Abstract
Chocolate is a rich dietary source of various bioactive flavonoid compounds. Despite being one of the most popular foods worldwide, the association between chocolate consumption and long-term mortality remains unclear. The objective of this study is to determine the associations between chocolate consumption and long-term overall and cause-specific mortality, to evaluate dose–response and potential mediators, and to conduct an updated meta-analysis based on prospective cohort studies. We performed a prospective analysis in the Alpha-Tocopherol, Beta-Carotene cancer prevention (ATBC) Study with a total of 27,111 men who were recruited between 1985 and 1988 and followed through 2015. Exposure data of daily chocolate consumption was obtained from validated baseline food frequency questionnaire. Hazard ratios (HRs) and 30-year absolute risk differences (ARDs) including 95% confidence intervals (CI) for overall and cause-specific mortality were estimated using multivariable-adjusted Cox proportional hazards regression models. An updated meta-analysis of cohort studies was also conducted. During 482,807 person-years of follow-up, a total of 22,064 men died. The multivariable analyses showed a statistically significant inverse association between chocolate consumption and risk of overall mortality, with HRs of 0.91, 0.89, 0.89, and 0.88 for the increasing categories 2–5 as compared with those in the lowest category (Ptrend < 0.0001, and P for nonlinearity < 0.0001). We observed significantly lower mortality from cardiovascular disease (CVD), heart disease and cancer, representing 13%, 16% and 12% risk reductions for the highest compared to lowest chocolate category, respectively (all Ptrend ≤ 0.002; all P for nonlinearity < 0.0001). The inverse associations of chocolate consumption with risk of overall, CVD and heart disease mortality were generally consistent across cohort subgroups (e.g., body mass index and serum cholesterol). Mediation analysis showed that 4.3% of the inverse association of chocolate and overall mortality was mediated through reducing blood pressure. Within the updated meta-analysis of cohort studies (21 risk estimates, 908,390 participants and 65,407 events), greater consumption of chocolate (per 5 g/day) was associated with a lower risk of CVD incidence and mortality (pooled relative risk = 0.98, P value < 0.001; P for nonlinearity < 0.001). The predefined subgroup analyses generally revealed consistent inverse chocolate-CVD risk associations. In this prospective study, calorie-balanced greater consumption of chocolate was inversely associated with lower overall, CVD, heart disease and cancer mortality. The systematic review and meta-analysis provide support for the inverse chocolate-CVD association. Our findings may provide evidence to partially allay concerns regarding adverse health outcomes from low-to-moderate chocolate consumption.
Keywords: Chocolate consumption, Overall mortality, Cause-specific mortality, Multivariate analysis, Mediation analysis, Systematic review, Meta-analysis
Introduction
Being one of the most popular foods worldwide, chocolate is a rich source of various bioactive flavonoid compounds, including catechin, epicatechin, procyanidins and theobromine [1]. Compared to other foods such as tea, apples and red wine, cocoa that mainly consumed as chocolate, contains significantly greater concentrations of flavonoids per serving [2] which has favorable implications for cardiovascular health benefits based on their anti-oxidant, anti-inflammatory, anti-hypertensive and anti-thrombotic properties [3, 4]. In addition, findings from small, short-term intervention trials demonstrated that greater chocolate consumption may improve lipid profiles by increasing HDL cholesterol, reducing LDL cholesterol and intimal peroxidation, and enhancing endothelial function [5, 6].
Despite such long-standing interest in the health effects of chocolate, whether or not to include it in dietary recommendations for cardiovascular disease (CVD) prevention remains controversial. The European Food Safety Authority (EFSA) suggested daily intake of 10 g high-flavanol dark chocolate might contribute to vasodilatation without compromising a balanced diet [7]. On the other hand, the fact that chocolate continues to be considered confectionaries and sweets in several food classification systems, and that lower consumption of this group has been associated with improved health outcomes, has led to dietary recommendations to consume less chocolate [8, 9].
Recent population-based prospective cohort studies and meta-analyses have examined the association between chocolate consumption and CVD risk [1, 8, 10–17]. Some studies observed inverse associations, including for coronary artery disease, coronary heart disease, stroke, and heart failure [1, 8, 10, 17], while others found no association for coronary heart disease, heart failure, stroke, or atrial fibrillation [14, 15]. Even so, data remain sparse for the long-term dose–response association between chocolate consumption and overall and cause-specific mortality risk, and mediation of the hypothesized inverse chocolate-mortality associations by serum biomarkers has not been evaluated.
We conducted the present study in order to comprehensively examine the relationships between chocolate consumption and risk of overall and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study of 27,000 participants followed for up to 30 years. A secondary mediation analysis assessed to what degree the chocolate-mortality associations were mediated through serum anti-oxidants, lipids and blood pressure. We further conducted an update meta-analysis of chocolate consumption and risk of CVD incidence and mortality that incorporate our new results.
Methods
Study population
The ATBC Study is a prospective cohort of 29,133 men who at baseline were 50 to 69 years of age, smoked five or more cigarettes per day, and were enrolled from 14 study centers in southwestern Finland between 1985 and 1988 [18]. Participants were asked to complete questionnaires related to lifestyle and behavioral characteristics, including age, smoking density and duration, physical activity, educational level, and medical history. Height, weight, and blood pressure were measured and overnight fasting blood samples were collected by professional study nurses at the baseline visit, and the blood samples were stored at − 70 °C until further assays. Written informed consent was provided by all participants at enrollment, and the approvals of the ATBC Study have been received from the Institutional Review Boards at the Finnish National Public Health Institute and the U.S. National Cancer Institute.
Assessment of dietary data
Data for usual diet over the preceding 12 months were collected through a self-administrated food frequency questionnaire that included a color picture booklet guide for portion size estimation, and the participants provided information on portion size and frequency for 203 food items and 73 mixed dishes. A previous study examined the reproducibility and validity of our dietary history questionnaire, and reported a range of 0.6–0.7 of intraclass correlation coefficients for most food items, and the correlation coefficients between values of candy intake (which included chocolate) from the food records and the food frequency questionnaire ranging from 0.51–0.59, which reflect good validity and reproducibility [19, 20]. The frequency consumption and portion size of chocolate candy from the dietary questionnaire (Supplemental Fig. 1) were used to calculate the amount of chocolate consumed per day for this study. In total, 93% of participants completed the food frequency questionnaire, leaving 27,111 participants in the final analysis.
Outcome ascertainment
The primary outcomes were all-cause and cause-specific mortality. Vital status was ascertained through linkage with the Causes of Death Registry, Statistics Finland. Details of cause of death are provided in Supplemental Table 1.
Statistical analysis
All the participants were followed from study enrollment in 1985–1988 until death or the end of follow-up (December 31, 2015), whichever occurred first. To control for the influence of total energy intake, we used the nutrient density method and divided all nutrients by total energy intake, and additionally adjusted for energy intake in the regression models. Category (C) 1 included men with no chocolate consumption and categories 2–5 equally divided into fourths of the distribution of daily chocolate consumption (nutrient density approach [unit = gram/kJ]: C2 > 0– < 0.11, C3 0.11– < 0.24, C4 0.24– < 0.47, and C5 ≥ 0.47). We used Cox proportional hazards regression models with person-time as the underlying time-metric and stratified by age group quintiles to examine the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between chocolate consumption (category) and risk of all-cause and cause-specific mortality. For the cause-specific analyses, mortality from causes other than the cause of interest was treated as a censored event at the time of death. The proportional hazards assumption was assessed through likelihood ratio tests that compared models with and without a cross-product interaction term between follow-up time and chocolate consumption (categories). Model 1 was adjusted for age at entry and daily energy intake. The multivariate Model 2 further adjusted for body mass index, cigarettes smoked per day, years of smoking, serum HDL and total cholesterol, intervention assignment, education, physical activity, alcohol consumption, and the Alternate Mediterranean Diet Score (details of which are provided in the Supplemental Table 2). The fully adjusted Model 3 additionally adjusted for systolic and diastolic blood pressure, history of cardiovascular disease and diabetes. Based on the adjusted survival curves and estimated HRs, adjusted absolute risk differences (ARD) were calculated for chocolate consumption at the maximum follow-up of 30 years, and the corresponding 95% CIs were estimated using a bootstrap method (n = 300 bootstrap samples). We used cubic-restricted spline regression to evaluate the possible nonlinear associations between chocolate and overall and cause-specific mortality by selecting four knots at the 5th, 25th, 75th, and 95th percentiles of the daily chocolate consumption. Based on the above-mentioned Cox proportional hazards regression models, we conducted mediation analyses to estimate the portion of the associations of chocolate consumption with risk of overall mortality and mortality from CVD and heart disease that were mediated through concentrations of the serum biomarkers alpha-tocopherol, retinol, total cholesterol, HDL cholesterol, as well as systolic and diastolic blood pressure [21].
We conducted stratified analyses by age at baseline (< 57, or ≥ 57 years, median split), number of cigarettes smoked daily (< 16, 16 to < 20, or ≥ 20), BMI (< 30, or ≥ 30 kg/m2), history of cardiovascular disease at baseline (no, or yes), trial intervention arm (alpha-tocopherol or no alpha-tocopherol; beta-carotene or no beta-carotene), eating a high-quality diet (defined by Alternate Mediterranean Diet Score: no [≤ 28], or yes [> 28]), saturated fatty acid intake (low/medium, or high), serum total cholesterol (< 205, or ≥ 205 mg/dL), serum alpha-tocopherol (< 11.5, or ≥ 11.5 mg/L), serum beta-carotene (< 172, or ≥ 172 μg/L), serum retinol (< 577, or ≥ 577 μg/L), and follow-up period (0 to < 13, 13 to < 23, or ≥ 23 years). Likelihood ratio tests were used to examine these interactions by comparing regression models with or without the interaction terms for the key factors (above categories) and chocolate consumption (5th versus 1st category).
We also conducted three sensitivity analyses. 1) To eliminate the potential influence from reverse causality, lag analyses excluded the first 5 years of follow-up. 2) To minimize the potential biases from preexisting disease on the chocolate consumption, we excluded participants who reported a history of CVD or diabetes at baseline. 3) In order to fully control for fruit and vegetable, we additionally adjusted for fruit and vegetable consumption in the sensitivity analyses.
All reported P values are two-sided at the type I error rate of 0.05. Missing values (less than 5% for any individual covariable) were treated as missing value indicators for each covariate included in the model. Multiple comparisons were controlled by the Bonferroni correction approach, with a threshold of 0.0063 for main analysis (8 outcomes) and 0.0038 for subgroup interaction analysis (13 subgroup tests). All the analyses were performed using SAS software, version 9.4 (SAS Institute Inc.).
Systematic review and meta-analysis of the relationship between chocolate consumption and CVD incidence and mortality
Based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [22], we conducted a systematic search and updated meta-analysis including the current study and all articles published in electronic databases (PubMed, Embase and Web of Science, up to 24 August 2021) that examined the association between chocolate consumption and risk of CVD and CVD mortality. Supplemental Table 3 documented details of the search strategy, and the systematic meta-analysis protocol was registered in the international prospective register of systematic reviews (PROSPERO 2021 CRD42021275088).
Studies were selected if they met the following criteria: prospective cohort studies with adult participants (≥ 18 years); at least 0.5-years duration of follow-up; examination of the association between chocolate consumption and incidence of CVD and CVD mortality; and, provided adjusted relative risks, hazard ratios, or odds ratios with 95% CIs. Information extracted from the eligible articles included: first author name; study population and cohort name; publication year; country where the cohort was established; sample size; follow-up duration; baseline age range of participants; chocolate consumption assessment methods; outcome ascertainment methods; number of endpoints; chocolate consumption categories; relative estimates and 95% CIs from the fully adjusted model; and, covariates included in the multivariable model. Chocolate consumption was quantified by calculating the median or midpoint for each category. When the top category was open (e.g., > 45 g/day), we multiplied the bottom bound of the top category by 1.75. To estimate the relative risk associated with a 5 g/day increment in consumption, the trend for log relative risk was used [23]. Person-years were imputed based on available data when person-years data were not presented for each consumption category [14, 16, 24–27]. When two relevant CVD events were available in one study (e.g., missing a total CVD event parameter), a fixed-effect model meta-analysis was performed to combine the relative risks accordingly [26].
We computed the relative risk of CVD and CVD mortality associated with daily chocolate consumption (per 5 g/day) for each eligible study and conducted random-effects models of meta-analysis for the pooled relative risk. Heterogeneity was assessed using Cochran Q-test and the I2 statistic. Each study was removed from the meta-analysis one at a time, and relative risks were re-computed to identify the influence of individual studies on heterogeneity. In the subgroup analysis, univariate meta-regression analyses based on study-level data were performed to evaluate possible sources of heterogeneity, including prior selected factors of adjustment for the following: sex, hypertension (yes or no), follow-up duration (< 10 or ≥ 10 years), number of events (< 1000 or ≥ 1000), and geographic location. Random effects models were also conducted to calculate the pooled relative risk of CVD for the highest versus the lowest category of chocolate consumption, stratified by BMI, smoking, alcohol consumption, and energy intake.
Egger’s tests and funnel plots were performed to explore potential publication bias; with evidence of publication bias of P < 0.10, we used the trim and fill method to adjust (R, version 4.1.1, meta). We applied the Newcastle–Ottawa Scale to assess the possibility of bias in the included studies. In the meta-analysis of prospective cohort studies, we also examined possible nonlinear dose–response associations using the cubic-restricted spline regression with four knots at the 5th, 25th, 75th, and 95th percentiles of the chocolate consumption per day [28]. Stata version 16.0 was used to conduct the meta-analysis.
Results
Chocolate consumption and lifestyle factors
This analysis included 27,111 men whose mean (SD) age was 57.0 (5.0) years at entry. Table 1 provides baseline characteristics according to categories of chocolate consumption (quartiles 1–4 vs a no chocolate category). Median chocolate consumption (categories 2–5) was 2.7 g per day (interquartile range, 1.3 to 5.7) and the mean (SD) was 4.8 (7.5) g per day. Compared to men with higher chocolate consumption, those in the lowest category (no consumption) tended to be physically inactive, to have higher BMI, lower educational level, and higher systolic blood pressure, and were more likely to have a self-reported history of CVD and diabetes mellitus. Chocolate consumption was also inversely related to alcohol consumption, and positively associated with serum beta-carotene and daily dietary intakes of energy, saturated fatty acids, fruit, vegetables, and the Alternate Mediterranean Diet.
Table 1.
Baseline characteristics of cohort participants by categories of daily chocolate consumption
| Chocolate consumption (categories) |
|||||
|---|---|---|---|---|---|
| 1 (n = 13,234) | 2 (n = 3509) | 3 (n = 3556) | 4 (n = 3331) | 5 (n = 3481) | |
| Chocolate consumption, g/day | 0 (0) | 0.87 (0.31) | 2.00 (0.80) | 3.83 (1.36) | 12.4 (11.9) |
| Age at baseline, y | 57.6 (5.1) | 56.5 (4.8) | 56.7 (4.9) | 56.8 (4.9) | 57.3 (5.1) |
| Cigarettes/day | 20.5 (8.8) | 20.2 (8.4) | 20.1 (8.7) | 20.2 (8.9) | 20.8 (9.2) |
| Years of smoking | 36.4 (8.4) | 35.0 (8.4) | 35.4 (8.2) | 35.4 (8.4) | 36.0 (8.5) |
| Systolic blood pressure, mm Hg | 143 (21) | 141 (19) | 141 (24) | 141 (24) | 140 (19) |
| Diastolic blood pressure, mm Hg | 88 (14) | 87 (11) | 88 (19) | 88 (19) | 87 (19) |
| Serum total cholesterol, mmol/L | 6.22 (1.19) | 6.30 (1.16) | 6.29 (1.14) | 6.25 (1.13) | 6.22 (1.12) |
| Serum HDL cholesterol, mmol/L | 1.35 (3.72) | 1.24 (2.35) | 1.32 (3.68) | 1.27 (2.95) | 1.40 (4.69) |
| Serum alpha-tocopherol, mg/L | 11.8 (3.83) | 12.0 (3.19) | 12.1 (3.29) | 12.1 (3.36) | 12.1 (3.35) |
| Serum beta-carotene, μg/L | 199 (178) | 217 (167) | 225 (168) | 227 (190) | 239 (237) |
| Serum retinol, mg/L | 588 (132) | 588 (130) | 590 (126) | 590 (124) | 586 (129) |
| BMI, kg/m2 | 26.5 (4.31) | 26.5 (4.2) | 26.3 (3.83) | 26.3 (4.61) | 25.6 (3.53) |
| Education, %, > elementary school | 16.0 | 16.7 | 24.6 | 30.0 | 37.6 |
| Physically active, % | 18.8 | 23.7 | 24.0 | 23.4 | 21.6 |
| History of CVD, % | 43.7 | 39.0 | 41.0 | 38.6 | 38.6 |
| History of diabetes mellitus, % | 6.5 | 2.5 | 1.8 | 2.0 | 1.6 |
| Daily dietary intake | |||||
| Energy, kcal | 2569 (730) | 2928 (743) | 2809 (788) | 2748 (734) | 2722 (748) |
| Saturated fatty acids, g | 49.9 (21.6) | 58.6 (22.1) | 54.9 (22.2) | 53.2 (21.0) | 53.6 (21.1) |
| Alcohol, g | 18.9 (22.7) | 17.7 (22.2) | 17.4 (20.5) | 17.0 (19.0) | 16.5 (19.8) |
| Fruit, g | 111 (94) | 140 (104) | 144 (105) | 149 (105) | 153 (112) |
| Vegetables, g | 104 (68) | 119 (70) | 124 (71) | 123 (70) | 123 (76) |
| Red meat, g | 68.7 (34.5) | 75.8 (34.0) | 74.2 (32.5) | 73.4 (32.3) | 71.6 (33.6) |
| Alternate Mediterranean Diet Score | 24.7 (5.2) | 24.7 (4.9) | 25.3 (5.0) | 25.5 (5.0) | 25.2 (4.9) |
The values are presented as means (SD) or percentage as noted. Category 1 was no chocolate consumption, and categories 2–5 were then equally divided into quartiles. Category (C) 1 included men with no chocolate consumption and categories 2–5 equally divided into fourths of the distribution of daily chocolate consumption (divided by energy intake [unit = gram/kJ]: C2 > 0–≤0.11, C3 0.11–≤0.24, C4 0.24–≤0.47, and Q5 > 0.47)
BMI body mass index, CVD cardiovascular disease, SD standard deviation
Chocolate consumption and overall and cause-specific mortality
During 482,807 person-years of follow-up (31 years of follow-up), there were 22,064 deaths, including 9121 due to CVD (7457 from heart disease and 1625 from stroke), 7224 from cancer, 1982 from respiratory disease, 1143 from injuries and accidents, and 2594 related to all other causes combined. After controlling for several potential confounding factors using Model 2, the multivariable analysis showed a statistically significant inverse association of chocolate consumption with risk of overall mortality, with HRs of 0.90, 0.87, 0.87, and 0.86 for categories 2–5 as compared with those in the lowest category (Ptrend < 0.0001). The findings remained unchanged when we further adjusted for CVD related risk factors including systolic and diastolic blood pressure, history of cardiovascular disease and diabetes (Model 3), with the HRs of 0.91, 0.89, 0.89, and 0.88 for categories 2–5 as compared with those in the lowest category (Table 2).
Table 2.
Risk of overall and cause-specific mortality associated with daily chocolate consumption in a prospective cohort study
| Causes of mortality | Chocolate consumption (categories) |
p for trenda | ||||
|---|---|---|---|---|---|---|
| 1 (n = 13,234) |
2 (n = 3509) HR (95% CI) |
3 (n = 3556) HR (95% CI) |
4 (n = 3331) HR (95% CI) |
5 (n = 3481) HR (95% CI) |
||
| All-cause | ||||||
| Deaths, n | 11,241 | 2767 | 2766 | 2578 | 2712 | |
| Model 1 | 1.00 | 0.86 (0.82, 0.89) | 0.83 (0.79, 0.86) | 0.82 (0.79, 0.86) | 0.81 (0.78, 0.85) | < 0.0001 |
| Model 2 | 1.00 | 0.90 (0.86, 0.94) | 0.87 (0.84, 0.91) | 0.87 (0.83, 0.91) | 0.86 (0.82, 0.90) | < 0.0001 |
| Model 3 | 1.00 | 0.91 (0.87, 0.95) | 0.89 (0.85, 0.93) | 0.89 (0.86, 0.93) | 0.88 (0.85, 0.92) | < 0.0001 |
| CVD | ||||||
| Deaths, n | 4777 | 1129 | 1135 | 1008 | 1072 | |
| Model 1 | 1.00 | 0.83 (0.78, 0.89) | 0.81 (0.76, 0.86) | 0.77 (0.72, 0.82) | 0.76 (0.71, 0.82) | < 0.0001 |
| Model 2 | 1.00 | 0.87 (0.81, 0.93) | 0.85 (0.80, 0.91) | 0.81 (0.76, 0.87) | 0.83 (0.78, 0.89) | < 0.0001 |
| Model 3 | 1.00 | 0.90 (0.84, 0.96) | 0.89 (0.83, 0.95) | 0.85 (0.79, 0.91) | 0.87 (0.82, 0.94) | 0.0002 |
| Heart disease | ||||||
| Deaths, n | 3939 | 910 | 941 | 822 | 845 | |
| Model 1 | 1.00 | 0.81 (0.76, 0.88) | 0.81 (0.76, 0.87) | 0.76 (0.70, 0.82) | 0.73 (0.68, 0.79) | < 0.0001 |
| Model 2 | 1.00 | 0.85 (0.79, 0.92) | 0.86 (0.80, 0.93) | 0.80 (0.75, 0.87) | 0.80 (0.74, 0.86) | < 0.0001 |
| Model 3 | 1.00 | 0.88 (0.82, 0.95) | 0.90 (0.84, 0.97) | 0.84 (0.78, 0.91) | 0.84 (0.78, 0.91) | < 0.0001 |
| Stroke | ||||||
| Deaths, n | 819 | 215 | 190 | 182 | 219 | |
| Model 1 | 1.00 | 0.92 (0.79, 1.07) | 0.78 (0.67, 0.92) | 0.80 (0.68, 0.94) | 0.90 (0.77, 1.04) | 0.17 |
| Model 2 | 1.00 | 0.95 (0.82, 1.11) | 0.82 (0.70, 0.96) | 0.84 (0.71, 0.99) | 0.95 (0.81, 1.10) | 0.49 |
| Model 3 | 1.00 | 0.99 (0.85, 1.15) | 0.85 (0.73, 1.00) | 0.88 (0.74, 1.03) | 1.00 (0.86, 1.16) | 0.97 |
| Cancer | ||||||
| Deaths, n | 3573 | 957 | 905 | 898 | 891 | |
| Model 1 | 1.00 | 0.93 (0.86, 1.00) | 0.85 (0.79, 0.92) | 0.90 (0.84, 0.97) | 0.85 (0.79, 0.91) | < 0.0001 |
| Model 2 | 1.00 | 0.97 (0.90, 1.04) | 0.89 (0.83, 0.96) | 0.95 (0.88, 1.02) | 0.87 (0.81, 0.94) | 0.0009 |
| Model 3 | 1.00 | 0.97 (0.90, 1.05) | 0.90 (0.84, 0.97) | 0.95 (0.88, 1.03) | 0.88 (0.82, 0.95) | 0.002 |
| Respiratory disease | ||||||
| Deaths, n | 1014 | 221 | 239 | 242 | 266 | |
| Model 1 | 1.00 | 0.78 (0.67, 0.90) | 0.80 (0.70, 0.92) | 0.87 (0.76, 1.00) | 0.88 (0.77, 1.01) | 0.19 |
| Model 2 | 1.00 | 0.84 (0.72, 0.97) | 0.85 (0.74, 0.98) | 0.93 (0.80, 1.07) | 0.88 (0.77, 1.02) | 0.20 |
| Model 3 | 1.00 | 0.84 (0.73, 0.97) | 0.85 (0.74, 0.98) | 0.93 (0.81, 1.07) | 0.89 (0.77, 1.02) | 0.24 |
| Injuries and accidents | ||||||
| Deaths, n | 597 | 147 | 126 | 126 | 147 | |
| Model 1 | 1.00 | 0.81 (0.68, 0.97) | 0.69 (0.57, 0.83) | 0.74 (0.61, 0.90) | 0.83 (0.69, 0.99) | 0.07 |
| Model 2 | 1.00 | 0.86 (0.71, 1.03) | 0.72 (0.60, 0.88) | 0.79 (0.65, 0.96) | 0.87 (0.73, 1.05) | 0.22 |
| Model 3 | 1.00 | 0.86 (0.72, 1.03) | 0.73 (0.60, 0.88) | 0.79 (0.65, 0.96) | 0.88 (0.73, 1.06) | 0.25 |
| Other causes | ||||||
| Deaths, n | 1280 | 313 | 361 | 304 | 336 | |
| Model 1 | 1.00 | 0.83 (0.73, 0.94) | 0.91 (0.81, 1.02) | 0.82 (0.72, 0.93) | 0.85 (0.75, 0.96) | 0.01 |
| Model 2 | 1.00 | 0.87 (0.77, 0.99) | 0.95 (0.84, 1.07) | 0.86 (0.76, 0.97) | 0.88 (0.78, 1.00) | 0.06 |
| Model 3 | 1.00 | 0.89 (0.79, 1.01) | 0.98 (0.87, 1.11) | 0.89 (0.78, 1.01) | 0.91 (0.81, 1.03) | 0.18 |
Model 1: adjusted for age at entry and daily dietary total energy. Model 2: adjusted for age at entry, body mass index, cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, education, physical activity, daily dietary total energy, alcohol, and Alternate Mediterranean Diet Score. Model 3: further adjusted for systolic and diastolic blood pressure, history of cardiovascular disease, diabetes in the models. Category (C) 1 was participants who reported no chocolate consumption, categories 2–5 were equally divided into quartiles (divided by energy intake [unit = gram/kJ]: C2 > 0–≤0.11, C3 0.11–≤0.24, C4 0.24–≤0.47, and C5 > 0.47)
CVD cardiovascular disease, HDL high-density lipoprotein, HR hazard ratio, SD standard deviation
P value for trend was tested according to the statistical significance of the coefficient of category variable (median value for the categories)
After adjustment for several potential confounding factors in the Model 2, the inverse associations of chocolate were observed for CVD, heart disease and cancer mortality, representing 17%, 20% and 13% risk reductions in participants for those in the highest category of daily consumption compared with the lowest, respectively (all Ptrend ≤ 0.0009). The findings remained relatively unchanged when we further adjusted for CVD related risk factors (Model 3), representing 13%, 16% and 12% risk reductions in participants for those in the highest category compared with the lowest, respectively (all Ptrend ≤ 0.002; Table 2). By contrast, we found no significant linear associations between chocolate consumption and risk of mortality from stroke, respiratory disease, injuries and accidents, and other causes (Table 2).
We also conducted a 4-knot restricted cubic spline analysis that modeled chocolate consumption as a continuous variable to examine dose–response associations and found that overall, CVD and heart disease mortality significantly decreased until daily consumption reached 2 g and then remained stable (Fig. 1). No association of chocolate consumption was found for stroke mortality in this dose–response analysis (Fig. 1).
Fig. 1.
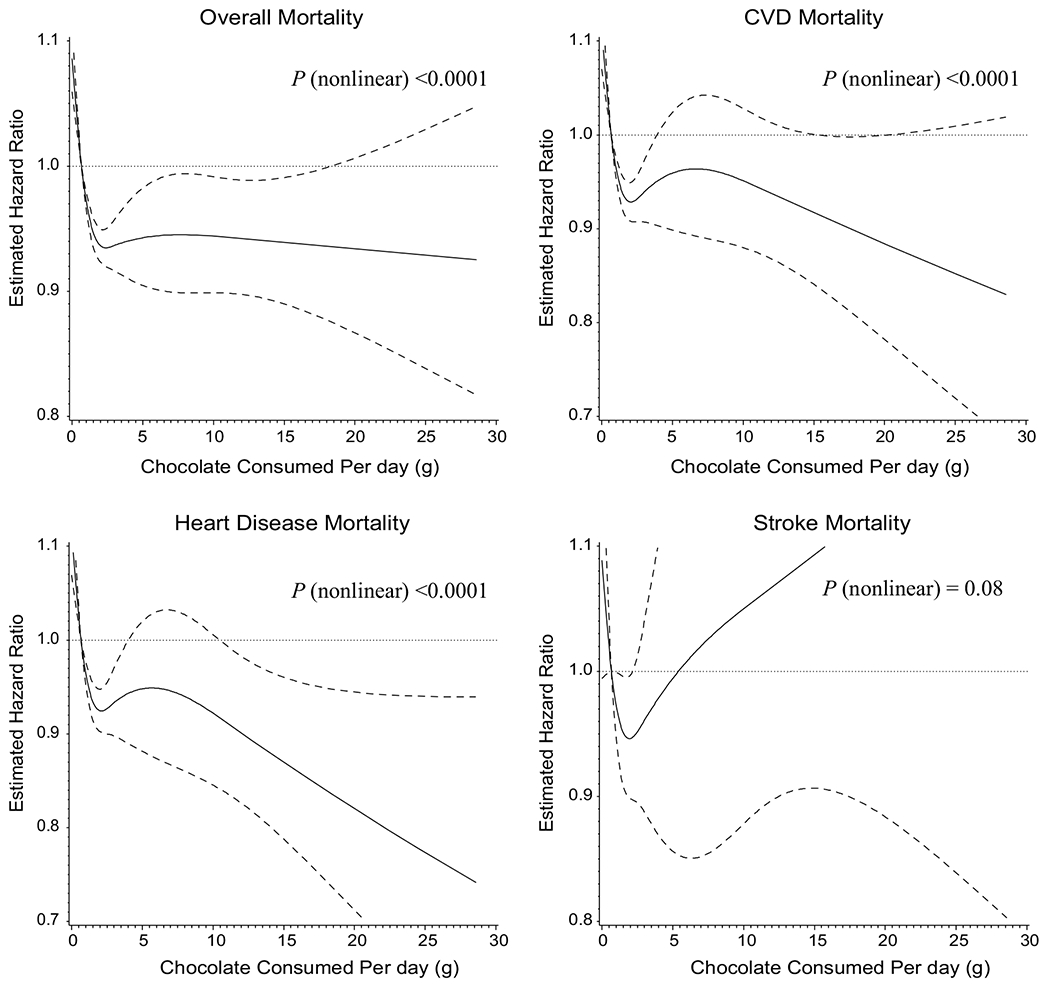
Risk of overall and cause-specific mortality according to daily chocolate consumption using cubic spline regression model. a Overall mortality. b Cardiovascular disease (CVD) mortality. c Heart disease mortality. d Stroke mortality. The solid line denotes the HR of mortality according to chocolate consumption with knot spline (four knots were selected at the 5th, 25th, 75th, and 95th percentiles of chocolate consumption; dashed lines were used to represent the 95% CIs). There were 27,111 participants in the final analyses. The number of events is 22,064, 9121, 7457 and 1625 for overall-, CVD-, heart disease- and stroke-mortality, respectively
Table 3 provides results from the mediation analyses of chocolate consumption that showed 4.3% and 1.5% of the chocolate-overall mortality, 4.1% and 1.4% of chocolate-CVD mortality, and 3.2% and 1.0% of the chocolate-heart disease mortality associations were mediated through systolic and diastolic blood pressure, respectively. By contrast, serum alpha-tocopherol (vitamin E) and total cholesterol played marginal roles and accounted for only 3.1% and 1.3% of the association with overall mortality. Other serum biomarkers we examined including retinol (vitamin A) and HDL cholesterol were unrelated to the chocolate-mortality association.
Table 3.
Mediating influence of serum biomarkers and blood pressure in the associations of daily chocolate consumption with risk of overall mortality
| Serum biomarkers or blood pressure | 1-SD increment HR (95% CI) | Proportion (%) of effect due to mediation through serum biomarkers or blood pressure (95% CIs) | P value for mediation |
|---|---|---|---|
| All-cause mortality | |||
| Model 2 | 0.98 (0.97, 0.99) | – | |
| Model 2 + Serum alpha-tocopherol | 0.98 (0.97, 0.99) | 3.1 (0.7, 12.9) | 0.087 |
| Model 2 + Serum retinol | 0.98 (0.97, 0.99) | None | – |
| Model 2 + Serum total cholesterol | 0.98 (0.97, 0.99) | 1.3 (0.2, 6.6) | 0.12 |
| Model 2 + Serum HDL cholesterol | 0.98 (0.97, 0.99) | None | – |
| Model 2: including systolic blood pressure | 0.98 (0.97, 0.99) | 4.3 (1.6, 11.1) | 0.0049 |
| Model 2: including diastolic blood pressure | 0.98 (0.97, 0.99) | 1.5 (0.5, 4.2) | 0.015 |
| CVD mortality | |||
| Model 2 | 0.96 (0.94, 0.99) | – | |
| Model 2 + Serum alpha-tocopherol | 0.97 (0.94, 0.99) | None | – |
| Model 2 + Serum retinol | 0.97 (0.94, 0.99) | None | – |
| Model 2 + Serum total cholesterol | 0.97 (0.94, 0.99) | None | – |
| Model 2 + Serum HDL cholesterol | 0.96 (0.94, 0.99) | None | – |
| Model 2: including systolic blood pressure | 0.96 (0.94, 0.99) | 4.1 (1.5, 10.5) | 0.0046 |
| Model 2: including diastolic blood pressure | 0.96 (0.94, 0.99) | 1.4 (0.5, 4.3) | 0.015 |
| Heart disease mortality | |||
| Model 2 | 0.95 (0.93, 0.98) | – | |
| Model 2 + Serum alpha-tocopherol | 0.95 (0.93, 0.98) | None | – |
| Model 2 + Serum retinol | 0.95 (0.93, 0.98) | None | – |
| Model 2 + Serum total cholesterol | 0.95 (0.93, 0.98) | None | – |
| Model 2 + Serum HDL cholesterol | 0.95 (0.93, 0.98) | None | – |
| Model 2: including systolic blood pressure | 0.95 (0.93, 0.98) | 3.2 (1.2, 8.0) | 0.0061 |
| Model 2: including diastolic blood pressure | 0.95 (0.93, 0.98) | 1.0 (0.3, 3.2) | 0.022 |
Model 2: adjusted for age at entry, body mass index, cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, and daily dietary total energy, alcohol, and Alternate Mediterranean Diet Score
Serum alpha-tocopherol, mg/L; serum retinol, μg/L; serum total cholesterol, mmol/L; serum HDL cholesterol, mmol/L; systolic and diastolic blood pressure, mm
Chocolate consumption and overall mortality in cohort subgroups
Figure 2 and Supplemental Figs. 2, 3 present findings from stratified analyses of key cohort subgroups mutually adjusted for several confounding factors (Model 3). The HRs of mortality for men in the highest category of chocolate consumption compared with those in the lowest category showed generally similar associations for overall, CVD and heart disease mortality across subgroups of age, smoking intensity, BMI, history of cardiovascular disease, trial intervention arm, consumption of a high-quality diet, saturated fat intake, serum concentrations of total cholesterol, alpha-tocopherol, serum beta-carotene and retinol, and length of follow-up. One exception was a stronger inverse association for overall mortality among men who were younger than 57 years at baseline (HR = 0.81, 95% CI, 0.75 to 0.87; the corresponding adjusted ARD = − 1.95%, 95% CI, − 3.09% to − 0.92%; men with age ≥ 57 years at baseline: HR = 0.94, 95% CI, 0.89 to 1.00; the corresponding adjusted ARD = − 0.20%, 95% CI, − 0.70% to 0.31%; Pinteraction = 0.001; Fig. 2).
Fig. 2.
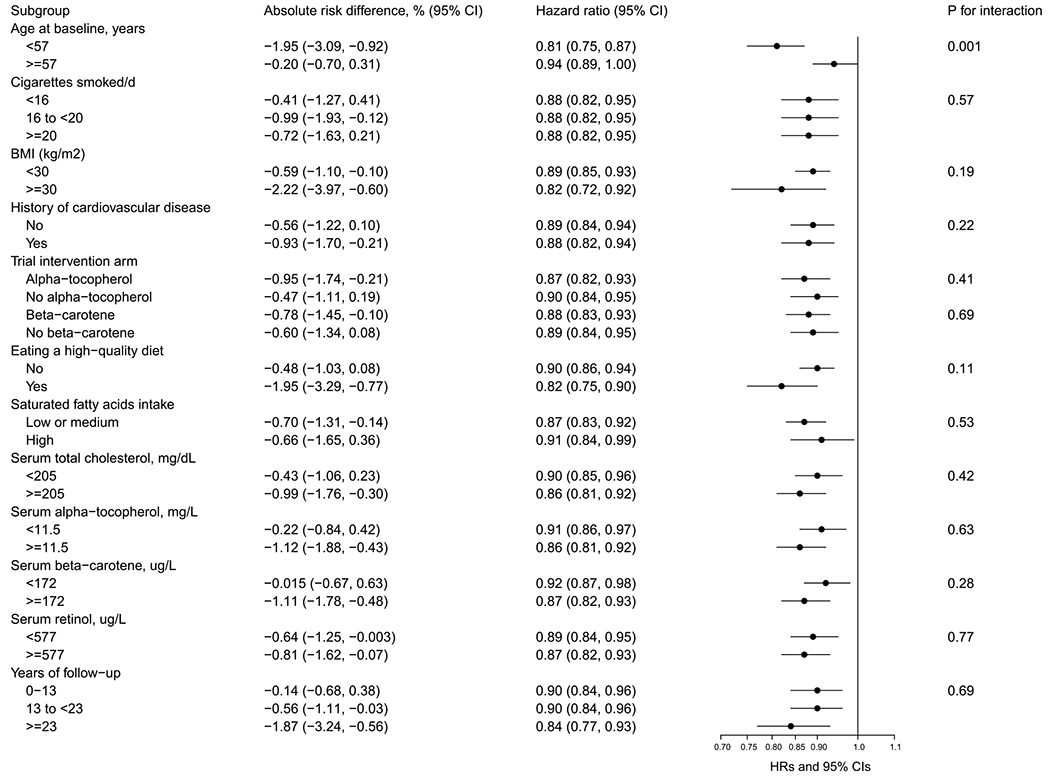
Risk of overall mortality associated with daily chocolate consumption by selected factors in the ATBC study. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention; HDL = high-density lipoprotein, HR = hazard ratio. Multivariable models were adjusted for age at entry, body mass index, cigarettes smoked per day, years of smoking, serum HDL cholesterol, intervention assignment, systolic and diastolic blood pressure, history of cardiovascular disease, diabetes, education, physical activity, and daily dietary total energy, alcohol, and Alternate Mediterranean Diet Score. Absolute risk differences and hazard ratios of overall mortality are for the 5th versus 1st category of chocolate consumption per day
Sensitivity analyses of chocolate consumption and overall and cause-specific mortality
Our findings remained unchanged when we excluded the first 5 years of cohort follow-up (overall mortality, fifth versus first category: HR = 0.88, 95% CI: 0.84, 0.92; Ptrend < 0.0001, Supplemental Table 4) or we excluded 11,626 men with a history of CVD or diabetes at baseline (overall mortality, fifth versus first category: HR = 0.89, 95% CI: 0.84, 0.95; Ptrend = 0.001, Supplemental Table 5). Similarly, the risk estimates were not materially altered when we additionally adjusted for fruit and vegetable consumption (overall mortality, fifth versus first category: HR = 0.89, 95% CI: 0.85, 0.93; Ptrend < 0.0001, Supplemental Table 6).
Systematic review and meta-analysis
Our initial search identified 334 articles after exclusion of duplicate studies. Following screening titles and abstracts, 55 articles were reviewed in full, of which 19 were included in the meta-analysis (Supplemental Table 3) [3, 14–17, 24–27, 29–38]. Supplemental Fig. 4 portrays the study selection process.
Characteristics of the studies included in the analysis are reported in Supplemental Tables 7 and 8. Of the 19 studies, 12 were from Europe, 5 from North America (United States), 1 from Asia (Japan), and 1 from Oceania (Australia). Based on the Newcastle–Ottawa quality assessment scale, 13 studies (including the present study) received a score of seven or eight, indicating a low risk of bias (Supplemental Table 9).
The 19 studies of chocolate consumption and risk of CVD and CVD mortality encompassed 21 risk estimates, 908,390 participants and 65,407 events. The summary effect size of CVD risk and mortality for consumption of 5 g additional chocolate per day was 0.98 (95% CI: 0.98, 0.99). We found significant heterogeneity between studies for CVD risk (I2 = 65.2%, P < 0.001; Fig. 3), although no single study disproportionately accounted for it (Supplemental Fig. 5). Visual inspection of funnel plot and Egger’s linear regression test demonstrated evidence of a possible publication bias (P value for Egger’s test = 0.001; Supplemental Fig. 6), whereas trim and fill method adjusted risk estimates suggested the results from meta-analysis were not remarkably influenced by publication bias with no change on the overall risk estimate (adjusted relative risk = 0.988, 95% CI: 0.976, 0.999; P = 0.04; Supplemental Fig. 7).
Fig. 3.
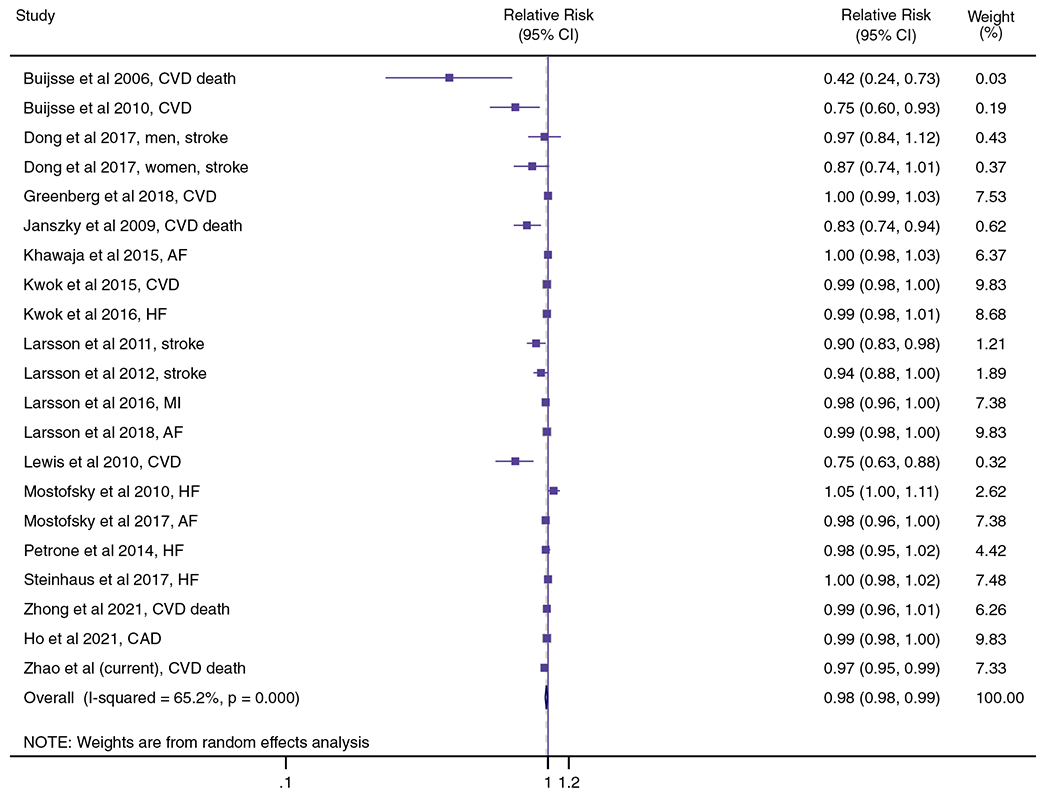
Association between chocolate consumption (per 5 g/day) and risk of CVD incidence and mortality using random effects meta-analysis. Squares denote study-specific relative risk. Grey square areas are proportional to each single study that weighted to the overall meta-analysis. Horizontal lines represent 95% CI. I2 reflects the heterogeneity among studies. AF = atrial fibrillation; BMI = body mass index; CAD = coronary artery disease; CHD = coronary heart disease; CVD = cardiovascular disease; HF = heart failure; MI = myocardial infarction
The predefined subgroup analyses showed consistent inverse chocolate-CVD risk associations in most subgroup comparisons, with the exception of smoking status (P for interaction = 0.02) where the inverse association appeared stronger in non-smoking participants (pooled relative risk = 0.77, 95% CI: 0.69, 0.86; I2 = 37.3%) as compared with current or former smoking participants (pooled relative risk = 0.89, 95% CI: 0.85, 0.93; I2 = 33.0%; Supplemental Tables 10 and 11). In the meta-analysis of 19 cohort studies, we performed a 4-knot restricted cubic spline regression analysis which found that CVD risk and CVD mortality decline with increasing daily chocolate consumption only up to 5 g, with relatively stable risk and mortality across higher consumption levels (Fig. 4).
Fig. 4.
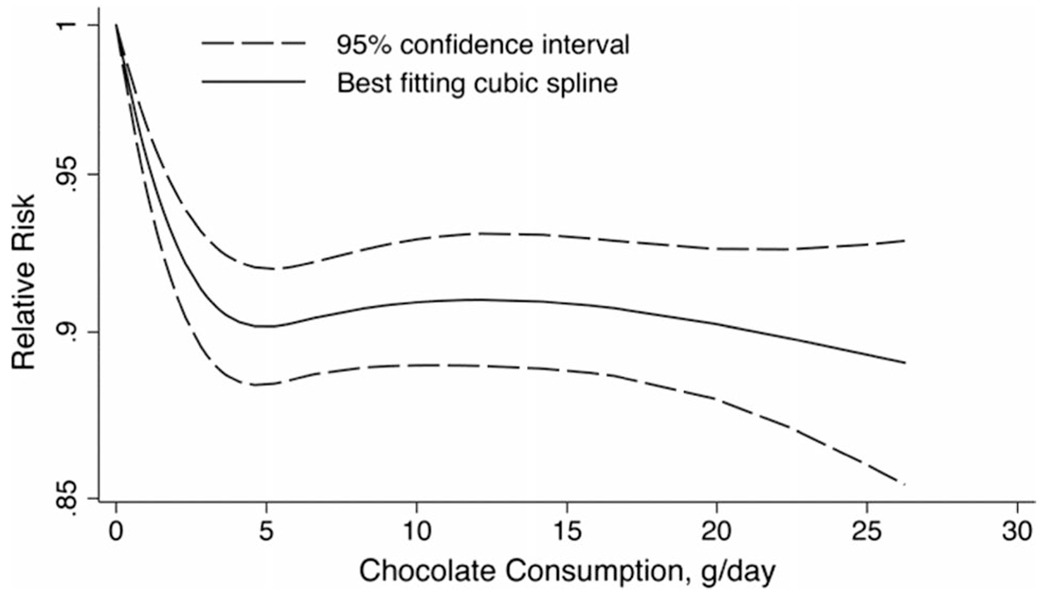
Association between daily chocolate consumption and risk of cardiovascular disease using a cubic spline model. The solid line denotes the relative risk of cardiovascular disease according to chocolate consumption, and the spline based on four knots selected at the 5th, 25th, 75th, and 95th percentiles of chocolate consumption; dashed lines were used to represent the 95% CIs (Pnon-linearity < 0.001). The analysis included 878,990 participants from 19 cohort studies
Discussion
In this large prospective Finnish cohort study with 30 years of follow-up, we found statistically significant inverse associations between chocolate consumption and overall mortality and mortality from cancer, CVD and heart disease (but not stroke), with a 12% risk reduction overall for participants having the highest (versus no) chocolate consumption. The inverse association was generally unchanged across key cohort subgroups but was somewhat stronger among younger men. Interestingly, the mediation analysis showed that 4.3% (1.6% to 11.1%) of the chocolate-overall mortality and 4.1% (1.5% to 10.5%) of the chocolate-CVD mortality associations were mediated through systolic blood pressure. Findings from this updated meta-analysis provided further evidence of a significant inverse association between chocolate consumption and CVD risk and CVD mortality, although considerable heterogeneity was observed between studies (I2 = 65.2%).
The restricted cubic spline analysis showed that as the consumption of chocolate increased, overall and CVD mortality was reduced, with the non-linear pattern of a mortality nadir for chocolate consumption at a modest dose of 2 g/day which might also reflect the frequency of consumption (i.e., 14 g once a week). In the meta-analysis of 19 studies, our results suggest a threshold association for CVD risk and CVD mortality, without additional benefit found beyond 5 g/day. This requires reexamination of the amount of daily chocolate consumption for the maximal risk reduction in other more diverse populations. Relatively few studies have evaluated the association between chocolate consumption and cause-specific mortality. The Leisure World Cohort Study of more than 13,000 U.S. participants showed that infrequent consumption of a few times/months or less (versus none) was associated with 6% reduced risk of mortality, while there was no association for frequent use (i.e., daily or at least a few times/week) [39]. Regarding CVD risk, a meta-analysis of 23 cohort studies representing 405,304 participants showed that chocolate consumption of less than 100 g/week (versus no consumption) was associated with lower risk [11], consistent with another recent meta-analysis that found small inverse associations for risk of coronary heart disease (n = nine studies) and stroke (n = eight studies) (4% and 10%, respectively), but not for heart failure (n = six studies) or type 2 diabetes (n = six studies; for each 10 g/day increase consumption) [8]. By contrast, the Women’s Health Initiative (WHI) cohort of 83,310 women followed for 13.4 years showed no association between chocolate and coronary heart disease or stroke risk [14]. There are relatively few studies of chocolate consumption and cancer mortality, with the WHI and PLCO studies not finding associations for cancer risk or mortality [27, 40]. By contrast, we observed a weak chocolate-cancer mortality association that is consistent with findings for the association between flavonoid intake and cancer-related mortality in the Danish Diet Cancer and Health Cohort [41]. In the Danish study, the authors reported that the threshold of flavonoid intake for the protective association was higher for cancer-related mortality (approximately 1000 mg/day) than for CVD mortality (500 mg/day). This association pattern is similar to that observed in the present study; i.e., an inverse chocolate-CVD mortality association for participants across the consumption categories of 2 to 5 (compared to the lowest), while the inverse association with cancer mortality appeared only for participants in the highest consumption category, suggesting a higher consumption threshold for cancer mortality reduction.
Underlying biological mechanisms for the observed inverse chocolate-CVD mortality associations are not known, although beneficial roles for some ingredients of chocolate products have been proposed. Polyphenolic flavanols are the primary bioactive compounds present in cocoa extracts [10], and evidence accumulating from laboratory experiments and clinical trials suggests mechanisms relevant to cardiometabolic health, including antioxidative and anti-inflammatory effects of flavonols and other constituents [6, 12, 42–44]. Greater flavanol intake may also decrease platelet activation and adhesion, of relevance to the pathogenesis of atherothrombosis [45–47]. A comprehensive meta-analysis of 42 acute or short-term randomized controlled trials demonstrated that chocolate, cocoa and flavanol intake can beneficially impact flow-mediated vasodilatation, increase HDL cholesterol, and decrease diastolic blood pressure, triglycerides, and mean arterial pressure [5]. In addition, findings from in vivo and in vitro studies suggest that flavonoids can exert antioxidant activities through regulation of nitrous oxide (NO) metabolism to improve endothelial NO synthase expression and activity and reduce reactive oxygen species [43, 48–50]. Decreased inflammatory cell infiltration and cytokines through the suppression of NF-κB signaling pathway has also been shown [51–53]. Aside from flavonoids, other bioactive substances in chocolate may also exert beneficial cardiovascular effects; for example, theobromine and beta-carotene. Theobromine appears to decrease blood pressure and LDL cholesterol and increase HDL cholesterol concentrations [8, 54, 55]. In the present study, our data provide evidence supporting the hypothesis that systolic and diastolic blood pressure may play a mediating role also in the observed inverse associations of chocolate with overall and CVD mortality (overall mortality: 1.6% to 11.1% for systolic blood pressure, and 0.5% to 4.2% for diastolic blood pressure). This is consistent with earlier findings showing that chocolate consumption was associated with reduced CVD risk in part by decreasing blood pressure [3, 56].
Strengths of our study include the large sample size and number of events, completeness of serum biomarkers measured within the cohort, and long-term follow-up with full ascertainment of cause-specific mortality through national registries. These study qualities enabled a robust examination of chocolate-cause-specific mortality associations and effect modification by population characteristics and risk factors, and permitted mediation analyses for important serum biomarkers as well as systolic and diastolic blood pressure. The large sample size of our meta-analysis afforded greater statistical power than do single studies (including our own), including for quantitative examination of the chocolate-CVD association in important population subgroups where we found generally similar risk estimates across strata of sex, hypertension, cohort geographic location, event numbers, and follow-up duration. Limitations of the study should also be noted. First, our dietary data for chocolate consumption was self-reported, but any measurement error from nondifferential misclassification would likely underestimate the associations (i.e., bias toward the null). Also, the associations remained materially stable when the follow-up was divided into early and later event periods. Second, despite the multivariable adjustment for multiple risk factors, we cannot completely preclude the possibility of potential residual confounding due to the observational nature of the study design. However, if the residual confounding from healthy lifestyle factors were responsible for the observed associations, we would not find the outcome specificity. Additionally, our findings are observational and do not represent cause and effect (i.e., causality). Third, generalization of the study findings to other populations might be limited due to the study having enrolled only male smokers of European ancestry. Given the fact that our study is based on a cohort with low-to-moderate chocolate consumption, generalization of our findings of benefit to substantially greater consumption should be limited, and requires re-examination in other populations.
In summary, this large prospective cohort analysis found that calorie-balanced greater chocolate consumption was significantly associated with lower risk of overall, CVD, heart disease and cancer mortality. The inverse chocolate-mortality associations were generally homogeneous across key subgroup factors and were partially mediated by reducing systolic and diastolic blood pressure. The systematic review and updated meta-analysis provide more comprehensive evidence and lends support to the inverse chocolate-CVD association. The optimal consumption level and maximal risk reduction should be further explored. Our findings may provide evidence to partially allay concerns regarding adverse health outcomes from low-to-moderate chocolate consumption, and these findings should be replicated in additional prospective studies.
Supplementary Material
Acknowledgements
We thank participants of the ATBC Study cohort for their contributions to this research.
Funding
The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10654-022-00858-5.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Gong F, Yao S, Wan J, Gan X. Chocolate consumption and risk of heart failure: a meta-analysis of prospective studies. Nutrients. 2017;9(4). 10.3390/nu9040402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51(25):7292–5. 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 3.Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31(13):1616–23. 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 4.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008;88(1):58–63. 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95(3):740–51. 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 6.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141(11):1982–8. 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 7. https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2012.2809 .
- 8.Morze J, Schwedhelm C, Bencic A, et al. Chocolate and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur J Nutr. 2020;59(1):389–97. 10.1007/s00394-019-01914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 dietary guidelines for Americans. 8th Edition. December 2015. https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015
- 10.Krittanawong C, Narasimhan B, Wang Z, et al. Association between chocolate consumption and risk of coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2020;2047487320936787. 10.1177/2047487320936787 [DOI] [PubMed] [Google Scholar]
- 11.Ren Y, Liu Y, Sun XZ, et al. Chocolate consumption and risk of cardiovascular diseases: a meta-analysis of prospective studies. Heart. 2019;105(1):49—55. 10.1136/heartjnl-2018-313131. [DOI] [PubMed] [Google Scholar]
- 12.Veronese N, Demurtas J, Celotto S, et al. Is chocolate consumption associated with health outcomes? An umbrella review of systematic reviews and meta-analyses. Clin Nutr. 2019;38(3):1101–8. 10.1016/j.clnu.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Kwok CS, Gulati M, Michos ED, et al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol. 2019;26(13):1415–29. 10.1177/2047487319843667. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg JA, Manson JE, Neuhouser ML, et al. Chocolate intake and heart disease and stroke in the Women’s Health Initiative: a prospective analysis. Am J Clin Nutr. 2018;108(1):41–8. 10.1093/ajcn/nqy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Drca N, Jensen-Urstad M, Wolk A. Chocolate consumption and risk of atrial fibrillation: two cohort studies and a meta-analysis. Am Heart J. 2018;195:86–90. 10.1016/j.ahj.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Kwok CS, Boekholdt SM, Lentjes MA, et al. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart. 2015;101(16):1279–87. 10.1136/heartjnl-2014-307050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrone AB, Gaziano JM, Djousse L. Chocolate consumption and risk of heart failure in the Physicians’ Health Study. Eur J Heart Fail. 2014;16(12):1372–6. 10.1002/ejhf.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 19.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am J Epidemiol. 1988;128(3):667–76. 10.1093/oxfordjournals.aje.a115014 [DOI] [PubMed] [Google Scholar]
- 20.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–66. 10.1093/oxfordjournals.aje.a115013 [DOI] [PubMed] [Google Scholar]
- 21.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE Macro. MA: Harvard T.H. Chan School of Public Health; 2012. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 24.Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med. 2009;266(3):248–57. 10.1111/j.1365-2796.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 25.Kwok CS, Loke YK, Welch AA, et al. Habitual chocolate consumption and the risk of incident heart failure among healthy men and women. Nutr Metab Cardiovasc Dis. 2016;26(8):722–34. 10.1016/j.numecd.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JR, Prince RL, Zhu K, Devine A, Thompson PL, Hodgson JM. Habitual chocolate intake and vascular disease: a prospective study of clinical outcomes in older women. Arch Intern Med. 2010;170(20):1857–8. 10.1001/archinternmed.2010.396. [DOI] [PubMed] [Google Scholar]
- 27.Zhong GC, Hu TY, Yang PF, et al. Chocolate consumption and all-cause and cause-specific mortality in a US population: a post hoc analysis of the PLCO cancer screening trial. Aging. 2021;13(14):18564–85. 10.18632/aging.203302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166(4):411–7. 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 30.Dong JY, Iso H, Yamagishi K, Sawada N, Tsugane S, Japan Public Health Center-based Prospective Study G. Chocolate consumption and risk of stroke among men and women: A large population-based, prospective cohort study. Atherosclerosis. 2017;260:8–12. doi: 10.1016/j.atherosclerosis.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 31.Khawaja O, Petrone AB, Kanjwal Y, Gaziano JM, Djousse L. Chocolate consumption and risk of atrial fibrillation (from the Physicians’ Health Study). Am J Cardiol. 2015;116(4):563–6. 10.1016/j.amjcard.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke in women. J Am Coll Cardiol. 2011;58(17):1828–9. 10.1016/j.jacc.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79(12):1223–9. 10.1212/WNL.0b013e31826aacfa. [DOI] [PubMed] [Google Scholar]
- 34.Larsson SC, Akesson A, Gigante B, Wolk A. Chocolate consumption and risk of myocardial infarction: a prospective study and meta-analysis. Heart. 2016;102(13):1017–22. 10.1136/heartjnl-2015-309203. [DOI] [PubMed] [Google Scholar]
- 35.Mostofsky E, Levitan EB, Wolk A, Mittleman MA. Chocolate intake and incidence of heart failure: a population-based prospective study of middle-aged and elderly women. Circ Heart Fail. 2010;3(5):612–6. 10.1161/CIRCHEARTFAILURE.110.944025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostofsky E, Berg Johansen M, Tjonneland A, Chahal HS, Mittleman MA, Overvad K. Chocolate intake and risk of clinically apparent atrial fibrillation: the Danish Diet, Cancer, and Health Study. Heart. 2017;103(15):1163–7. 10.1136/heartjnl-2016-310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhaus DA, Mostofsky E, Levitan EB, et al. Chocolate intake and incidence of heart failure: findings from the Cohort of Swedish Men. Am Heart J. 2017;183:18–23. 10.1016/j.ahj.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho YL, Nguyen XT, Yan JQ, et al. Chocolate consumption and risk of coronary artery disease: the Million Veteran Program. Am J Clin Nutr. 2021;113(5):1137–44. 10.1093/ajcn/nqaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med. 2007;44(4):305–10. 10.1016/j.ypmed.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg JA, Neuhouser ML, Tinker LF, et al. Chocolate candy and incident invasive cancer risk in the women’s health initiative: an observational prospective analysis. J Acad Nutr Diet. 2020. 10.1016/j.jand.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Bondonno NP, Dalgaard F, Kyro C, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat Commun. 2019;10(1):3651. 10.1038/s41467-019-11622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark JL, Zahradka P, Taylor CG. Efficacy of flavonoids in the management of high blood pressure. Nutr Rev. 2015;73(12):799–822. 10.1093/nutrit/nuv048. [DOI] [PubMed] [Google Scholar]
- 43.Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nutr Rev. 2015;73(4):216–35. 10.1093/nutrit/nuu014. [DOI] [PubMed] [Google Scholar]
- 44.Heptinstall S, May J, Fox S, Kwik-Uribe C, Zhao L. Cocoa flavanols and platelet and leukocyte function: recent in vitro and ex vivo studies in healthy adults. J Cardiovasc Pharmacol. 2006;47 Suppl 2:S197–205 (discussion S6–9). 10.1097/00005344-200606001-00015 [DOI] [PubMed] [Google Scholar]
- 45.Hermann F, Spieker LE, Ruschitzka F, et al. Dark chocolate improves endothelial and platelet function. Heart. 2006;92(1):119–20. 10.1136/hrt.2005.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rein D, Paglieroni TG, Wun T, et al. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72(1):30–5. 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 47.Pearson DA, Paglieroni TG, Rein D, et al. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106(4–5):191–7. 10.1016/s0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- 48.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–41. 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 49.Croft KD. Dietary polyphenols: antioxidants or not? Arch Biochem Biophys. 2016;595:120–4. 10.1016/j.abb.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55(6):1398–405. 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–84. 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lv D, Cheng X, Tang L, Jiang M. The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed Pharmacother. 2017;88:277–84. 10.1016/j.biopha.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 53.Prince PD, Fischerman L, Toblli JE, Fraga CG, Galleano M. LPS-induced renal inflammation is prevented by (−)-epicatechin in rats. Redox Biol. 2017;11:342–9. 10.1016/j.redox.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Pinilla E, Onatibia-Astibia A, Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front Pharmacol. 2015;6:30. 10.3389/fphar.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neufingerl N, Zebregs YE, Schuring EA, Trautwein EA. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: a randomized controlled trial. Am J Clin Nutr. 2013;97(6):1201–9. 10.3945/ajcn.112.047373. [DOI] [PubMed] [Google Scholar]
- 56.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290(8):1029–30. 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


