy for Chronic Pain. Ongoing study September 2009.
NCT00678548 {published data only}
- Effect of Positive Guided Imagery on Patients With in or Below‐Level Chronic Pain Related to Spinal Cord Injury. Ongoing study October 2008.
NCT01087918 {published data only}
- Effects of Automated Treadmill Training and Lower Extremity Strength Training on Walking‐related and Other Outcomes in Subjects With Chronic Incomplete Spinal Cord Injury. Ongoing study July 2009.
NCT01112774 {published data only}
- Investigation of the Mechanisms of Transcranial Direct Current Stimulation of Motor Cortex for the Treatment of Chronic Pain in Spinal Cord Injury. Ongoing study April 2010.
NCT01236976 {published data only}
- SCIPA Full‐On: Intensive Exercise Program After Spinal Cord Injury. Ongoing study December 2010.
Additional references
Ballinger 2000
- Ballinger DA, Rintala DH, Hart KA. The relation of shoulder pain and range‐of‐motion problems to functional limitations, disability, and perceived health of men with spinal cord injury: a multifaceted longitudinal study. Archives of Physical Medicine and Rehabilitation 2000;81(12):1575‐81. [DOI] [PubMed] [Google Scholar]
Bryce 2007
- Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence‐based review for clinical practice and research. Report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures Meeting. The Journal of Spinal Cord Medicine 2007; Vol. 30, issue 5:421‐40. [DOI] [PMC free article] [PubMed]
Cardenas 2006
- Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: a survey study. The Journal of Spinal Cord Medicine 2006;29(2):109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dijkers 2009
- Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. Journal of Rehabilitation Research and Development 2009;46(1):13‐29. [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Heutink 2011
- Heutink M, Post MW, Wollaars MM, Asbeck FW. Chronic spinal cord injury pain: pharmacological and non‐pharmacological treatments and treatment effectiveness. Disability and Rehabilitation 2011;33(5):433‐40. [DOI] [PubMed] [Google Scholar]
Hicken 2002
- Hicken BL, Putzke JD, Richards JS. Classification of spinal cord injury pain: literature review and future directions. In: Yezierski RP, Burchiel KJ editor(s). Spinal Cord Injury Pain: Assessment, Mechanisms, Management. Seattle: International Association for the Study of Pain/IASP Press, 2002:25‐38. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1 [updated March 2011]. www.cochrane‐handbook.org 2011.
Hróbjartsson 2010
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003974.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
IMMPACT Recommendations 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9‐19. [DOI] [PubMed] [Google Scholar]
Jensen 2005
- Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord 2005;43(12):704‐12. [DOI] [PubMed] [Google Scholar]
Jensen 2007
- Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health‐related quality of life: review and implications. Neurology 2007;68(15):1178‐82. [DOI] [PubMed] [Google Scholar]
Merbitz 1989
- Merbitz C, Morris J, Grip JC. Ordinal scales and foundations of misinference. Archives of Physical Medicine and Rehabilitation 1989;70(4):308‐12. [PubMed] [Google Scholar]
Merskey 1994
- Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd Edition. Seattle: International Association for the Study of Pain/IASP Press, June 1994. [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Nayak 2001
- Nayak S, Matheis RJ, Agostinelli S, Shifleft SC. The use of complementary and alternative therapies for chronic pain following spinal cord injury: a pilot survey. The Journal of Spinal Cord Medicine 2001;24(1):54‐62. [DOI] [PubMed] [Google Scholar]
Newby 2009
- Newby VA, Conner GR, Grant CP, Bunderson CV. The Rasch model and additive conjoint measurement. Journal of Applied Measurement 2009;10(4):348‐54. [PubMed] [Google Scholar]
Norrbrink 2004
- Norrbrink Budh C, Lundeberg T. Non‐pharmacological pain‐relieving therapies in individuals with spinal cord injury: a patient perspective. Complementary Therapies in Medicine 2004;12(4):189‐97. [DOI] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connell 2010
- O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non‐invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008208.pub2] [DOI] [PubMed] [Google Scholar]
Putzke 2002
- Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain 2002;100(3):231‐42. [DOI] [PubMed] [Google Scholar]
Salisbury 2003
- Salisbury SK, Choy NL, Nitz J. Shoulder pain, range of motion, and functional motor skills after acute tetraplegia. Archives of Physical Medicine and Rehabilitation 2003;84(10):1480‐5. [DOI] [PubMed] [Google Scholar]
SCIRE 2010
- Teasell RW, Mehta S, Aubut J, Foulon BL, Wolfe DL, Hsieh JTC, et al. Pain following spinal cord injury. Spinal Cord Injury Rehabilitation Evidence SCIRE 2010; Vol. Version 3.0.
Siddall 2000
- Siddall PJ, Yezierski RP, Loeser JD. Pain following spinal cord injury: clinical features, prevalence, and taxonomy. International Association for the Study of Pain Newsletter 2000 Vol. 3.
Siddall 2001
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord 2001;39(2):63‐73. [PUBMED: 11402361] [DOI] [PubMed] [Google Scholar]
Siddall 2006
- Siddall PJ, Finnerup NB. Chapter 46. Pain following spinal cord injury. In: Vinken PJ, Bruyn GW editor(s). Handbook of Clinical Neurology. Vol. 81, Baltimore: University Park Press, 2006:689‐703. [DOI] [PubMed] [Google Scholar]
Siddall 2006a
- Siddall PJ, Middleton JW. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord 2006;44(2):67‐77. [DOI] [PubMed] [Google Scholar]
Sie 1992
- Sie IH, Waters RL, Adkins RH, Gellman H. Upper extremity pain in the postrehabilitation spinal cord injured patient. Archives of Physical Medicine and Rehabilitation 1992;73:44. [PubMed] [Google Scholar]
Stucki 1996
- Stucki G, Daltroy L, Katz JN, Johannesson M, Liang MH. Interpretation of change scores in ordinal clinical scales and health status measures: the whole may not equal the sum of the parts. Journal of Clinical Epidemiology 1996;49(7):711‐7. [DOI] [PubMed] [Google Scholar]
van den Berg 2010
- Berg ME, Castellote JM, Mahillo‐Fernandez I, Pedro‐Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 2010;34(3):184‐92; discussion 192. [DOI] [PubMed] [Google Scholar]
Widerstrom‐Noga 2003
- Widerström‐Noga EG, Turk DC. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord 2003;41(11):600‐9. [DOI] [PubMed] [Google Scholar]
Widerstrom‐Noga 2008
- Widerström‐Noga E, Biering‐Sørensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set. Spinal Cord 2008;46:818‐23. [DOI] [PubMed] [Google Scholar]
Widerström‐Noga 2009
- Widerström‐Noga EG, Finnerup NB, Siddall PJ. Biopsychosocial perspective on a mechanisms‐based approach to assessment and treatment of pain following spinal cord injury. Journal of Rehabilitation Research and Development 2009;46:1. [PubMed] [Google Scholar]


