Abstract
Background
Transarterial chemo(embolization) is preferred for treating unresectable hepatocellular carcinoma (uHCC); however, because of emerging immune-targeted therapies, its efficacy is at stake. This systematic review pioneers to evaluate the clinical efficacy and safety of transarterial chemo(embolization) combined with immune-targeted therapy for uHCC patients.
Methods
PubMed, Embase, and Cochrane Library were searched for studies comparing immune-targeted therapy with or without transarterial chemo(embolization) until 31 May 2024. The complete response (CR) rate, objective response rate (ORR), and disease control rate (DCR) were considered to be the primary outcomes calculated for the clinical outcomes of transarterial chemo(embolization) combined with immune-targeted therapy, along with progression-free survival (PFS) and overall survival (OS). The incidence of treatment-related severe adverse events was set as the major measure for the safety outcome.
Results
Sixteen studies, encompassing 1,789 patients receiving transarterial chemo(embolization) plus immune-targeted therapy and 1,215 patients receiving immune-targeted therapy alone, were considered eligible. The combination of transarterial chemo(embolization) and immune-targeted therapy demonstrated enhanced outcomes in CR (OR = 2.12, 95% CI = 1.35–3.31), ORR (OR = 2.78, 95% CI = 2.15–3.61), DCR (OR = 2.46, 95% CI = 1.72–3.52), PFS (HR = 0.59, 95% CI = 0.50–0.70), and OS (HR = 0.51, 95% CI = 0.44–0.59), albeit accompanied by a surge in ALT (OR = 2.17, 95% CI = 1.28–3.68) and AST (OR = 2.28, 95% CI = 1.42–3.65). The advantages of additional transarterial chemo(embolization) to immune-targeted therapy were also verified in subgroups of first-line treatment, intervention techniques, with or without extrahepatic metastasis, Child–Pugh grade A or B, and with or without tumor thrombus.
Conclusion
The combination of transarterial chemo(embolization) and immune-targeted therapy seems to bolster local control and long-term efficacy in uHCC, albeit at the expense of hepatic complications.
Systematic review registration
http://www.crd.york.ac.uk/PROSPERO/, identifier 474669.
Keywords: transarterial chemo(embolization), unresectable hepatocellular carcinoma, targeted agents, immunotherapy, systematic review
Introduction
In 2020, primary liver cancer was recognized as the sixth most prevalent malignant tumor globally, among which hepatocellular carcinoma (HCC) accounts for more than 90% of the cases (1). The majority of HCC cases have lost the chance of radical hepatectomy mainly because HCC generally progresses asymptomatically (2). It is diagnosed at an intermediate to advanced stage, also termed unresectable HCC (uHCC). The inception of the IMbrave150 trial heralded a new epoch in the utilization of targeted agents and immunotherapy for uHCC management, boasting an objective response rate (ORR) of 28% (3). This regimen, along with apatinib and camrelizumab (4) and lenvatinib and pembrolizumab (5), signifies a promising stride, albeit with an unsatisfactory median overall survival (OS).
Transcatheter arterial chemoembolization (TACE), as one of the classical transarterial therapies, is considered the standard treatment for uHCC (6). Conversely, hepatic artery infusion chemotherapy (HAIC), an emerging transarterial therapeutic modality, demonstrates non-inferior local control compared to TACE but superior long-term outcomes (7, 8). Despite these advancements, the advent of targeted agents and immunotherapy warrants re-evaluating the role of transarterial chemo(embolization) in HCC management. The IMbrave150 trial demonstrated the potential of integrating transarterial chemo(embolization) with targeted agents and immunotherapy (3, 9), hinting at a synergistic interaction. In theory, transarterial chemo(embolization) could enhance tumor antigen release and immunogenicity; bolster the infiltration of CD4+ T, CD8+ T, and NK cells; and elicit proinflammatory responses (10, 11), thereby fostering a conducive microenvironment for immune checkpoint inhibitors (ICIs). Concurrently, it can increase the expression of vascular endothelial growth factor (12, 13), hinting at a viable partnership with angiogenic blockers.
Preliminary studies have witnessed the promise of immune-targeted therapy with transarterial chemo(embolization) for uHCC in the recent three years (14–16), which was reiterated by a systematic review (17). However, most of the studies were retrospective, single-center, non-comparative analyses. In the recent two years, researchers have reported encouraging results upon comparing immune-targeted therapy with transarterial chemo(embolization) for uHCC (18–20); nonetheless, adding transarterial therapy to the targeted agents and immunotherapy appears debatable (21). Consequently, we embarked on this meta-analysis to juxtapose the efficacy and toxicity profiles of immune-targeted therapy with or without transarterial therapies for uHCC.
Materials and methods
Literature search
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline, which was also registered at http://www.crd.york.ac.uk/PROSPERO/ (Review registry 474669). An ethics statement was not required because this study was based exclusively on published research. A comprehensive search was executed in PubMed, Medline, Embase, the Cochrane Library, and Web of Science to identify publications concerning immune-targeted therapy with or without transarterial chemo(embolization) for uHCC. Supplementary Table S1 summarizes the search strategy. A supplementary search in gray literature was conducted by reviewing conference proceedings and reference lists of key articles. The publications were not confined to any specific language, provided that they had an abstract in English to ensure data reproducibility. The literature search was independently conducted by two researchers from 1 February 2023 to 31 May 2024, based on predefined search strategies.
Literature screening and data acquisition
First, data collected through electronic or manual searches were imported to EndNote version X9 software (Clarivate) to detect duplicate records. Then, two reviewers (Huipeng Fang and Qiao Ke) conducted literature screening based on the inclusion and exclusion criteria ( Supplementary Table S2 ). In case of any discrepancy between reviewers, a third-party reviewer was consulted to reach a final decision.
Information of the eligible studies was extracted directly by two independent researchers (Huipeng Fang and Qiao Ke) using a predefined format, encompassing data on publication, study design, baseline characteristics in each study, and endpoints. Data were cross-validated between researchers, and discrepancies were resolved through a multidisciplinary team (MDT) discussion, including at least one senior doctor.
Endpoints in this meta-analysis included the complete response (CR) rate, objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs). Tumor response was evaluated based on the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) or Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (22). ORR was calculated as the proportion of patients with the best response of CR or partial response (PR). DCR was calculated as the proportion of patients with the best response of ORR or stable disease (SD). PFS was defined as the duration from the initiation of treatment to the onset of disease progression or mortality from any cause. OS was defined as the time from treatment initiation to cancer-related death. AEs were evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 or 5.0, with a grade ≥3 indicating severe AEs.
Quality assessment
Considering the retrospective nature of the included studies, the quality was evaluated using a modified Newcastle-Ottawa Scale (NOS) (23). The risk of bias was graphically represented for the following elements: i) clarity in the objective definition; ii) provision of a clear triple combination of TACE/HAIC, TKIs, and ICIs; iii) provision of response assessment criteria (i.e., RECIST or mRECIST); and iv) clear definition of outcomes including CR, ORR, DCR, and AEs.
Statistical analysis
Comparison analysis between two groups was conducted using RevMan Version 5.3. The odds ratio (OR) was calculated to compare the effect size of CR, ORR, DCR, and AEs with 95% confidence interval (CI), as well as the hazard ratio (HR) for OS and PFS. The χ² test and I 2 statistics were used to evaluate the heterogeneity among the included studies. P >0.10 and I 2 <50% suggested no apparent heterogeneity, and the fixed-effects model was used to estimate the effect size; otherwise, the random-effects model was used (24). Sensitivity analysis was carried out by removing each of the included studies sequentially to determine the reliability of the results. Additionally, subgroup analyses were also conducted to decrease the heterogeneity among the included studies. Publication bias was determined using the funnel plot with Egger’s and Begg’s tests (25, 26). In this study, a P-value <0.05 indicated statistical significance.
Results
Search results
Initially, 2,683 records were identified through electronic database search, apart from 11 records via manual searching. We excluded 108 duplicate studies, 2,586 studies upon screening titles and abstracts, and 92 studies after full-text review. Finally, 16 studies were considered eligible for this meta-analysis ( Figure 1 ). Potential time and center crossover were noted among the studies, particularly between the studies of Mei et al. (27) and Fu et al. (28) from similar single-center and multicenter studies because of numerous participations by some centers.
Figure 1.
Flowchart of the study inclusion.
All of the included studies originated from China; six were multicentered (16, 29–33) and five underwent PSM analysis (34–38) and one underwent sIPTW analysis (33). A total of 3,004 patients were included in this meta-analysis, encompassing 1,789 patients administered with transarterial chemo(embolization) plus immune-targeted therapy and 1,215 patients receiving immune-targeted therapy alone, respectively. Table 1 summarizes the baseline characteristics and quality assessment outcomes. Supplementary Table S3 summarizes the treatment regimens, considering no consensus on the transarterial chemo(embolization) plus immune-targeted therapy. Supplementary Figure S1 illustrates the quality of each study. Supplementary Table S4 summarizes the scoring rules of each study.
Table 1.
Basic characteristics and quality assessment of the included studies.
| Study | Design | Treatment | Patients | Age, years | Sex, M/F | HBV, P/N | Child–Pugh, A/B | AFP (ng/ml), <400/≥400 | MVI, yes/no | Extrahepatic metastasis, yes/no | BCLC stage, A/B/C | CR, N (%) | ORR, N (%) | DCR, N (%) | Median PFS, months | Median OS, months | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai 2021 | R single center | TACE + Sor + sintilimab | 35 | 56.5 ± 10.2 | 30/5 | 27/8 | 19/16 | NA | 17/18 | 6/29 | 0/14/21 | 6 (17) | 10 (29) | 28 (80) | 5 | 13 | H |
| Sor + sintilimab | 23 | 54.0 ± 15.0 | 21/2 | 18/5 | 12/11 | NA | 16/7 | 5/18 | 0/5/18 | 4 (17) | 6 (26) | 17 (74) | 4 | 9 | |||
| Mei 2021 | R single center | HAIC + Len+ ICIs | 45 | 49.1 ± 10.6 | 38/7 | 37/8 | 44/1 | 4,106.0 (72.8–121,000.0) | 36/9 | 15/30 | 0/5/40 | 0 (0) | 18 (40) | 38 (84) | 8.8 | 15.9 | H |
| Len + ICIs | 25 | 50.1 ± 12.3 | 18/7 | 19/6 | 22/3 | 767.6 (23.3–21,940.5) | 18/7 | 13/12 | 0/3/22 | 0 (0) | 4 (14) | 11 (44) | 5.4 | 8.6 | |||
| Chen 2021 | R multi-center | HAIC + Len + pembrolizumab | 84 | 52 (42–67) | 72/12 | 45/39 | 71/13 | 3,984.0 (82.0–49,534.0) | 49/35 | 20/64 | 0/22/62 | 13 (15) | 50 (60) | 74 (88) | 10.9 | 17.7 | H |
| Len + pembrolizumab | 86 | 53 (43–69) | 71/15 | 48/38 | 75/11 | 4,022.0 (79.0–51,462.0) | 55/31 | 24/62 | 0/21/65 | 8 (9) | 36 (42) | 71 (83) | 6.8 | 12.6 | |||
| Guo 2022 | R single center | cTACE+ MTDs + camrelizumab | 31 | 24/7 <60/≥60 |
26/5 | 29/2 | 21/10 | 17/14 | 20/11 | 17/14 | 2/5/24 | 2 (6) | 16 (52) | 28 (90) | 11.7 | 19.8 | H |
| MTDs + camrelizumab | 23 | 12/11 <60/≥60 |
22/1 | 20/3 | 14/9 | 12/11 | 11/12 | 14/9 | 1/3/19 | 0 (0) | 5 (22) | 15 (65) | 4 | 11.6 | |||
| Huang 2022 after PSM | R single center | TACE + immune-targeted therapy | 24 | 58.0 ± 10.7 | 20/4 | 20/4 | 18/6 | 12/12 | 18/6 | 9/15 | 0/0/24 | 1 (4) | 10 (42) | 19 (79) | 7.4 | 17.3 | H |
| Immune-targeted therapy | 24 | 56.5 ± 14.0 | 21/3 | 20/4 | 14/10 | 9/15 | 18/6 | 13/11 | 0/0/24 | 0 (0) | 3 (13) | 12 (50) | 6.7 | 11.8 | |||
| Dong 2022 | R dual center | TACE/HAIC + immune-targeted therapy | 66 | 52 (40–65) | 57/9 | 54/12 | 50/16 | 39/27 | 25/41 | 29/37 | 0/0/66 | 2 (3) | 40 (61) | 56 (85) | 8.4 | 11.6 | H |
| Immune-targeted therapy + TACE/HAIC | 56 | 52 (41–64) | 51/5 | 52/4 | 42/14 | 28/28 | 27/29 | 29/27 | 0/0/56 | 0 (0) | 18 (32) | 42 (75) | 5.3 | 10.0 | |||
| Immune-targeted therapy | 41 | 57 (47–67) | 34/7 | 36/5 | 31/10 | 20/21 | 16/25 | 24/17 | 0/0/41 | 0 (0) | 9 (22) | 33 (80) | 6.3 | 11.3 | |||
| Wang 2023 after PSM | R single center | TACE + Len + ICIs | 43 | 57.07 ± 10.53 | 38/5 | 42/4 | 39/4 | 25/18 | 19/24 | 22/21 | 0/8/35 | 0 (0) | 24 (56) | 37 (86) | 10.2 | 20.5 | H |
| Len + ICIs | 43 | 58.00 ± 10.52 | 37/6 | 52/7 | 36/7 | 21/22 | 18/25 | 25/18 | 0/7/36 | 0 (0) | 13 (30) | 28 (65) | 7.4 | 12.6 | |||
| Xin 2023 | R single center | TACE + Len + ICIs | 60 | 37/23 <60/≥60 |
54/6 | 56/4 | 60/0 | 32/28 | 28/32 | 18/42 | 0/21/39 | 10 (17) | 46 (77) | 58 (97) | 16.2 | 29 | H |
| Len + ICIs | 58 | 40/18 <60/≥60 |
51/7 | 51/7 | 58/0 | 28/30 | 17/41 | 26/32 | 0/23/35 | 3 (5) | 26 (45) | 44 (76) | 10.2 | 17.8 | |||
| Yang 2023 after PSM |
R single center | TACE + regorafenib + ICIs | 23 | 53 (43.0–65.0) | 20/3 | 19/4 | 22/1 | 15/8 | 8/15 | 11/12 | 0/19/14 | 0 (0) | 8 (35) | 16 (70) | 5.8 | 13.6 | H |
| Regorafenib + ICIs | 23 | 49 (45.0–56.0) | 19/4 | 16/7 | 18/5 | 14/9 | 10/13 | 12/11 | 0/5/18 | 0 (0) | 1 (4) | 10 (44) | 2.6 | 7.5 | |||
| Fu 2023 | R single center | HAIC + Len + ICIs | 89 | 51.9 ± 10.5 | 83/6 | 79/10 | 88/1 | 37/52 | 89/0 | 21/68 | 0/0/89 | 17 (19) | 55 (62) | 77 (87) | 11.5 | 26.3 | M |
| Len + ICIs | 53 | 53.5 ± 10.5 | 50/3 | 45/8 | 47/6 | 20/33 | 53/0 | 26/27 | 0/0/53 | 2 (4) | 11 (21) | 30 (57) | 5.5 | 13.8 | |||
| Pan 2023 after PSM | R multicenter | TACE/HAIC + immune-targeted therapy | 131 | 54.0 (48.5–61.0) | 118/13 | 117/14 | 127/4 | 20,461.84 ± 36,365.99 | 102/29 | 48/83 | 0/19/112 | 2 (2) | 48 (37) | 112 (85) | NA | 23.9 | H |
| Immune-targeted therapy | 131 | 54.0 (47.5–60.5) | 119/12 | 112/19 | 122/9 | 20,331.47 ± 85,642.76 | 83/48 | 48/83 | 0/19/112 | 6 (5) | 43 (33) | 109 (83) | NA | Not reached | |||
| Lang 2023 after PSM | R single center | TACE + Len + sintilimab | 75 | 57/18 ≤60/>60 |
66/9 | 69/6 | 59/16 | 45/30 | 23/52 | 26/49 | 0/32/43 | 2 (3) | 33 (44) | 47 (63) | 11.1 | Not reached | H |
| Len+ sintilimab | 39 | 29/10 ≤60/>60 |
34/5 | 35/4 | 30/9 | 23/16 | 9/30 | 19/20 | 0/14/25 | 0 (0) | 9 (23) | 17 (44) | 5.1 | 14.0 | |||
| Li 2023 | R multicenter | TACE + immune-targeted therapy | 62 | 50/12 <65/≥65 |
55/7 | 46/16 | 48/13/1 A/B/C |
24/38 | 28/34 | 14/48 | 6/9/46/1 A/B/C/D |
NA | N | NA | 7.4 | 20.3 | M |
| Immune-targeted therapy | 83 | 46/37 <65/≥65 |
71/12 | 58/35 | 65/17/1 A/B/C |
43/40 | 43/40 | 32/51 | 6/8/68/1 A/B/C/D |
NA | NA | NA | 5.0 | 13.6 | |||
| Hu 2023 | R single center | TACE + immune-targeted therapy | 98 | 52 (42–62) | 87/11 | 85/13 | 75/23 | 39/59 ≤200/>200 |
73/25 | 49/49 | 0/12/86 | 22 (22) | 73 (74) | 89 (91) | 9.7 | 19.5 | H |
| Immune-targeted therapy | 49 | 53 (47–63) | 47/2 | 43/6 | 33/16 | 22/27 ≤200/>200 |
30/19 | 26/23 | 0/7/42 | 4 (8) | 20 (41) | 36 (73) | 7.7 | 10.8 | |||
| Cao 2023 | R dual center | TACE + Atez/Bev | 62 | 55.8 ± 11.2 | 52/10 | 44/18 | 40/22 | 30/32 | 34/28 | 33/29 | NA | 1 (2) | 24 (39) | 43 (69) | 10 | 14 | H |
| Atez/Bev | 77 | 52.8 ± 11.0 | 65/12 | 59/18 | 51/26 | 41/36 | 43/34 | 45/32 | NA | 1 (1) | 13 (17) | 49 (64) | 6 | 10 | |||
| Jin 2024 after sIPTW | R multicenter | TACE + immune-targeted therapy | 805 | 54 (48–63) | 693/112 | 681/124 | 659/146 | 394/354 | 570/235 | 471/334 | NA | NA | 332 (41.2) | NA | 9.9 | 22.6 | H |
| Immune-targeted therapy | 437 | 56 (47–62) | 378/59 | 374/63 | 357/80 | 208/197 | 308/129 | 258/179 | NA | NA | 100 (22.9) | NA | 7.4 | 15.9 |
TACE, transcatheter arterial chemoembolization; HAIC, hepatic artery infusion chemotherapy; MTDs, molecularly targeted drugs; ICIs, immune checkpoint inhibitors; Len, lenvatinib; Sor, sorafenib; Atez, atezolizumab; Bev, bevacizumab; R, retrospective; M, male; F, female; HBV, hepatitis B virus; P, positive; N, negative; S, single; M, multiple; MVI, macrovascular invasion; BCLC, Barcelona Clinic Liver Cancer stage; CR, complete response; PR, partial response; ORR, objective response rate; DCR, disease control rate; OS, overall survival; PFS, progression-free survival; H, high; M, medium; NA, not available; PSM, propensity score matching; sIPTW, stabilized inverse probability of treatment weighting.
Short-term endpoints
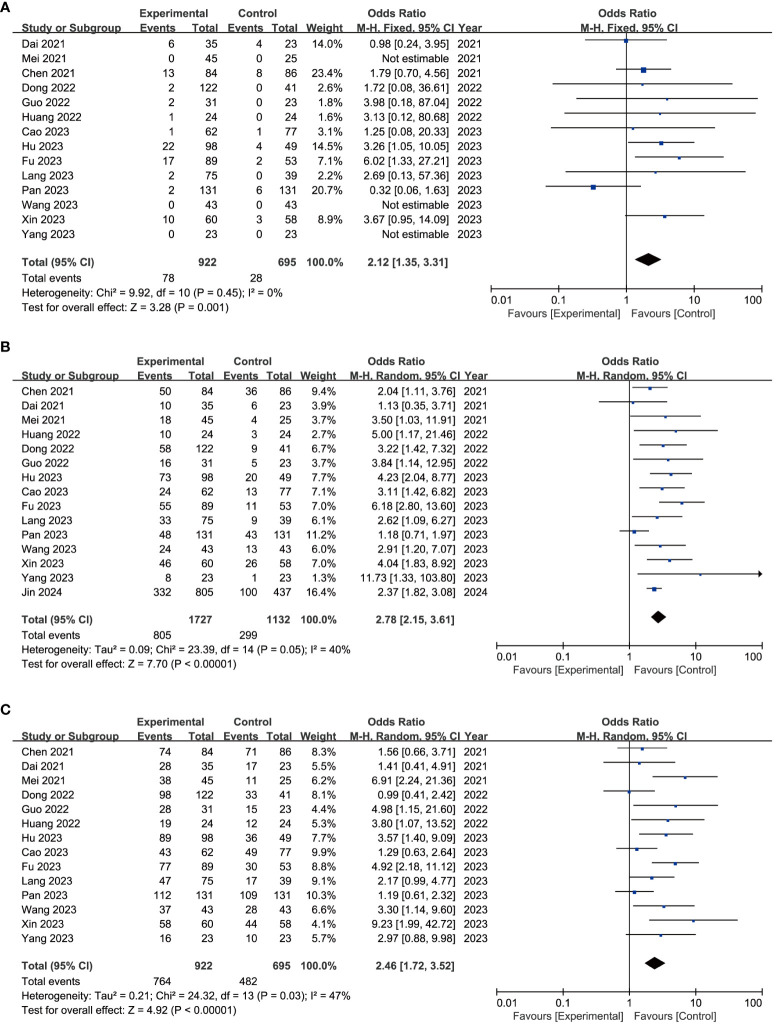
CR was evaluated in 14 included trials (16, 19, 20, 27–29, 32, 34, 35, 37–41), without significant heterogeneity (I 2 = 0%, P = 0.45, Figure 2A ). Using the fixed-effects model, the pooled CR rate was in favor of the experiment group over the control group (8.5% vs. 4.0%) with an OR of 2.12 (95% CI = 1.35–3.31, Figure 2A ). Sensitivity analysis showed that the results did not change greatly after removing any included single study ( Supplementary Figure S2A ). Asymmetry was absent in the funnel plot ( Supplementary Figure S3A ), with P-values of 0.9756 and 0.6971 for Egger’s test and Begg’s test, respectively ( Supplementary Table S5 ).
Figure 2.
Forest plot of complete response (A), disease control rate (B), and objective response rate (C) of immune-targeted therapy with or without transarterial chemo(embolization).
ORR was evaluated in 15 included trials (16, 19, 20, 27–29, 32–35, 37–41), among which significant heterogeneity was observed (I 2 = 40%, P = 0.05, Figure 2B ). Using the random-effects model, the pooled ORR rate was in favor of the experiment group over the control group (46.6% vs. 26.4%) with an OR of 2.78 (95% CI = 2.15–3.61, Figure 2B ). The robustness of these results was confirmed by sensitivity analysis ( Supplementary Figure S2B ). Asymmetry was observed in the funnel plot ( Supplementary Figure S3B ), with P-values of 0.1017 and 0.2160 for Egger’s test and Begg’s test, respectively ( Supplementary Table S5 ).
Similarly, DCR was evaluated in 14 studies (16, 19, 20, 27–29, 32, 34, 35, 37–41) with significant heterogeneity (I 2 = 47%, P = 0.03, Figure 2C ). Using the random-effects model, the pooled DCR rate was in favor of the experiment group over the control group (82.9% vs. 69.4%) with an OR of 2.46 (95% CI = 1.72–3.52, Figure 2C ). Sensitivity analysis validated the consistency of these findings ( Supplementary Figure S2C ). Asymmetry was observed by funnel plot ( Supplementary Figure S3C ), with P-values of 0.0195 and 0.0328 for Egger’s test and Begg’s test, respectively ( Supplementary Table S5 ). The trim-and-fill method identified five additional publications, without any significant impact on the results ( Supplementary Table S5 ).
Long-term endpoints
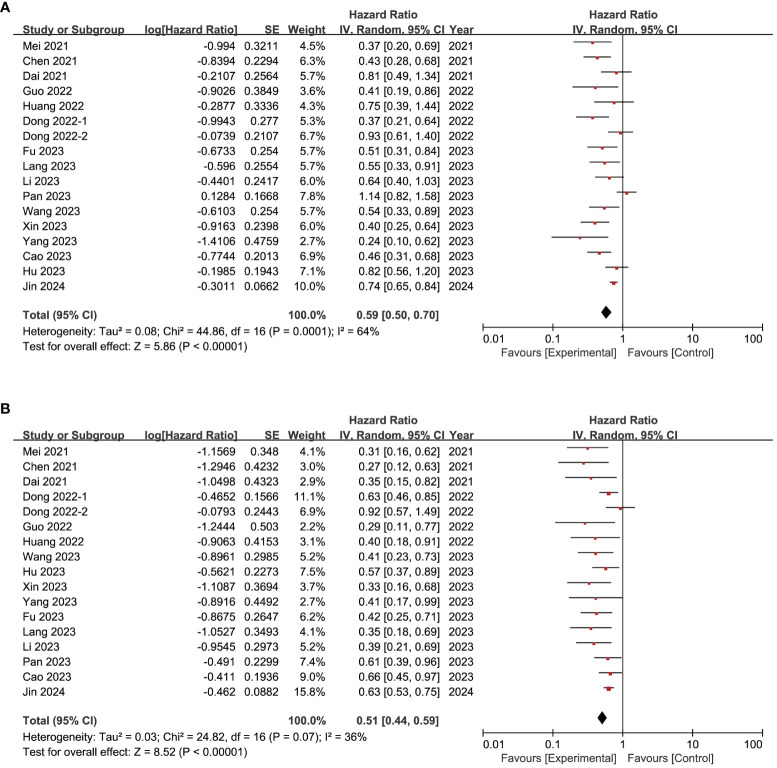
PFS was evaluated in 16 studies (16, 19, 20, 27–29, 31–35, 37–41), among which significant heterogeneity was observed (I 2 = 64%, P < 0.05, Figure 3A ). Using the random-effects model, the pooled HR was in favor of the experiment group over the control group (HR = 0.59, 95% CI = 0.50–0.70, Figure 3A ), a finding upheld by sensitivity analysis ( Supplementary Figure S2D ). Asymmetry was observed by funnel plot ( Supplementary Figure S3D ) with P-values of 0.0239 and 0.0581 for Egger’s test and Begg’s test, respectively ( Supplementary Table S5 ). Six additional studies were identified through the trim-and-fill method, without substantial alteration in the results ( Supplementary Table S5 ).
Figure 3.
Forest plot of progression-free survival (A) and overall survival (B) of immune-targeted therapy with or without transarterial chemo(embolization).
OS was evaluated in 16 studies (16, 19, 20, 27–29, 31–35, 37–41), with significant heterogeneity (I 2 = 36%, P = 0.07, Figure 3B ). Using the random-effects model, the pooled HR was in favor of the experiment group over the control group (HR = 0.51, 95% CI = 0.44–0.59, Figure 3B ), confirmed by sensitivity analysis ( Supplementary Figure S2E ). Funnel plot analysis showed asymmetry ( Supplementary Figure S3E ), with P-values of 0.0006 and 0.0084 for Egger’s test and Begg’s test, respectively ( Supplementary Table S5 ). The trim-and-fill method identified six more publications, with no significant change in the results ( Supplementary Table S5 ).
Subgroup analysis
Ten of the included studies (16, 19, 20, 27, 29, 32, 33, 35, 39, 41) enrolled uHCC patients who did not receive prior treatment. Results revealed a superior outcome of combination therapy of transarterial chemo(embolization) and immune-targeted therapy in terms of CR (OR = 1.69, 95% CI = 1.05–2.73, Supplementary Table S6 ), ORR (OR = 2.34, 95% CI = 1.96–2.81, Supplementary Table S6 ), DCR (OR = 2.00, 95% CI = 1.29–3.10, Supplementary Table S6 ), median PFS (HR = 0.62, 95% CI = 0.50–0.77, Supplementary Table S6 ), and median OS (HR = 0.55, 95% CI = 0.46–0.66, Supplementary Table S6 ).
In China, TACE and HAIC are the two most common modalities of transarterial therapies (42). In this meta-analysis, TACE was adopted in 11 studies (19, 20, 31, 33–35, 37–41), whereas HAIC was adopted in three studies (16, 27, 28), respectively. Results confirmed the advantage of additional TACE to immune-targeted therapy in terms of CR (OR = 2.32, 95% CI = 1.26–4.26, Supplementary Table S6 ), ORR (OR = 2.72, 95% CI = 2.22–3.33, Supplementary Table S6 ), DCR (OR = 2.58, 95% CI = 1.84–3.61, Supplementary Table S6 ), median PFS (HR = 0.60, 95% CI = 0.49–0.72, Supplementary Table S6 ), and median OS (HR = 0.55, 95% CI = 0.48–0.63, Supplementary Table S6 ). Similarly, the advantage of additional HAIC to immune-targeted therapy was also verified in terms of CR, ORR, DCR, median PFS, and median OS (all P < 0.05, Supplementary Table S6 ).
Advanced HCC often coexists with extrahepatic metastasis (6, 42), making additional local treatment debatable. Herein, nine studies (27–29, 32–35, 37, 39) conducted subgroup analysis for patients with or without extrahepatic metastasis. Expectedly, in patients without extrahepatic metastasis, the experiment group outperformed the control group in median PFS and OS (HR = 0.67, 95% CI = 0.57–0.79; HR = 0.57, 95% CI = 0.47–0.68, respectively, Supplementary Table S6 ). Compared with the control group, the pooled HR for median PFS and OS favored the experiment in patients with extrahepatic metastasis (HR = 0.78, 95% CI = 0.68–0.89; HR = 0.66, 95% CI = 0.57–0.77, respectively, Supplementary Table S6 ).
Liver function is the bottleneck of additional transarterial chemo(embolization) to immune-targeted therapy (43). In this meta-analysis, seven studies (27, 28, 32–35, 37) compared patients with a Child–Pugh grade of A and B. Compared with the control group, the pooled HRs for both PFS and OS were in favor of the experiment group among patients with a Child–Pugh grade of A or B (all P < 0.05, Supplementary Table S6 ).
Transarterial chemo(embolization) improves the long-term prognosis of patients with tumor thrombus (44, 45), which is an aggressive characteristic of HCC (6, 42). Herein, eight studies (27, 28, 32–35, 37, 39) enrolled patients with tumor thrombus and seven studies (27, 32–35, 37, 39) enrolled patients without tumor thrombus. Compared with the control group, the pooled HRs for both PFS and OS were in favor of the experiment group among patients with or without tumor thrombus (all P < 0.05, Supplementary Table S6 ).
Adverse events
Table 2 delineates treatment-related AEs. No treatment-related deaths were reported. The most prevalent all-grade AEs included fatigue, diarrhea, rash, and elevated alanine transaminase (ALT) and aspartate aminotransferase (AST). In aggregate, the addition of transarterial therapies heightened the risk of certain AEs including elevated ALT, AST, and gamma-glutamyl transpeptidase (GGT); fever; nausea; and vomiting (all P < 0.05, Table 2 ). Likewise, severe AEs mirrored those of all-grade AEs, with transarterial chemo(embolization) additionally elevating the risk of severe elevated ALT and AST (ALT: OR = 2.17, 95% CI = 1.28–3.68; AST: OR = 2.28, 95% CI = 1.42–3.65; both P < 0.05, Table 2 ).
Table 2.
Treatment-related adverse events.
| Events | All grade | Grade ≥3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Included studies | Participants | Effect model | OR (95 CI) | P-value | Included studies | Participants | Effect model | OR (95 CI) | P-value | |
| Elevated ALT | 12 | 2,316 | Random | 2.33 [1.48, 3.67] | <0.001 | 11 | 2,146 | Fixed | 2.17 [1.28, 3.68] | 0.004 |
| Elevated AST | 12 | 2,316 | Random | 2.20 [1.41, 3.42] | <0.001 | 11 | 2,146 | Fixed | 2.28 [1.42, 3.65] | <0.001 |
| Elevated GGT | 2 | 172 | Fixed | 2.37 [1.09, 5.16] | 0.03 | 2 | 172 | Fixed | 0.98 [0.24, 3.95] | 0.98 |
| Anemia | 4 | 429 | Random | 2.03 [0.70, 5.86] | 0.19 | 4 | 429 | Fixed | 0.98 [0.26, 3.65] | 0.97 |
| Neutropenia | 3 | 302 | Random | 2.70 [0.74, 9.86] | 0.13 | 3 | 302 | Fixed | 1.29 [0.34, 4.95] | 0.71 |
| Lymphopenia | 2 | 172 | Random | 1.65 [0.52, 5.26] | 0.4 | 2 | 172 | Fixed | 0.98 [0.24, 3.95] | 0.98 |
| Thrombocytopenia | 11 | 2,373 | Random | 1.21 [0.71, 2.06] | 0.47 | 11 | 2,492 | Fixed | 1.25 [0.75, 2.11] | 0.39 |
| Hypoleukemia | 8 | 2,007 | Random | 1.38 [0.79, 2.44] | 0.26 | 8 | 2,126 | Fixed | 1.38 [0.61, 3.10] | 0.44 |
| Hypoalbuminemia | 4 | 500 | Fixed | 0.97 [0.62, 1.51] | 0.89 | 3 | 330 | Fixed | 1.16 [0.41, 3.27] | 0.78 |
| Nausea and vomiting | 9 | 1,077 | Random | 3.71 [1.48, 9.34] | 0.005 | 8 | 1,054 | Fixed | 1.53 [0.61, 3.85] | 0.37 |
| Hand-foot syndrome | 9 | 1,929 | Fixed | 1.07 [0.82, 1.41] | 0.62 | 10 | 2,218 | Fixed | 1.02 [0.57, 1.82] | 0.94 |
| Hypertension | 11 | 2,331 | Fixed | 0.94 [0.76, 1.15] | 0.53 | 10 | 2,308 | Fixed | 0.97 [0.66, 1.41] | 0.86 |
| Hyperthyroidism | 5 | 548 | Fixed | 1.11 [0.43, 2.86] | 0.83 | 4 | 378 | – | Not estimable | – |
| Hypothyroidism | 10 | 2,160 | Fixed | 0.97 [0.70, 1.36] | 0.88 | 9 | 1,990 | Fixed | 0.97 [0.40, 2.33] | 0.94 |
| Rash | 13 | 2,479 | Fixed | 0.96 [0.74, 1.25] | 0.76 | 12 | 2,309 | Fixed | 1.00 [0.51, 1.98] | 1.00 |
| RCCEP | 5 | 1,510 | Fixed | 1.49 [0.90, 2.47] | 0.12 | 5 | 1,510 | Fixed | 1.10 [0.33, 3.63] | 0.88 |
| Urine protein | 9 | 2,052 | Fixed | 0.87 [0.64, 1.20] | 0.40 | 8 | 1,910 | Fixed | 0.76 [0.33, 1.75] | 0.52 |
| Diarrhea | 13 | 2,570 | Fixed | 1.03 [0.80, 1.33] | 0.82 | 11 | 2,258 | Fixed | 0.88 [0.47, 1.66] | 0.69 |
| Fatigue | 13 | 2,564 | Fixed | 0.95 [0.76, 1.19] | 0.63 | 11 | 2,254 | Fixed | 1.33 [0.70, 2.53] | 0.38 |
| Decreased appetite | 10 | 2,169 | Fixed | 0.98 [0.74, 1.30] | 0.88 | 9 | 1,999 | Fixed | 0.87 [0.40, 1.90] | 0.72 |
| Fever | 10 | 1,848 | Random | 4.23 [2.05, 8.71] | <0.001 | 9 | 1,978 | Fixed | 1.36 [0.65, 2.82] | 0.42 |
| Pain | 4 | 1,532 | Random | 2.40 [0.62, 9.32] | 0.21 | 3 | 1,362 | Random | 1.77 [0.41, 7.56] | 0.44 |
| Pruritus | 6 | 1,798 | Fixed | 1.00 [0.57, 1.77] | 1.00 | 5 | 1,628 | Fixed | 2.76 [0.13, 57.70] | 0.51 |
| Muscle soreness | 2 | 168 | Fixed | 1.12 [0.31, 4.11] | 0.86 | 2 | 168 | Fixed | 1.44 [0.20, 10.32] | 0.72 |
| Cough | 3 | 298 | Fixed | 1.21 [0.48, 3.04] | 0.69 | 2 | 128 | – | Not estimable | – |
| Pneumonia | 6 | 1,919 | Fixed | 0.85 [0.50, 1.46] | 0.56 | 6 | 1,919 | Fixed | 0.85 [0.33, 2.18] | 0.74 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; RCCEP, reactive cutaneous capillary endothelial proliferation; HR, hazard ratio; OR, odds ratio; CI, confidence interval.
Discussion
Traditionally, transarterial chemo(embolization) has been the preferred option for uHCC (6, 46, 47); however, its role is debatable in the era of immune-targeted therapy. To the best of our knowledge, this is the first meta-analysis to compare the clinical efficacy and safety of transarterial chemo(embolization) plus immune-targeted therapy versus immune-targeted therapy. This meta-analysis consisted of 16 studies, encompassing 1,789 patients who received transarterial chemo(embolization) plus immune-targeted therapy and 1,215 patients who received immune-targeted therapy. The results elucidated that transarterial chemo(embolization) plus immune-targeted therapy outperformed immune-targeted therapy alone in terms of CR, ORR, DCR, PFS, and OS, albeit at the cost of escalated AEs concerning liver function.
Additional TACE has been introduced to amplify the local control effect, considering the promising results of immune-targeted therapy including IMbrave150 (3, 9). Since the first report by Liu et al. (48) in 2021, a plethora of pertinent studies regarding transarterial chemo(embolization) combined with immune-targeted therapy, both comparative (16, 19, 28, 29, 32, 34) and non-comparative (48, 49), have emerged. Supplementary Table S7 summarizes the ongoing trials (all from China). Notably, the application spectrum of transarterial chemo(embolization) in China diverges from Western practices (6, 50), extending to downstaging or bridge therapy for resectable HCC (51), conversion therapy for uHCC (52), adjuvant postoperative treatment for high-risk HCC (8, 53), and salvage therapy for recurrence (54–56). Consistent with a 2022 systematic review (17), all studies originated from China.
A meta-analysis confirmed the superiority of transarterial chemo(embolization) combined with immune-targeted therapy over transarterial chemo(embolization) combined with TKIs regarding the short- and long-term outcomes (57). Unlike TACE combined with TKIs, immune-targeted therapy is preferred for uHCC management globally (6, 58). Our analysis demonstrated that a combination of TACE and immune-targeted therapy significantly bolstered the CR, ORR, and DCR and extended PFS and OS, compared with immune-targeted therapy alone. Noteworthy, the advantage of additional transarterial chemo(embolization) was also corroborated across various clinical scenarios (first-line treatment, TACE or HAIC, with or without extrahepatic metastasis, Child–Pugh A or B, and with or without tumor thrombus, Supplementary Table S6 ). These findings suggested that additional transarterial chemo(embolization) could potentially ameliorate the prognosis of uHCC, albeit necessitating higher-tier evidence from future studies.
CR and subsequent conversion hepatectomy have gained attention for uHCC (30, 59). Previous non-comparative studies have demonstrated a CR rate and conversion rate of 48% and 60%, respectively (60). However, in this meta-analysis, the CR rate was only reported in 14 studies and the conversion rate was reported in three studies (28, 29, 39), respectively. Moreover, the CR rate ranged from 0% to 22%, which was far beyond people’s expectations. This paucity of data warrants a deeper exploration, particularly concerning whether a larger sample size may diminish the perceived benefits of additional transarterial chemo(embolization).
Researchers have underscored the potential of TACE to exacerbate liver damage (61, 62); hence, it is primarily recommended for patients with robust liver function (50, 63). Studies have demonstrated the tolerability of adjunctive TACE to immune-targeted therapy across both single-center (14, 31, 64) and multicenter settings (16, 30), consistent with systematic reviews (17, 57). However, a significant uptick in AEs was revealed in six studies (27, 28, 33, 37, 40, 41), predominantly centering on impaired liver function. Furthermore, we found that the pooled rates of elevated ALT and AST were significantly higher in the transarterial chemo(embolization) plus targeted immunotherapy group than in immune-targeted therapy alone (31.3% vs. 21.6%, 32.2% vs. 24.3%, P < 0.05, Table 2 ). The larger sample size in this analysis unveils these AEs, which are scarcely highlighted in single studies, underscoring the need for safety assessments in larger cohorts. However, other liver function-related indexes such as total bilirubin and prothrombin time and the occurring timepoint of AEs were rarely reported, which deserve more attention in ongoing RCTs. Considering that the safety profile of immune-targeted therapy has been fully inspected in both large RCTs and real-world studies, additional transarterial chemo(embolization) might be the choke point of safety.
Nonetheless, there were several limitations in this meta-analysis. First, the retrospective design of the included studies may have resulted in confounding bias, despite five studies (29, 34, 35, 37, 38) utilizing PSM. Second, reporting bias, notably regarding CR rate and conversion rate, was also inevitable. Third, the inherent heterogeneity within the uHCC patient population would potentially circumscribe the generalizability of our findings beyond this demographic, aside from the differences in the regimen of transarterial chemo(embolization) and immune-targeted therapy. Fourth, immune-targeted therapy is initiated immediately after transarterial chemo(embolization); therefore, the timing of AEs concerning liver function needs to be described. AST and ALT were possibly elevated after transarterial chemo(embolization), suggesting its therapeutic effect. Finally, all studies were from China, and the findings would be applicable only in China.
Conclusion
With the available data, the combination of transarterial chemo(embolization) and immune-targeted therapy surpasses immune-targeted therapy alone regarding local control and long-term efficacy. However, the adjunctive use of transarterial chemo(embolization) escalates the incidence of liver function-related AEs.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
HF: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. QK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. SW: Data curation, Methodology, Supervision, Validation, Writing – review & editing. QT: Conceptualization, Resources, Writing – review & editing. LW: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Jiangxi Province Natural Science Foundation (20234BAB206086).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1421520/full#supplementary-material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (London England). (2022) 400:1345–62. doi: 10.1016/s0140-6736(22)01200-4 [DOI] [PubMed] [Google Scholar]
- 3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (Rescue): A nonrandomized, open-label, phase ii trial. Clin Cancer Res an Off J Am Assoc Cancer Res. (2021) 27:1003–11. doi: 10.1158/1078-0432.Ccr-20-2571 [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:2960–70. doi: 10.1200/jco.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. Bclc strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li S, Mei J, Wang Q, Shi F, Liu H, Zhao M, et al. Transarterial infusion chemotherapy with folfox for advanced hepatocellular carcinoma: A multi-center propensity score matched analysis of real-world practice. Hepatobiliary Surg Nutr. (2021) 10:631–45. doi: 10.21037/hbsn.2020.03.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with folfox in hepatocellular carcinoma with microvascular invasion: A multicenter, phase iii, randomized study. J Clin Oncol Off J Am Soc Clin Oncol. (2023) 41:1898–908. doi: 10.1200/jco.22.01142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, et al. Atezolizumab plus bevacizumab versus sorafenib in the chinese subpopulation with unresectable hepatocellular carcinoma: phase 3 randomized, open-label imbrave150 study. Liver Cancer. (2021) 10:296–308. doi: 10.1159/000513486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh P, Toom S, Avula A, Kumar V, Rahma OE. The immune modulation effect of locoregional therapies and its potential synergy with immunotherapy in hepatocellular carcinoma. J hepatocellular carcinoma. (2020) 7:11–7. doi: 10.2147/jhc.S187121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J immunotherapy Cancer. (2021) 9(9):e003311. doi: 10.1136/jitc-2021-003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta radiologica (Stockholm Sweden 1987). (2008) 49:523–9. doi: 10.1080/02841850801958890 [DOI] [PubMed] [Google Scholar]
- 13. Abou-Alfa GK. Tace and sorafenib: A good marriage? J Clin Oncol Off J Am Soc Clin Oncol. (2011) 29:3949–52. doi: 10.1200/jco.2011.37.9651 [DOI] [PubMed] [Google Scholar]
- 14. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of tace combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol. (2021) 11:783480. doi: 10.3389/fonc.2021.783480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus tace with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring pd-L1 expression: A retrospective study. J Cancer Res Clin Oncol. (2022) 148:2115–25. doi: 10.1007/s00432-021-03767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen S, Xu B, Wu Z, Wang P, Yu W, Liu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting pd-L1 staining: A multicenter retrospective study. BMC Cancer. (2021) 21:1126. doi: 10.1186/s12885-021-08858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial chemo(Embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: A systematic review. Front Immunol. (2022) 13:913464. doi: 10.3389/fimmu.2022.913464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, et al. Transarterial chemoembolization with pd-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (Chance001). Signal transduction targeted Ther. (2023) 8:58. doi: 10.1038/s41392-022-01235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao F, Shi C, Zhang G, Luo J, Zheng J, Hao W. Improved clinical outcomes in advanced hepatocellular carcinoma treated with transarterial chemoembolization plus atezolizumab and bevacizumab: A bicentric retrospective study. BMC Cancer. (2023) 23:873. doi: 10.1186/s12885-023-11389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xin Y, Zhang X, Liu N, Peng G, Huang X, Cao X, et al. Efficacy and safety of lenvatinib plus pd-1 inhibitor with or without transarterial chemoembolization in unresectable hepatocellular carcinoma. Hepatol Int. (2023) 17:753–64. doi: 10.1007/s12072-023-10502-3 [DOI] [PubMed] [Google Scholar]
- 21. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. (2021) 18:293–313. doi: 10.1038/s41575-020-00395-0 [DOI] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised recist guideline (Version 1.1). Eur J Cancer (Oxford Engl 1990). (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 27. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with pd-1 inhibitors plus lenvatinib versus pd-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. (2021) 11:618206. doi: 10.3389/fonc.2021.618206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu Y, Peng W, Zhang W, Yang Z, Hu Z, Pang Y, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. (2023) 58:413–24. doi: 10.1007/s00535-023-01976-x [DOI] [PubMed] [Google Scholar]
- 29. Pan Y, Zhu X, Liu J, Zhong J, Zhang W, Shen S, et al. Systemic therapy with or without transcatheter intra-arterial therapies for unresectable hepatocellular carcinoma: A real-world, multi-center study. Front Immunol. (2023) 14:1138355. doi: 10.3389/fimmu.2023.1138355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-pd-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J hepatocellular carcinoma. (2021) 8:1233–40. doi: 10.2147/jhc.S332420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li H, Su K, Guo L, Jiang Y, Xu K, Gu T, et al. Pd-1 inhibitors combined with antiangiogenic therapy with or without transarterial chemoembolization in the treatment of hepatocellular carcinoma: A propensity matching analysis. J hepatocellular carcinoma. (2023) 10:1257–66. doi: 10.2147/jhc.S415843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong H, Jian Y, Wang M, Liu F, Zhang Q, Peng Z, et al. Hepatic artery intervention combined with immune-targeted therapy is superior to sequential therapy in bclc-C hepatocellular carcinoma. J Cancer Res Clin Oncol. (2023) 149:5405–16. doi: 10.1007/s00432-022-04386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin ZC, Chen JJ, Zhu XL, Duan XH, Xin YJ, Zhong BY, et al. Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma (Chance2201): A target trial emulation study. EClinicalMedicine. (2024) 72:102622. doi: 10.1016/j.eclinm.2024.102622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang JT, Zhong BY, Jiang N, Li WC, Zhang S, Yin Y, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors plus tyrosine kinase inhibitors versus immune checkpoint inhibitors plus tyrosine kinase inhibitors for advanced hepatocellular carcinoma. J hepatocellular carcinoma. (2022) 9:1217–28. doi: 10.2147/jhc.S386672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lang M, Gan L, Ren S, Han R, Ma X, Li G, et al. Lenvatinib plus sintilimab with or without transarterial chemoembolization for intermediate or advanced stage hepatocellular carcinoma: A propensity score-matching cohort study. Am J Cancer Res. (2023) 13:2540–53. [PMC free article] [PubMed] [Google Scholar]
- 36. Qin J, Huang Y, Zhou H, Yi S. Efficacy of sorafenib combined with immunotherapy following transarterial chemoembolization for advanced hepatocellular carcinoma: A propensity score analysis. Front Oncol. (2022) 12:807102. doi: 10.3389/fonc.2022.807102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Zhao M, Han G, Han X, Shi J, Mi L, et al. Transarterial chemoembolization combined with pd-1 inhibitors plus lenvatinib showed improved efficacy for treatment of unresectable hepatocellular carcinoma compared with pd-1 inhibitors plus lenvatinib. Technol Cancer Res Treat. (2023) 22:15330338231166765. doi: 10.1177/15330338231166765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang X, Deng H, Sun Y, Zhang Y, Lu Y, Xu G, et al. Efficacy and safety of regorafenib plus immune checkpoint inhibitors with or without tace as a second-line treatment for advanced hepatocellular carcinoma: A propensity score matching analysis. J hepatocellular carcinoma. (2023) 10:303–13. doi: 10.2147/jhc.S399135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu Y, Pan T, Cai X, He QS, Zheng YB, Huang MS, et al. Addition of transarterial chemoembolization improves outcome of tyrosine kinase and immune checkpoint inhibitors regime in patients with unresectable hepatocellular carcinoma. J gastrointestinal Oncol. (2023) 14:1837–48. doi: 10.21037/jgo-23-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo Z, Zhu H, Zhang X, Huang L, Wang X, Shi H, et al. The efficacy and safety of conventional transcatheter arterial chemoembolization combined with pd-1 inhibitor and anti-angiogenesis tyrosine kinase inhibitor treatment for patients with unresectable hepatocellular carcinoma: A real-world comparative study. Front Oncol. (2022) 12:941068. doi: 10.3389/fonc.2022.941068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai L, Cai X, Mugaanyi J, Liu Y, Mao S, Lu C, et al. Therapeutic effectiveness and safety of sintilimab-dominated triple therapy in unresectable hepatocellular carcinoma. Sci Rep. (2021) 11:19711. doi: 10.1038/s41598-021-98937-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong JK, Lim HJ, Tam VC, Burak KW, Dawson LA, Chaudhury P, et al. Clinical consensus statement: establishing the roles of locoregional and systemic therapies for the treatment of intermediate-stage hepatocellular carcinoma in Canada. Cancer Treat Rev. (2023) 115:102526. doi: 10.1016/j.ctrv.2023.102526 [DOI] [PubMed] [Google Scholar]
- 44. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: A biomolecular exploratory, randomized, phase iii trial (Fohaic-1). J Clin Oncol Off J Am Soc Clin Oncol. (2022) 40:468–80. doi: 10.1200/jco.21.01963 [DOI] [PubMed] [Google Scholar]
- 45. Xiang X, Lau WY, Wu ZY, Zhao C, Ma YL, Xiang BD, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. (2019) 45:1460–7. doi: 10.1016/j.ejso.2019.03.042 [DOI] [PubMed] [Google Scholar]
- 46. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase iii trial. J Clin Oncol Off J Am Soc Clin Oncol. (2022) 40:150–60. doi: 10.1200/jco.21.00608 [DOI] [PubMed] [Google Scholar]
- 47. Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Trans Hepatol. (2023) 11:480–9. doi: 10.14218/jcth.2022.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. (2021) 12:709060. doi: 10.3389/fphar.2021.709060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu SX, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-pd-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. (2021) 13:1395–405. doi: 10.2217/imt-2021-0192 [DOI] [PubMed] [Google Scholar]
- 50. IBoCMDA SGoID . [Chinese expert consensus on intra-arterial drug and combined drug administration for primary hepatocellular carcinoma]. Zhonghua nei ke za zhi. (2023) 62:785–801. doi: 10.3760/cma.j.cn112138-20230202-00049 [DOI] [PubMed] [Google Scholar]
- 51. Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatol (Baltimore Md). (2018) 67:381–400. doi: 10.1002/hep.29485 [DOI] [PubMed] [Google Scholar]
- 52. Hu Z, Yang Z, Wang J, Fu Y, Hu Z, Zhou Z, et al. Survival benefit of neoadjuvant hepatic arterial infusion chemotherapy followed by hepatectomy for hepatocellular carcinoma with portal vein tumor thrombus. Front Pharmacol. (2023) 14:1223632. doi: 10.3389/fphar.2023.1223632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for hbv-related hepatocellular carcinoma after resection: A randomized controlled study. Clin Cancer Res an Off J Am Assoc Cancer Res. (2018) 24:2074–81. doi: 10.1158/1078-0432.Ccr-17-2899 [DOI] [PubMed] [Google Scholar]
- 54. Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: A propensity score matching study. Eur Radiol. (2018) 28:3522–31. doi: 10.1007/s00330-017-5166-4 [DOI] [PubMed] [Google Scholar]
- 55. Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. (2018) 287:705–14. doi: 10.1148/radiol.2018171541 [DOI] [PubMed] [Google Scholar]
- 56. Lu J, Zhao M, Arai Y, Zhong BY, Zhu HD, Qi XL, et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of international society of multidisciplinary interventional oncology (Ismio). Hepatobiliary Surg Nutr. (2021) 10:661–71. doi: 10.21037/hbsn-21-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu J, Wang P, Shang L, Zhang Z, Tian Y, Chen X, et al. Tace plus tyrosine kinase inhibitors and immune checkpoint inhibitors versus tace plus tyrosine kinase inhibitors for the treatment of patients with hepatocellular carcinoma: A meta-analysis and trial sequential analysis. Hepatol Int. (2024) 18:595–609. doi: 10.1007/s12072-023-10591-0 [DOI] [PubMed] [Google Scholar]
- 58. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022 [DOI] [PubMed] [Google Scholar]
- 59. Wu JY, Zhang ZB, Zhou JY, Ke JP, Bai YN, Chen YF, et al. Outcomes of salvage surgery for initially unresectable hepatocellular carcinoma converted by transcatheter arterial chemoembolization combined with lenvatinib plus anti-pd-1 antibodies: A multicenter retrospective study. Liver Cancer. (2023) 12:229–37. doi: 10.1159/000528356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-pd-1 antibodies, and hepatic arterial infusion chemotherapy: A retrospective study. Front Oncol. (2021) 11:729764. doi: 10.3389/fonc.2021.729764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lao XM, Wang D, Shi M, Liu G, Li S, Guo R, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res Off J Japan Soc Hepatol. (2011) 41:553–63. doi: 10.1111/j.1872-034X.2011.00796.x [DOI] [PubMed] [Google Scholar]
- 62. Miksad RA, Ogasawara S, Xia F, Fellous M, Piscaglia F. Liver function changes after transarterial chemoembolization in us hepatocellular carcinoma patients: the livert study. BMC Cancer. (2019) 19:795. doi: 10.1186/s12885-019-5989-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cho Y, Choi JW, Kwon H, Kim KY, Lee BC, Chu HH, et al. Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the korean liver cancer association. Clin Mol Hepatol. (2023) 29:521–41. doi: 10.3350/cmh.2023.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and safety of tace combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced tace-refractory hepatocellular carcinoma: A retrospective study. Front Mol Biosci. (2020) 7:609322. doi: 10.3389/fmolb.2020.609322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.