Abstract
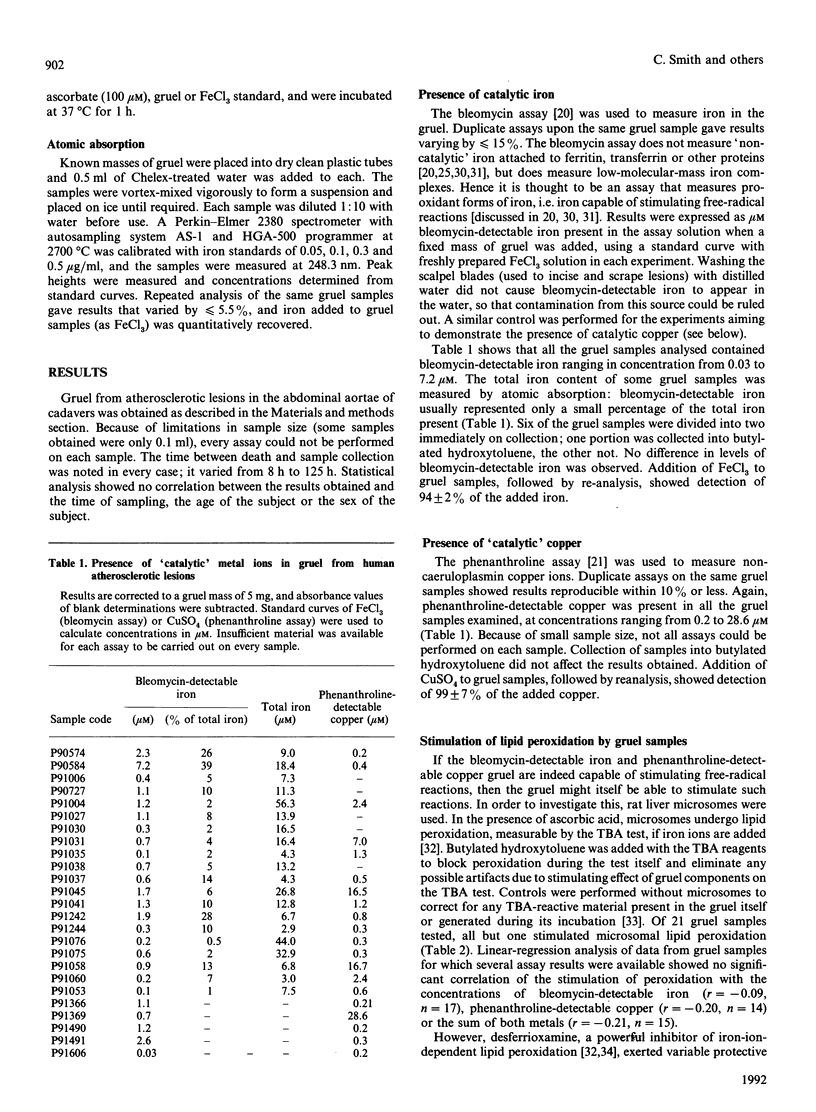
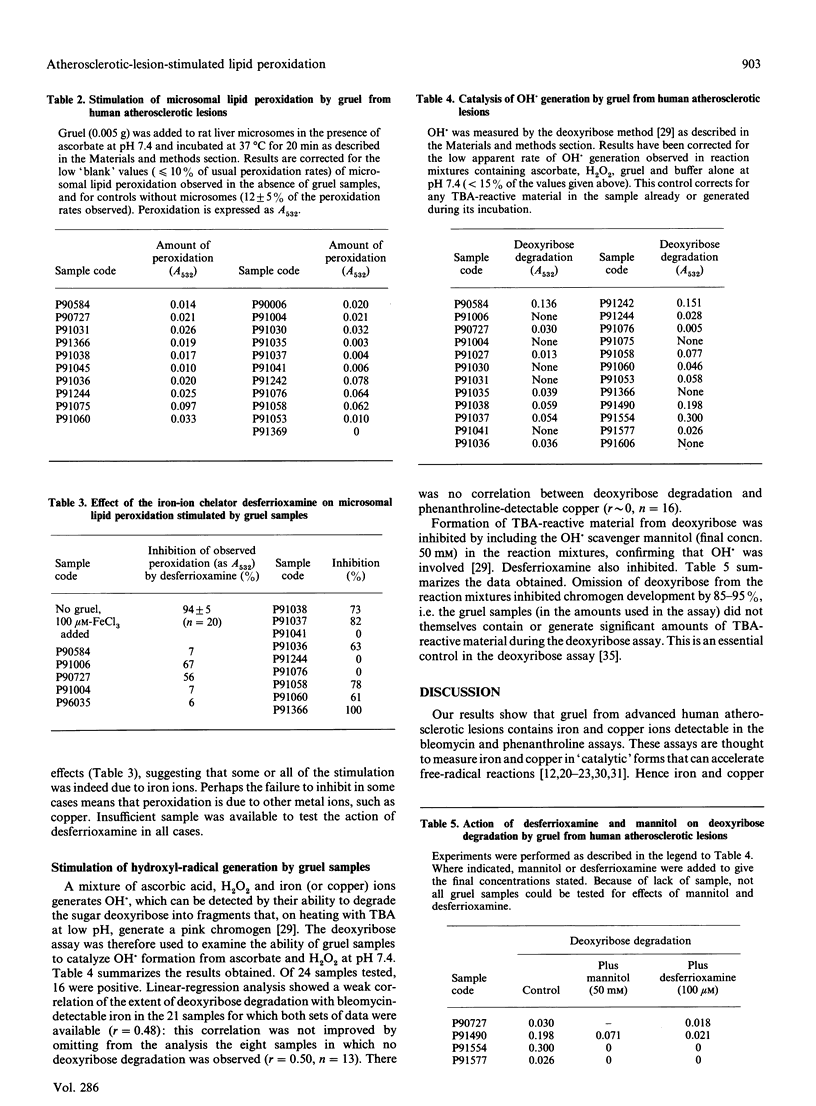
Lipid peroxidation within human arterial lesions is thought to play an important role in the development of atherosclerosis. Peroxidation can be accelerated by the presence of 'catalytic' iron or copper ions. Gruel samples from advanced atherosclerotic lesions in the abdominal aortae of human cadavers were tested for pro-oxidant properties. All samples contained bleomycin-detectable iron and phenanthroline-detectable copper. Almost all gruel samples stimulated peroxidation of rat liver microsomes, and this was usually inhibited by the iron-ion chelator desferrioxamine. Some samples stimulated formation of hydroxyl radicals from H2O2 in the presence of ascorbate, a reaction again inhibited by desferrioxamine. We conclude that the interior of human advanced atherosclerotic lesions is a highly pro-oxidant environment, and that the use of copper or iron ions to promote peroxidation of low-density lipoproteins in vitro may be a valid model for events in the arterial wall.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Barnhart R. L., Busch S. J., Jackson R. L. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: probucol degradation precedes lipoprotein oxidation. J Lipid Res. 1989 Nov;30(11):1703–1710. [PubMed] [Google Scholar]
- Bellamy M. F., Nealis A. S., Aitken J. W., Bruckdorfer K. R., Perkins S. J. Structural changes in oxidised low-density lipoproteins and of the effect of the anti-atherosclerotic drug probucol observed by synchrotron X-ray and neutron solution scattering. Eur J Biochem. 1989 Aug 1;183(2):321–329. doi: 10.1111/j.1432-1033.1989.tb14932.x. [DOI] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini R., Aruoma O. I., Halliwell B. The action of hydrogen peroxide on the formation of thiobarbituric acid-reactive material from microsomes, liposomes or from DNA damaged by bleomycin or phenanthroline. Artefacts in the thiobarbituric acid test. Free Radic Res Commun. 1990;10(4-5):245–258. doi: 10.3109/10715769009149893. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Dieber-Rotheneder M., Waeg G., Striegl G., Jürgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990 Mar-Apr;3(2):77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Bomford A., Halliwell B. Non-caeruloplasmin copper and ferroxidase activity in mammalian serum. Ferroxidase activity and phenanthroline-detectable copper in human serum in Wilson's disease. Free Radic Res Commun. 1989;7(1):55–62. doi: 10.3109/10715768909088162. [DOI] [PubMed] [Google Scholar]
- Gey K. F., Puska P., Jordan P., Moser U. K. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am J Clin Nutr. 1991 Jan;53(1 Suppl):326S–334S. doi: 10.1093/ajcn/53.1.326S. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J. 1984 Mar 15;218(3):983–985. doi: 10.1042/bj2180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Hou Y. Y. Iron complexes and their reactivity in the bleomycin assay for radical-promoting loosely-bound iron. Free Radic Res Commun. 1986;2(3):143–151. doi: 10.3109/10715768609088066. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Winyard P. G., Blake D. R., Lunec J., Brailsford S., Halliwell B. The behaviour of caeruloplasmin in stored human extracellular fluids in relation to ferroxidase II activity, lipid peroxidation and phenanthroline-detectable copper. Biochem J. 1985 Sep 1;230(2):517–523. doi: 10.1042/bj2300517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I., Mufti G., Bomford A. Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett. 1988 Dec 5;241(1-2):202–204. doi: 10.1016/0014-5793(88)81061-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Aruoma O. I. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987 Aug 15;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984 Jun 23;1(8391):1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990 Jul;280(1):1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9(1):1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide, iron, vascular endothelium and reperfusion injury. Free Radic Res Commun. 1989;5(6):315–318. doi: 10.3109/10715768909073413. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J. Extracts of human atherosclerotic lesions can modify low density lipoproteins leading to enhanced uptake by macrophages. Atherosclerosis. 1988 Mar;70(1-2):29–41. doi: 10.1016/0021-9150(88)90097-4. [DOI] [PubMed] [Google Scholar]
- Jessup W., Darley-Usmar V., O'Leary V., Bedwell S. 5-Lipoxygenase is not essential in macrophage-mediated oxidation of low-density lipoprotein. Biochem J. 1991 Aug 15;278(Pt 1):163–169. doi: 10.1042/bj2780163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya M., Naito M., Funaki C., Hayashi T., Asai K., Kuzuya F. Lipid peroxide and transition metals are required for the toxicity of oxidized low density lipoprotein to cultured endothelial cells. Biochim Biophys Acta. 1991 Feb 22;1096(2):155–161. doi: 10.1016/0925-4439(91)90054-d. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Hagaman K. A. Effect of probucol on the physical properties of low-density lipoproteins oxidized by copper. Biochemistry. 1989 Jan 10;28(1):321–327. doi: 10.1021/bi00427a043. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals in biological systems. Does myoglobin stimulate hydroxyl radical formation from hydrogen peroxide? Free Radic Res Commun. 1988;4(6):415–422. doi: 10.3109/10715768809066910. [DOI] [PubMed] [Google Scholar]
- Quinlan G. J., Halliwell B., Moorhouse C. P., Gutteridge J. M. Action of lead(II) and aluminium (III) ions on iron-stimulated lipid peroxidation in liposomes, erythrocytes and rat liver microsomal fractions. Biochim Biophys Acta. 1988 Sep 23;962(2):196–200. doi: 10.1016/0005-2760(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Regnström J., Walldius G., Carlson L. A., Nilsson J. Effect of probucol treatment on the susceptibility of low density lipoprotein isolated from hypercholesterolemic patients to become oxidatively modified in vitro. Atherosclerosis. 1990 May;82(1-2):43–51. doi: 10.1016/0021-9150(90)90142-6. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Salonen R., Seppänen K., Kantola M., Suntioinen S., Korpela H. Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. BMJ. 1991 Mar 30;302(6779):756–760. doi: 10.1136/bmj.302.6779.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Wilkins G. M., Leake D. S. Free radicals and low-density lipoprotein oxidation by macrophages. Biochem Soc Trans. 1990 Dec;18(6):1170–1171. doi: 10.1042/bst0181170. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]


