Abstract
Despite advances in screening, diagnosis, and treatment for prostate cancer (PCa), Black men tend to be diagnosed at younger ages, have higher mortality rates, and are at increased risk of recurrence or metastasis compared to their White counterparts. PCa disparities among Black men are caused by a complex interaction of social, behavioral, and biological factors across the public policy, community, organizational, interpersonal, and individual levels. Key contributing factors include mistrust in the health care system, poor communication between patients and providers, low awareness of screening guidelines, and high medical costs. These disparities are further exacerbated by the low representation of Black men in clinical trials, which limits access to high-quality cancer care and generalizability for PCa treatments. In this narrative review of the existing literature, we examined the epidemiology and identified contributing factors, and propose multi-level strategies to address and mitigate disparities among Black men with PCa.
Keywords: (5-6): prostate cancer, healthcare disparities, racial disparities, ethnic disparities, screening guidelines, provider bias
Introduction
Despite improvements in prostate cancer (PCa) screening, diagnostics, and treatment, Black men are diagnosed at younger ages, have higher mortality rates, and are at greater risk of recurrence and metastasis than White men.1-4 Furthermore, Black men also have less access to PCa treatment and often experience longer treatment delays than White men. Prostate-specific antigen (PSA) screening is a critical component of PCa management, yet significant screening disparities exist, particularly for Black men. Black men are less likely to undergo PSA screening despite being more likely to present with higher PSA levels than White men. 5 Consequently, this gap in screening rates contributes to delayed diagnoses and treatment, ultimately leading to higher mortality rates for Black men. Studies have shown that Black men are more likely to have genomically aggressive cancer, even with clinically low-risk disease; as such, they tend to benefit more from PSA testing and more targeted screening for clinically significant tumors using molecular markers and MRI-targeted biopsies. 3 Notably, screening guidelines from the US Preventive Services Task Force (USPSTF) currently recommend against routine PSA screening; this has led to a decrease in PCa screening frequency, with Black men experiencing the most significant reduction. 3 This has implications for the early detection of PCa among Black men, who are 2.2 times more likely to die from PCa than White men. 3 For this study, Black men are defined as men of African or Caribbean ancestry living in the United States, unless otherwise specified.
The racial disparities seen with PCa are multifaceted and include social, behavioral, and biological factors. 6 Examples of social and behavioral factors include mistrust in the health care system, poor patient-provider communication, limited awareness about screening guidelines and treatment options, high costs of medical care, and institutional racism. 3 Biological factors include genetic predispositions, differences in tumor biology, variations in hormone levels, and potentially differential responses to treatments. 3 Black men are also underrepresented in PCa clinical trials of novel therapeutics, resulting in a lack of access to high-quality cancer care and approval of PCa treatments that may not be generalizable.3,6 Furthermore, the lack of representation in studies that inform screening guidelines negatively impacts Black men. 7 Factors that keep Black men from participating in PCa studies and clinical trials are also complex and include lack of insurance or knowledge of clinical trials, mistrust, provider bias, flaws in study design, and lack of diversity among medical and research teams.
Given the significant racial PCa disparities and their profound impact on outcomes for Black men, this review is necessary to understand the contributing factors and propose effective strategies comprehensively. Here, we will discuss the epidemiology of racial disparities across the continuum of PCa care to understand the causes of screening, diagnosis, and treatment disparities among Black men in the United States. As the factors contributing to these racial disparities are complex, their solutions must include multilevel strategies and interventions. We propose strategies to address these factors at the public policy, community, organizational, interpersonal, and individual levels.
The Epidemiology of PCa Disparities
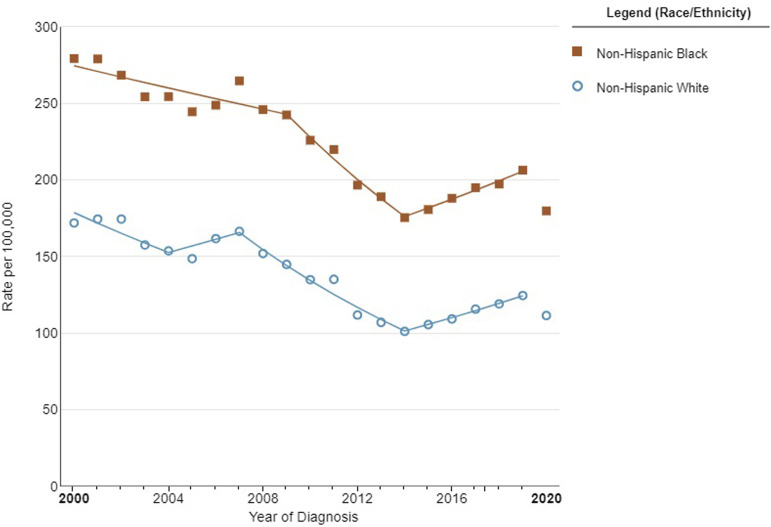
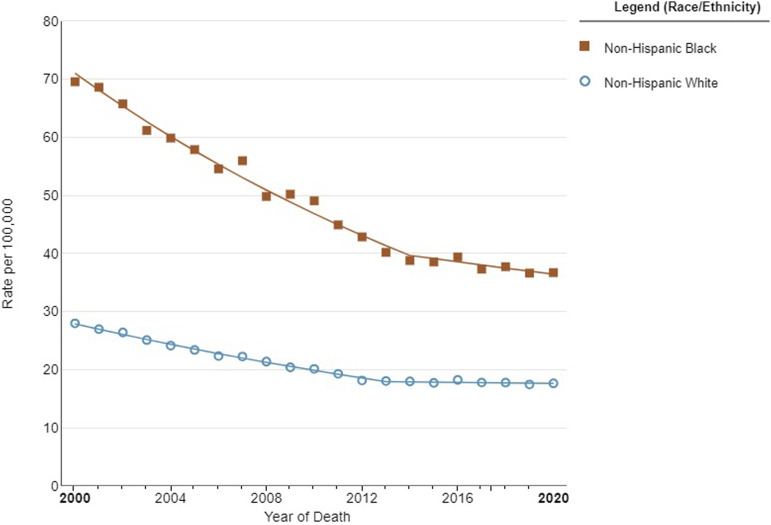
There are striking variances in PCa prevalence and incidence rates among various racial and ethnic groups (Figure 1). Black men appear to be notably more affected by PCa than their White, Asian, or Hispanic counterparts. Studies have shown that almost 1 in 3 new cancer diagnoses among men is PCa, and it continues to be the second leading cause of cancer-related death. 8 Black men have higher PCa incidence and mortality rates than men of other ethnic and racial backgrounds, with a 2.5-fold higher risk of being diagnosed with a more aggressive form of PCa (Figure 2). 9
Figure 1.
Prostate cancer incidence stratified by race between 2000 and 2020 in the United States. Image created in SEER*Explore using SEER Incidence Data, November 2022 Submission (1975-2020), SEER 22.
Figure 2.
Prostate cancer mortality rates stratified by race in the United States between 2000 and 2020. Image created in SEER*Explore using SEER Incidence Data, November 2022 Submission (1975-2020), SEER 22.
We and others have reported that Black men continue to endure a disproportionate cancer burden, with the highest death rate and shortest survival of any racial or ethnic group for most cancers.4,10 Black men are more likely to experience more aggressive diseases, have higher mortality rates, and be diagnosed at a younger age than men of any other racial or ethnic background. 11 Further research is needed to understand these disparities and how effective interventions could reduce this burden.
Incidence and Mortality of PCa
PCa is 1 of the primary causes of male mortality worldwide, with mortality rates varying across ethnic and racial groups. Black men consistently demonstrate 1.6 to 2.4 times higher incidence rates than Asian, Hispanic, and White men.12-14 Black men also experience much higher PCa-specific mortality rates than other racial and ethnic groups (38.4/100 000 men), followed by White (18.2/100 000), Hispanic (15.6/100 000), and Asian men (13.7/100 000).8,13
PCa incidence rates vary for other racial and ethnic groups. White men exhibit an intermediate incidence rate for PCa. 12 For Asian populations, previous studies consistently report PCa incidence rates ranging between 30% and 50% lower than White or Black populations. 12 It should be noted that variance exists within Asian populations because of specific subgroup characteristics, lifestyle factors, and geographical regions. 12 Hispanic men typically exhibit lower incidence rates than Black or White populations, and disparities may not be as pronounced as those seen among Black populations. 12 However, the available data aggregate all Hispanic subgroups into 1 broad group, including Mexican, Puerto Rican, Cuban, South or Central American, and Dominican subpopulations. Interestingly, Puerto Rican men showed much higher PCa-specific mortality rates than other Hispanic groups. 15 Indeed, PCa is the most common type of cancer and accounts for the most cancer-specific deaths among Puerto Rican men.16,17
Disparities Among Black Subpopulations
Though this paper’s primary objective is to highlight PCa disparities among Black men in the United States, it is also essential to acknowledge the significant variations in incidence and outcomes across different Black populations worldwide.18,19 For example, Caribbean men of African descent experience higher PCa incidence rates compared to African American men. 19 Sub-Saharan African men exhibit varied incidence rates that can be affected by factors like genetic predispositions, lifestyle differences, and disparities in health care access and quality. 20 Such variations demonstrate the complexity of PCa disparities and emphasize the need for research that includes all men affected by the African Diaspora.18,21,22
A cohort study demonstrated that Black men in the UK had significantly higher PSA values and were more likely to be diagnosed with prostate cancer within 1 year of receiving their PSA test than White or Asian men. Furthermore, an study of PCa among immigrants in the U.S. indicated that Caribbean men from Guyana and Trinidad experienced significantly greater mortality risks than US-born Black men. 18
Most available data on PCa incidence and mortality among Black men are limited by the lack of stratification by country or region of origin. Understanding the differences in PCa incidence and mortality between Caribbean, African, and African American men is essential to crafting targeted interventions that address specific risk factors and barriers. 23 Further research must explore the genetic, environmental, and sociocultural influences that lead to these disparities and create more effective screening and treatment plans for all populations worldwide. By expanding our understanding of PCa among Black men globally, we will also reduce its burden among this population.
Understanding the Causes of PCa Disparities
Disparities in PCa mortality between ethnic and racial populations can be explained by various factors, including genetic and biological factors, socioeconomic status (SES), health care access barriers, cultural beliefs and attitudes, and health behaviors. 17 Limited health care access and use may result in delayed diagnoses, with more advanced disease stages at diagnosis; this may lead to worse survival outcomes. Multiple interlinked factors can explain racial and ethnic disparities in PCa prevalence and incidence. Genetic mutations could influence the aggressiveness of prostate tumors as well as their response to treatment, thus contributing to the observed disparities. Socioeconomic issues, such as access and use gaps, impact early detection and timely treatment, which affects incidence and prevalence rates.
Genetic and Biological Factors
Genetic predisposition plays a key role in PCa disparities across ethnic and racial groups. Genetic variations, including inherited mutations or polymorphisms, can influence the incidence, aggressiveness, and treatment outcomes associated with PCa. Certain genetic mutations, specifically BRCA1 and BRCA2, have been linked with an increased risk of PCa among men, particularly those affected at a young age. 24 Hereditary mutations of BRCA1 and BRCA2 genes, which have long been associated with breast and ovarian cancers, can also increase PCa risks. 25 The BRCA1 and BRCA2 mutations are associated with more aggressive forms of PCa and poorer outcomes, underscoring the significance of genetic screening and personalized treatment plans tailored specifically for each person. Research on these mutations has significantly advanced our understanding of their causes and lead to the creation of targeted therapies. Men carrying BRCA mutations may especially benefit from regular screenings and therapies tailored specifically to their genetic profile. Understanding the prevalence and effect of BRCA1 and BRCA2 mutations across various populations is critical for creating effective prevention and treatment strategies. Recently, somatic mutations in driver genes, such as NCOA2, STK19, DDX11L1, and PCAT1, were found more often among men with African ancestry with PCa compared to men with European ancestry. These ancestral attributes of patients may contribute to PCa disparities. 26
Ancestral genetic factors play a significant role in PCa, with 278 genetic risk variants having been identified through genome-wide association studies (GWAS)27,28; however, it is crucial to highlight the significant disparity in sample representation for such studies, with the majority of samples being from patients with European ancestry (95.85%) as compared to African American or Afro-Caribbean ancestry (0.49%). 28 Additional research by Wang et al 27 further revealed 187 previously unreported genetic risk variants for PCa, bringing the total number of identified variants to 451. 27 Notably, the genetic risk scores derived from this multi-ancestry GWAS were associated with a higher risk of aggressive vs non-aggressive disease among men with African ancestry. This emphasizes the importance of exploring the genetic landscape of PCa among Black men to better understand and address the disparities observed across different ancestral groups. Further research is needed to understand the role genetics may have on to PCa health disparities across the African Diaspora. 28
Hormonal factors also influence PCa disparities, with variations in hormone levels, metabolism, and androgen receptor activity potentially leading to differences in PCa incidence, development, aggressiveness, progression, and response to hormone therapies. 29 The metabolism of androgens, like testosterone and dihydrotestosterone (DHT), is affected by genetic variations, such as polymorphisms in genes encoding enzymes involved with androgen metabolism; this can lead to ethnic and racial variations that impact androgen metabolism and disparities in PCa risk. 30 Furthermore, higher testosterone levels have been associated with an increased risk of PCa. 31 A previous study suggested that testosterone levels decrease more significantly with age in Black males than in White males, potentially contributing to racial disparities in PCa; these findings warrant further investigation. 31
Socioeconomic Status
SES, income, education, occupation, and environmental exposures influence PCa outcomes and differences in access to health care services, environmental carcinogens, and health literacy. 32 Lower income levels often prevent access to health care resources, including preventive screenings and early diagnosis of PCa. 32 This results in PCa being diagnosed at more advanced stages among Black patients than their White counterparts. 13 Furthermore, a lack of education and low health literacy may result in delays in seeking medical help and poorer outcomes. 32
In addition, certain racial and ethnic groups may have a higher likelihood of being exposed to occupational hazards like heavy metals, pesticides, and industrial chemicals, further compounding health disparities, including increasing their risk of PCa. 33 Environmental exposure refers to individuals’ contact with potentially hazardous substances in their surroundings; this exposure is more prevalent among lower-SES populations. Exposures could include occupational hazards and residential proximity to pollutant sources. 34 Lower SES populations face an array of risk factors that increase their vulnerability to PCa while complicating overall health outcomes. 34 Furthermore, working in stressful environments with long hours, job strain, and limited control may contribute to negative health outcomes, including PCa-related outcomes. 34 By understanding the relationship between SES and environmental exposures, we can more efficiently address the root causes of health disparities and design targeted interventions to mitigate them.
Healthcare Access Barriers
Access to health care is 1 of the main factors contributing to PCa disparities across ethnic and racial groups, leading to delayed diagnoses, suboptimal treatments, and poor survival rates. 35 uninsured patients face additional barriers when seeking preventive screenings and early diagnosis services like PCa screenings. 10 Racial and ethnic minority populations, and Black men in particular, may face additional difficulties accessing high-quality health care because of a shortage of providers within a geographical location or limited availability of specialized services. 35
Disparities also exist in PSA testing rates, an essential screening tool for PCa. Black men may be less likely to undergo PSA testing than other racial and ethnic groups, which may delay diagnosis until their disease has already begun to progress. 10 Furthermore, variations in guidelines and recommendations could contribute to these disparities; recent revisions emphasize shared decision-making as part of screening practices. 10
Cultural Beliefs and Attitudes
Differences in cultural beliefs and attitudes contribute significantly to disparate PCa outcomes by influencing how individuals view illness, approach medical interventions, and engage with health care services. The impact of cultural factors on PCa outcomes in Black men is multifaceted. 36 Cultural factors include mistrust of the health care system, poor patient-physician communication, stigma, and fear of PCa diagnosis. 3 There is extensive evidence that fear, mistrust, and negative opinions of health care professionals can contribute to the lower rates of screening practices among Black men than White men.36,37 Feelings of fear and shame associated with PCa can lead to reluctance to discuss symptoms or side effects with health care professionals; this can result in individuals seeking informal medical advice from family, friends, or other members from the church and community, potentially perpetuating misinformation and misperceptions. 36 Fear of invasive procedures, such as biopsies, may also deter men from undergoing necessary screening or diagnostic tests. This avoidance can result in delayed diagnoses and more advanced disease stages at the time of treatment.
Conflicting messages in the media and among health care professionals on screening guidelines for PCa can be difficult for patients to navigate. For Black men who already face barriers in accessing health care and may harbor mistrust due to historical injustices and lived discrimination, these conflicting guidelines can be problematic. They may interpret changes in messages as evidence of uncertainty within the medical community, reinforcing doubts about the reliability of medical advice. The lack of clear, culturally appropriate communication about screening guidelines can leave Black men unsure about what actions to take to protect their health. Distrust in the health care system can manifest in a reluctance to engage with health care providers or participate in clinical research, which are essential for advancing cancer treatments. 36
Addressing these cultural factors requires a concerted effort from health care providers, policymakers, and community advocates. Education and outreach programs tailored to specific cultural beliefs and concerns for Black men can help reduce stigma, build trust, and encourage the uptake of PCa screenings and treatment. Additionally, incorporating cultural competency training for health care providers can improve communication and the quality of patient care. By acknowledging and addressing the role of cultural beliefs and attitudes, it is possible to mitigate disparities in PCa outcomes and improve the health of Black men.
Health Behaviors
Diet, physical activity levels, and tobacco/alcohol use can significantly alter the risk, progression, and outcomes associated with PCa. Diets characterized by increased red meat consumption and reduced intake of fruits and vegetables have been linked with an increased PCa risk. 38 Additionally, low physical activity levels, prolonged inactivity, and cigarette smoking have been associated with an increased risk of aggressive PCa and poor outcomes. As such, variations in smoking prevalence and cessation rates between racial and ethnic groups also contribute to PCa disparities.39,40
We and others have additionally reported significant associations between alcohol consumption and risk for PCa aggressiveness.41,42 This association may differ between racial and ethnic groups, which could perpetuate disparities.43,44 We also reported that the potential impact of alcohol intake on PCa aggressiveness may not be universal among PCa patients because of the effects of genetic heterogeneity. 41 Interactions between alcohol and single nucleotide polymorphisms may explain the inconsistent results of alcohol’s impact on PCa aggressive in studies that do not consider genetic profiles. Taken together, these results suggest that excessive alcohol intake significantly impacts PCa aggressiveness, particularly among specific genetic and racial subgroups.
Diagnosis and Screening Disparities
Access to screening and early diagnosis plays an integral role in PCa prognostication and management for Black men who are at increased risk of developing aggressive disease. However, in 2012, the US Preventative Services Task Force (USPSTF) advised against routine PSA-based screening for all men, regardless of age.6,45 This resulted in a 9.5% decrease in the frequency of PSA screenings from 2012 to 2018, with the most significant reduction observed among Black men (11.6%, compared to 9.3% among White men). In a recent study, Basourakos et al 46 conducted a harm-to-benefit analysis of PCa screening among Black men and concluded that there was a higher net benefit with PSA screening for Black men than for the general population. Black men often present with clinically aggressive disease, higher PSA levels and Gleason scores, and more advanced T stages than their White counterparts. 3 , 47 , 48
The importance of PSA screening for detecting and managing PCa among Black men cannot be overstated. Given the high incidence and mortality rates among this population, early-stage detection has proven essential in timely intervention and improving outcomes; however, the limitations and possible contradictions of PSA screening must also be acknowledged.
Notably, a recent nationwide study among veterans demonstrated a higher incidence of de novo metastasis rates among Black men than White men, even when they have equal rates of PSA screening. This is highly important, as PCa disparities are driven by socioeconomic factors and genomic attributes.6,49 Though PSA screening can be beneficial in many respects, it alone may not be enough to address disparate PCa outcomes; tumor biology, genetic predispositions, socioeconomic variables, and other factors also play crucial roles in these disparities. Given the complexity of PCa disparities, researchers must investigate and validate additional screening tools to supplement or amplify the efficacy of PSA testing.
Expanding the Screening Paradigm
Internationally, researchers are evaluating multiparametric MRI (mpMRI) as an alternative or adjunct screening modality. 50 Results are promising, particularly with its application to improving PCa detection accuracy by identifying clinically significant tumors that would not be detected via PSA screening alone. 50 Furthermore, the PROMIS study demonstrated that mpMRI performed better than transrectal ultrasound biopsies at detecting significant PCa, thus decreasing unnecessary biopsies and improving diagnostic precision. 50 Other emerging biomarkers and imaging techniques are being evaluated to provide more comprehensive and reliable screening options. 50
Adopting a multifaceted screening approach that includes PSA testing and other advanced diagnostic tools like mpMRI is crucial to effectively address disparities in PCa outcomes. Such an approach can enable early detection of aggressive tumors and more accurate risk evaluation, ultimately enabling timely and appropriate treatment interventions. Future research should focus on exploring and validating alternative screening methods across diverse populations to ensure that their benefits are universally applicable and address the specific challenges experienced by Black men and other high-risk groups. By broadening the PCa screening paradigm, we can enhance early detection and management of PCa, thus decreasing disparities in outcomes.
Risk Stratification
Additionally, the tools currently used for PCa risk stratification and treatment assignment may fail to consider the genomic diversity among Black men. 17 A prospective study by Awasthi et al 49 on the Decipher Score reclassification for low-intermediate risk PCa patients demonstrated that Black men were twice as likely as non-Black men to experience genomic reclassification (relative risk [RR], 2.23 [95% CI, 1.02-4.90]) and were at the highest risk of genomic metastasis. Additionally, another large population-based cohort study demonstrated that Black men with clinically low-risk PCa are likely to experience Gleason reclassification (RR, 1.30 [95% CI, 1.12-1.50]), leading to poorer outcomes. 48
Suboptimal disease risk classification for Black men can be partially attributed to the lack of minority representation in the clinical trials that are used to inform clinical guidelines. 51 Consequently, these clinical guidelines may not adequately represent ideal PCa disease characterization, treatment recommendations, and prognostication for Black men. Javier-DesLoges et al analyzed data from NCI-funded clinical trials from 2015 to 2019 and reported that Black men were significantly less likely to be included in PCa clinical trials than their non-Hispanic White counterparts (odds ratio [OR], 0.85 [95% CI, 0.79-0.92]). 51 Thus, the collective body of evidence suggests that existing guidelines on PCa risk stratification and treatment recommendations for Black men need to be carefully reviewed.
Treatment Disparities
Access to care
Timely intervention with PCa treatment is critical to achieve positive long-term outcomes, and intervention timeliness is closely tied with patients’ access to care and SES. Compared to White men, Black men with PCa across all D’Amico risk classification categories are less likely to receive definite treatment. 52 Black men also tend to wait longer to receive PCa treatment (incidence rate ratio [IRR] 1.19; 95% confidence interval [CI], 1.04-1.36). 53 How these disparities in access to the best care translate into considerable differences in outcomes remains an active area of investigation.
Treatment
There are multiple options for PCa treatment, and their use can depend on the tumor’s clinical stage and the patient’s age and health status. For localized cases, surgery or radiation treatments are the main options. Emerging evidence has shown that outcome-related disparities tend to dissipate when patients are treated in relatively equal-access settings, such as treatment from Veterans Affairs.6,54,55 Moreover, Black men are likely to have more favorable outcomes than their White counterparts in these settings.6,54,55 These findings indicate that treatment-related outcome disparities are eliminated by providing equitable access to care; however, residual disparities related to metastasis continued to exist among Black patients because of their higher incidence of advanced PCa cases. This association was further explored in a study by Yamoah et al, which reported that, despite having similar metastasis burden after treatment, Black veterans were nearly twice as likely to experience metastasis than White veterans across all National Comprehensive Cancer Network (NCCN) risk categories (low risk, 4 vs 2 per 100 000; intermediate risk, 13 vs 6 per 100 000; high risk, 19 vs 9 per 100 000). Additional studies have suggested a that there is a similar mortality from PCa seen between Black and White men who received curative-intent treatment. 55
Current research also suggests that Black men experience more favorable outcomes with radiation treatment than their White counterparts.6,56 In an extensive Veterans Health Administration study (N = 31 131), the 10-year cumulative death from PCa was lower among Black than White men (P = 0.004). 56 However, these favorable outcomes were not observed among Black patients who received a surgical treatment. A multicenter open-labeled observational study by Sartor et al 57 reported that Black men treated with Sipuleucel-T injections experienced better overall survival than their White counterparts. The observed differences have been linked to the genomic diversity of PCa tumors among Black men, which may make the tumors more sensitive to immune radiation modalities.58,59 Awasthi et al 58 explored this hypothesis via an extensive comparative genomic study and highlighted immune-related differences between the tumors of Black and White men. The authors hypothesized that the complex interplay between immune response markers and DNA damage repair mechanism markers may explain the favorable response to immune radiation therapies observed among Black men.
For some cases, physicians may recommend active surveillance or tracking low-risk PCa progression over time, typically while undergoing routine monitoring (such as PSA testing, prostate biopsy, or MRI). A main concern with active surveillance is whether the program is equally safe for Black patients as their White counterparts. Multiple studies have reported that Black patients in an active surveillance program showed higher rates of progression and within shorter periods of time. 60 Additionally, 1 study reported that White patients were placed on active surveillance programs more frequently than Black men, but the lack of a clearly defined active surveillance strategy may be a potential reason for this disparity. 61
Participation in Clinical Trials
The Black population is under-represented in most cancer clinical trials that inform NCCN treatment guidelines. Improving Black patients’ enrollment in clinical trials is important to ensure generalizability of clinical trial results and recommendations. Overall differences in PCa treatment outcomes may be influenced by the diversity of prostate tumors of Black men; therefore, personalized guidelines that integrate both sociocultural factors and PCa genomic features should be developed to achieve durable and equitable outcomes for PCa patients across racial groups.
Additionally, Black men who participate in such studies tend to have higher health-seeking behavior and may not represent the general Black population, resulting in potential selection bias for the existing Black representation in currently available clinical studies.17,62 There are several potential reasons for low representation of Black patients in clinical trials. First, the design of clinical trials have strict inclusion and exclusion criteria, and many Black patients are not eligible based on exclusion criteria because of their co-existing comorbidities. 63 Further, many clinical trials do not consider racial differences in standard lab test values, which may exclude appropriate candidates because of differences in normal laboratory value ranges between racial groups. 64 Second, previous studies reported that Black men are influenced by religious beliefs; lack of knowledge about clinical trials; and physical barriers, such as transportation. 65 Third, many Black men are skeptical about health care system and especially clinical research studies because of institutional racism and research misconduct. 66
Addressing PCa Disparities
PCa disparities result from complex interactions among various factors, requiring comprehensive and multilevel solutions and strategies. 7 PCa disparities must be seen as interlinked systems influencing each other. As such, the socioecological model (SEM) can provide an excellent framework to address PCa disparities on policy, community, organizational, interpersonal, and individual levels (Table 1).
Table 1.
Multilevel Strategies to Address PCa Disparities.
| Level | Description |
|---|---|
| Public policy | - Implement evidence-based policies improving prevention, screening, clinical trial diversity, and health care access for Black men |
| - Follow recommendations from AACR and USPSTF to enhance funding, data collection, clinical trials, and insurance coverage | |
| Community | - Engage community stakeholders through participatory research via trusted institutions like barber shops and churches for education |
| - Involve women and spouses in educating communities about prostate cancer | |
| Organizational | - Address structural racism within organizations to improve diversity in the research and health care workforce |
| - Emphasize the importance of patient-provider racial/ethnic concordance for better communication and trust | |
| Interpersonal | - Establish effective communication between patients and providers to overcome mistrust barriers |
| - Understand the historical context of mistrust due to racism and unethical research practices | |
| Individual (patient) | - Provide education tailored to Black men’s needs about prostate cancer screenings, treatment options, and disparities |
| - Use patient navigators to address structural barriers and guide patients through the health care system | |
| Individual (provider) | - Provide cultural competency, implicit bias, and empathy training to address personally mediated racism |
| - Understand the role of structural racism and power imbalances in health care |
Abbreviations: AACR, American association for cancer Research; PCa, prostate cancer; USPTF, US preventive services Task Force.
Public Policy Level
Evidence-based public policies can improve PCa disparities by improving prevention and early detection screening, improving diversity in PCa clinical trial enrollment, and increasing access to high-quality care for Black men. 7 The American Association for Cancer Research (AACR) has called on policymakers to (1) provide more funding for the federal agencies and programs that aim to reduce cancer-related health disparities; (2) ensure that racial and minority populations are included in data collection; (3) improve representation in clinical trials; (4) expand Medicaid and access to high-quality, affordable health insurance; and (5) increase diversity in the cancer research and care workspaces. 7
In the United States, The US Preventive Services Task Force (USPSTF) is the primary government body that makes recommendations for PCa screening and prevention. In 2018, the USPSTF identified gaps in the research for PCa screening among Black men, 7 including the lack of studies that have developed or validated tools or targeted therapies with analyses of specificity, sensitivity, and positive/negative predictive values among racial/ethnic subpopulations. There is also a need for evidence-based PCa screening guidelines designed for Black men and other at-risk groups. 7 To create these guidelines, there must be increased minority participation, especially among Black men, in screening and clinical trials. This would allow researchers to better understand the etiology of PCa in marginalized populations, which could then be translated into customized screening criteria for at-risk populations. 7
Increasing insurance coverage is another policy-level strategy to address PCa disparities. One proposed strategy to reduce screening disparities is requiring both public and private insurance companies to cover cancer screenings as essential health benefits. 7 However, if the screening guidelines don’t include specific recommendations for subpopulations who are most at risk, insurance companies may refuse to cover the cost of PCa screenings for Black men who are younger than the currently recommended ages of 55 to 69 years.
Community Level
To better address screening and treatment disparities, researchers must engage community stakeholders. 2 Community-based participatory research engages key community members to help develop and implement interventions that increase screening, seeking and adhering to treatment, and participation in clinical trials. For example, interventions that involve barber shops and churches as locations to administer PCa education have succeeded because these institutions are considered trustworthy in the Black community. 2 Another strategy is to engage women and the spouses of Black men by educating them on the signs and symptoms of PCa as well as the importance of screening, the types of screening tests, treatment options, and participation in PCa clinical trials. When properly educated, spouses and trusted community leaders can be trustworthy advocates to encourage routine screenings. Researchers must collaborate with community leaders to ensure the community’s needs are being met and trustworthy information about PCa is accessible and culturally appropriate.
Organizational Level
Organizations must consider how past and present structural racism creates barriers to screening and participation in clinical trials among Black men, and they must address institutional racism as a fundamental cause of racial disparities in PCa. Structural racism influences diversity within the medical institutions conducting research and among study teams (leadership, investigators, nurses, study coordinators, patient navigators, etc.) At the organizational level, increasing the representation of Black men in the cancer research and care workforce can address PCa disparities by enhancing patients’ quality of care, improving patient satisfaction, and fostering trust. 7 Patient-provider racial/ethnic concordance is associated with better communication and patient satisfaction; therefore, it can potentially result in increased trust, adherence to PCa screening procedures, and participation in PCa clinical trials among Black men. 67
Interpersonal Level
Effective communication between patients and providers is paramount for making informed decisions about participating in screening activities or clinical trials. Many health disparities are caused by Black patients’ deeply rooted mistrust of research and the health care system, which can be a major barrier to participation in both screening procedures and in PCa clinical trials. Mistrust is often described as an individual patient–level barrier; however, its effects reach across all levels of the SEM. We include mistrust as an interpersonal-level factor to acknowledge the role it plays in preventing effective communication between patients and providers, but it is important to remember that mistrust is complex, and interventions to address mistrust must be multilevel.
Black men have reported having higher general mistrust in health care systems than any other racial/ethnic group. 3 In a study assessing attitudes toward PCa care, Black men expressed mistrust of the health care system as a reason for the lack of medical testing and PCa research participation. 68 Lack of trust in the health care system and in research within the Black community stems from both general experiences with racism and discrimination and from the historical abuses of Black participants in research, such as in the United States Public Health Service Syphilis Study. These unethical experiments on Black men have created fear and distrust of research and medical professionals. Black men have expressed fear that PCa screenings would not be thorough and that results would be misused.3,68 Black patients who deeply mistrust research may interpret PCa disparities among Black men as a rationale for their mistrust, whereas patients who are neutral to or favor research may perceive these disparities as motivation to participate. 68
Poor communication has been reported as a barrier contributing to PCa disparities; improving communication between Black men and their providers can enhance trust and improve participation in PCa screenings as well as clinical research. In 1 study, Black men were less likely to report good patient-provider communication than White men (60% vs 71%; P < .001), and conversations regarding PSA screenings were described as infrequent and suboptimal.3,69 Interventions to enhance patient-provider communication include training for providers to improve their communication skills. Such trainings should include education on mistrust and its role as a barrier to screening, treatment, and clinical trial participation among racial/ethnic minority groups as well as cultural competency and ways to foster better patient-provider communication to become more trustworthy.
Individual (patient) Level
Black men should be educated on the importance of screening, diagnosis, treatment, and follow-up on PCa care, as well as the disparities associated with PCa. 3 Nearly half of Black men across various ages and educational and SES levels report being undereducated about PCa screenings. 3 1 way to address these disparities at the individual patient–level is to ensure that Black men are provided adequate education and resources so they can better understand their treatment and support options. Strategies may include providing culturally and ethnically specific educational materials to improve Black men’s knowledge of PCa. Importantly, a patient-level strategy of providing targeted PCa education for Black men should involve the input of community stakeholders to ensure the material is culturally appropriate. 2
Another patient-level strategy to address PCa disparities is employing patient navigators, individuals who help patients understand the health care system, access screening, and guide them through treatment. 7 Patient navigators can address structural barriers Black men face when accessing PCa care by connecting Black men with tailored resources necessary to mitigate personal barriers to accessing screening procedures, treatment, and participation in PCa clinical trials.
Individual (Provider) Level
Treating physicians should be aware of the treatment disparities for Black men with PCa. PCa disparity interventions need to integrate cultural competency, implicit bias, and empathy training to address personally mediated racism. 70 Cultural competence training and education about the barriers Black men experience at all phases of the cancer continuum can help providers and researchers mitigate their implicit bias and improve patient-provider interactions when discussing the importance of PCa screening, treatment, and participation in clinical trials.3,71 Researchers should be trained to understand structural racism and how it impacts PCa disparities. However, these individual provider–level strategies alone will not address the power imbalance present in medical care, and they are therefore not sufficient to address structural racism alone. 70 These strategies must be coupled with public policy and organizational interventions.
Strengths and Limitations
This review addresses a critical need in cancer research by presenting a comprehensive analysis of the disparities in PCa diagnosis, treatment, and outcomes among Black men. The multidisciplinary approach, involving experts from various backgrounds, provides a broad perspective of the complex factors contributing to PCa disparities. This paper proposes evidence-based strategies at multiple levels, from public policy to individual patient care, that can serve as a roadmap for stakeholders to address these issues.
However, it is essential to recognize the study’s limitations and consider these when developing and implementing strategies to improve health outcomes for this population. This review is limited by the existing literature and does not present any original research findings. Given that this review focused on Black men, the findings may not be generalizable to other racial and ethnic groups. Additionally, this paper does not address the practical challenges in implementing the proposed strategies. Future research must evaluate the effectiveness and feasibility of any proposed interventions.
Future Research
The health care community must make a commitment to sustained systemic change at all levels of influence to address prostate cancer disparities among Black men. Multidisciplinary teams, including the community, must be involved in future research to continue unraveling the complexities of genetic, environmental, and social factors contributing to these disparities. This will contribute to the larger goal of health equality. This vision for health equity will require innovative funding and collaboration between policymakers and health care providers.
Conclusion
Black men are particularly at risk for PCa, with earlier presentation, more aggressive disease, and higher mortality rates than their White counterparts. Furthermore, Black men have reduced access to high-quality PCa treatments and often experience treatment delays. Understanding the drivers of racial inequities in PCa incidence and mortality has proven challenging because of the complex interplay among genetic, social, environmental, patient-related, provider-related, and systemic factors influencing PCa outcomes among Black men. Examples of factors driving racial disparities among Black men with PCa include systemic and institutional racism, mistrust in research and health care systems, a lack of participation in clinical trials, poor patient-provider communication, not being informed about screening options or treatment possibilities, and costs involved with care that are not covered by insurance.
Solutions to PCa disparities must include public policy, community, organization, interpersonal, and individual strategies. The SEM can serve as an organizing framework to identify the many complex factors influencing PCa outcomes and create interventions to mitigate racial inequalities. This integrative approach to PCa disparities considers multiple levels of influences on health outcomes (public policy, community, organizational, interpersonal, and individual), allowing us to achieve greater efficacy when combatting PCa disparities. At an individual level, we can offer education and resources to empower patients to take control of their health. At an interpersonal level, health care providers should specifically engage with Black men to address their unique challenges. At the organizational level, organizations should tackle the impact of institutional and structural racism on the engagement of Black men in PCa screening practices and clinical trials while also striving to enhance the representation of Black men in cancer research. At the community and public policy levels, we must expand access to health care and support policies that facilitate PCa screening and treatment.
Acknowledgments
Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Publishing by Daley White and Gerard Hebert; no compensation was given beyond their regular salaries.
Appendix.
List of Abbreviations
Abbreviation definition
- AACR
American Association for Cancer Research
- CI
Confidence Interval
- DHT
Dihydrotestosterone
- DNA
Deoxyribonucleic Acid
- IRR
Incidence Rate Ratio
- MRI
Magnetic Resonance Imaging
- NCCN
National Comprehensive Cancer Network
- NCI
National Cancer Institute
- OR
Odds Ratio
- PCa
Prostate Cancer
- PCF
Prostate Cancer Foundation
- PSA
Prostate Specific Antigen
- RR
Relative Risk
- SEM
Socioecological Model
- SES
Socioeconomic Status
- USPSTF
United States Preventative Service Task Force
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
ORCID iDs
Anastasia Murphy https://orcid.org/0009-0002-0983-7969
Kosj Yamoah https://orcid.org/0009-0001-0987-0972
References
- 1.Borno H, George DJ, Schnipper LE, Cavalli F, Cerny T, Gillessen S. All men are created equal: Addressing disparities in prostate cancer care. Am Soc Clin Oncol Educ Book. 2019;39:302-308. doi: 10.1200/edbk_238879 [DOI] [PubMed] [Google Scholar]
- 2.Carthon B, Sibold HC, Blee S, R DP. Prostate cancer: Community education and disparities in diagnosis and treatment. Oncol. 2021;26(7):537-548. doi: 10.1002/onco.13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillard JW, Jr., Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: a literature review. Cancer. 2022;128(21):3787-3795. doi: 10.1002/cncr.34433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietro GD, Chornokur G, Kumar NB, Davis C, Park JY. Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J. 2016;20(Suppl 2):S112-S119. doi: 10.5213/inj.1632722.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari AK, Gold HT, Demers RY, et al. Effect of socioeconomic factors on long-term mortality in men with clinically localized prostate cancer. Urology. 2009;73(3):624-630. doi: 10.1016/j.urology.2008.09.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamoah K, Lee KM, Awasthi S, et al. Racial and ethnic disparities in prostate cancer outcomes in the veterans Affairs health care system. JAMA Netw Open. 2022;5(1):e2144027. doi: 10.1001/jamanetworkopen.2021.44027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PA, Zaidi SK, Sengupta R. AACR cancer disparities progress report 2022. Cancer Epidemiol Biomarkers Prev. 2022;31(7):1249-1250. doi: 10.1158/1055-9965.Epi-22-0542 [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA A Cancer J Clin. 2023;73(1):17-48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 9.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290-1314. doi: 10.1002/cncr.28509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA A Cancer J Clin. 2016;66(4):290-308. doi: 10.3322/caac.21340 [DOI] [PubMed] [Google Scholar]
- 11.Tsodikov A, Gulati R, de Carvalho TM, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer. 2017;123(12):2312-2319. doi: 10.1002/cncr.30687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA A Cancer J Clin. 2011;61(2):69-90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA A Cancer J Clin. 2023;73(1):17-48. DOI: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 15.Chinea FM, Patel VN, Kwon D, et al. Ethnic heterogeneity and prostate cancer mortality in Hispanic/Latino men: A population-based study. Oncotarget. 2017;8(41):69709-69721. doi: 10.18632/oncotarget.19068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tortolero-Luna GZ-ZD, Pérez-Ríos N, Torres-Cintrón CR. et al. Cancer in Puerto Rico; 2013:2006-2010. [Google Scholar]
- 17.Mahal BA, Gerke T, Awasthi S, et al. Prostate cancer racial disparities: A systematic review by the prostate cancer foundation panel. Eur Urol Oncol. 2022;5(1):18-29. doi: 10.1016/j.euo.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 18.Mutetwa B, Taioli E, Attong-Rogers A, Layne P, Roach V, Ragin C. Prostate cancer characteristics and survival in males of African Ancestry according to place of birth: data from Brooklyn-New York, Guyana, Tobago and Trinidad. Prostate. 2010;70(10):1102-1109. doi: 10.1002/pros.21144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JR, Woods-Burnham L, Hooker SE, Jr., Batai K, Kittles RA. Genetic contributions to prostate cancer disparities in men of west african descent. Front Oncol. 2021;11:770500. doi: 10.3389/fonc.2021.770500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebbeck TR, Devesa SS, Chang BL, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer. 2013;2013:560857. doi: 10.1155/2013/560857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebbeck TR. Prostate cancer disparities by race and ethnicity: From nucleotide to neighborhood. Cold Spring Harb Perspect Med 2018;8(9). doi: 10.1101/cshperspect.a030387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JR, Kittles RA. Genetic ancestry and racial differences in prostate tumours. Nat Rev Urol. 2022;19(3):133-134. doi: 10.1038/s41585-021-00544-3 [DOI] [PubMed] [Google Scholar]
- 23.Weise N, Shaya J, Javier-Desloges J, Cheng HH, Madlensky L, McKay RR. Disparities in germline testing among racial minorities with prostate cancer. Prostate Cancer Prostatic Dis. 2022;25(3):403-410. doi: 10.1038/s41391-021-00469-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234. doi: 10.1038/bjc.2011.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193. doi: 10.1016/j.eururo.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 26.Jaratlerdsiri W, Jiang J, Gong T, et al. African-specific molecular taxonomy of prostate cancer. Nature. 2022;609(7927):552-559. doi: 10.1038/s41586-022-05154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A, Shen J, Rodriguez AA, et al. Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants. Nat Genet. 2023;55(12):2065-2074. doi: 10.1038/s41588-023-01534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soh PXY, Mmekwa N, Petersen DC, et al. Prostate cancer genetic risk and associated aggressive disease in men of African ancestry. Nat Commun. 2023;14(1):8037. doi: 10.1038/s41467-023-43726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng I, Plummer SJ, Neslund-Dudas C, et al. Prostate cancer susceptibility variants confer increased risk of disease progression. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2124-2132. doi: 10.1158/1055-9965.Epi-10-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haiman CA, Patterson N, Freedman ML, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39(5):638-644. doi: 10.1038/ng2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H, Odedina FT, Reams RR, Lissaker CTK, Xu X. Racial differences in age-related variations of testosterone levels among US males: potential implications for prostate cancer and personalized medication. Journal of Racial and Ethnic Health Disparities. 2015;2(1):69-76. doi: 10.1007/s40615-014-0049-8 [DOI] [PubMed] [Google Scholar]
- 32.Reyes-Ortiz CA, Eschbach K, Zhang DD, Goodwin JS. Neighborhood composition and cancer among Hispanics: Tumor stage and size at time of diagnosis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2931-2936. doi: 10.1158/1055-9965.Epi-07-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ash M, Boyce JK. Racial disparities in pollution exposure and employment at US industrial facilities. Proc Natl Acad Sci U S A. 2018;115(42):10636-10641. doi: 10.1073/pnas.1721640115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krstev S, Knutsson A. Occupational risk factors for prostate cancer: A meta-analysis. J Cancer Prev. 2019;24(2):91-111. doi: 10.15430/jcp.2019.24.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19(2):146-155. doi: 10.1111/j.1525-1497.2004.30209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vapiwala N, Miller D, Laventure B, et al. Stigma, beliefs and perceptions regarding prostate cancer among Black and Latino men and women. BMC Publ Health. 2021;21(1):758. doi: 10.1186/s12889-021-10793-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray TF, Cudjoe J, Murphy J, Thorpe RJ, Jr., Wenzel J, Han HR. Disparities in cancer screening practices among minority and underrepresented populations. Semin Oncol Nurs. 2017;33(2):184-198. doi: 10.1016/j.soncn.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 38.Gathirua-Mwangi WG, Zhang J. Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev. 2014;23(2):96-109. doi: 10.1097/CEJ.0b013e3283647394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy AB, Akereyeni F, Nyame YA, et al. Smoking and prostate cancer in a multi-ethnic cohort. Prostate. 2013;73(14):1518-1528. doi: 10.1002/pros.22699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816-825. doi: 10.1001/jamainternmed.2016.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin HY, Wang X, Tseng TS, et al. Alcohol intake and alcohol-SNP interactions associated with prostate cancer aggressiveness. J Clin Med. 2021;10(3):553 doi: 10.3390/jcm10030553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demoury C, Karakiewicz P, Parent ME. Association between lifetime alcohol consumption and prostate cancer risk: a case-control study in Montreal, Canada. Cancer Epidemiol. 2016;45:11-17. doi: 10.1016/j.canep.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 43.D'Ecclesiis O, Pastore E, Gandini S, et al. Association between alcohol intake and prostate cancer mortality and survival. Nutrients. 2023;4:15. doi: 10.3390/nu15040925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macke AJ, Petrosyan A. Alcohol and prostate cancer: time to draw conclusions. Biomolecules 2022;12(3). doi: 10.3390/biom12030375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian Z, Al Khatib K, Chen X, et al. Investigating the racial gap in prostate cancer screening with prostate-specific antigen among younger men from 2012 to 2020. JNCI Cancer Spectr. 2023;7(2). doi: 10.1093/jncics/pkad003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basourakos SP, Gulati R, Vince RA, Jr., et al. Harm-to-Benefit of three decades of prostate cancer screening in black men. NEJM Evid. 2022;1(6). doi: 10.1056/evidoa2200031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71(9):985-997. doi: 10.1002/pros.21314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awasthi S, Mahal BA, Park JY, et al. Substantial Gleason reclassification in Black men with national comprehensive cancer network low-risk prostate cancer - a propensity score analysis. Prostate Cancer Prostatic Dis. 2022;25(3):547-552. doi: 10.1038/s41391-022-00510-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awasthi S, Grass GD, Torres-Roca J, et al. Genomic testing in localized prostate cancer can identify subsets of african Americans with aggressive disease. J Natl Cancer Inst. 2022;114(12):1656-1664. doi: 10.1093/jnci/djac162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822. doi: 10.1016/s0140-6736(16)32401-1 [DOI] [PubMed] [Google Scholar]
- 51.Javier-DesLoges J, Nelson TJ, Murphy JD, et al. Disparities and trends in the participation of minorities, women, and the elderly in breast, colorectal, lung, and prostate cancer clinical trials. Cancer. 2022;128(4):770-777. doi: 10.1002/cncr.33991 [DOI] [PubMed] [Google Scholar]
- 52.Moses KA, Orom H, Brasel A, Gaddy J, Underwood W, 3rd. Racial/ethnic disparity in treatment for prostate cancer: Does cancer severity matter? Urology. 2017;99:76-83. doi: 10.1016/j.urology.2016.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinlock BL, Thorpe RJ, Jr., Howard DL, et al. Racial disparity in time between first diagnosis and initial treatment of prostate cancer. Cancer Control. 2016;23(1):47-51. doi: 10.1177/107327481602300108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 2020;126(8):1683-1690. doi: 10.1002/cncr.32666 [DOI] [PubMed] [Google Scholar]
- 55.Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(7):975-983. doi: 10.1001/jamaoncol.2019.0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi: 10.1002/cncr.33224 [DOI] [PubMed] [Google Scholar]
- 57.Sartor O, Armstrong AJ, Ahaghotu C, et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: Outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 2020;23(3):517-526. doi: 10.1038/s41391-020-0213-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awasthi S, Berglund A, Abraham-Miranda J, et al. Comparative genomics reveals distinct immune-oncologic pathways in african American men with prostate cancer. Clin Cancer Res. 2021;27(1):320-329. doi: 10.1158/1078-0432.Ccr-20-2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan Z, Fernandez D, Dhillon J, et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis. 2021;24(1):140-149. doi: 10.1038/s41391-020-0254-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gökce MI, Sundi D, Schaeffer E, Pettaway C. Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis. 2017;20(2):127-136. doi: 10.1038/pcan.2016.56 [DOI] [PubMed] [Google Scholar]
- 61.Krishna S, Fan Y, Jarosek S, Adejoro O, Chamie K, Konety B. Racial disparities in active surveillance for prostate cancer. J Urol. 2017;197(2):342-349. doi: 10.1016/j.juro.2016.08.104 [DOI] [PubMed] [Google Scholar]
- 62.Awidi M, Al Hadidi S. Participation of black Americans in cancer clinical trials: Current challenges and proposed solutions. JCO Oncol Pract. 2021;17(5):265-271. doi: 10.1200/op.21.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: Study design barriers. J Clin Oncol. 2004;22(4):730-734. doi: 10.1200/jco.2004.03.160 [DOI] [PubMed] [Google Scholar]
- 64.Vastola ME, Yang DD, Muralidhar V, et al. Laboratory eligibility criteria as potential barriers to participation by black men in prostate cancer clinical trials. JAMA Oncol. 2018;4(3):413-414. doi: 10.1001/jamaoncol.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivers D, August EM, Sehovic I, Lee Green B, Quinn GP. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials. 2013;35(2):13-32. doi: 10.1016/j.cct.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 66.Banda DR, Germain DS, McCaskill-Stevens W, Ford JG, Swain SM. A critical review of the enrollment of black patients in cancer clinical trials. 2012:153-157. doi: 10.14694/EdBook_AM.2012.32.88 [DOI] [PubMed] [Google Scholar]
- 67.Ford ME, Siminoff LA, Pickelsimer E, et al. Unequal burden of disease, unequal participation in clinical trials: Solutions from African American and Latino community members. Health Soc Work. 2013;38(1):29-38. doi: 10.1093/hsw/hlt001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers CR, Rovito MJ, Hussein M, et al. Attitudes toward genomic testing and prostate cancer research among black men. Am J Prev Med. 2018;55(5 Suppl 1):S103-s111. doi: 10.1016/j.amepre.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollack CE, Armstrong KA, Mitra N, et al. A multidimensional view of racial differences in access to prostate cancer care. Cancer. 2017;123(22):4449-4457. doi: 10.1002/cncr.30894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Best AL. Anti-Black racism and power: Centering black scholars to achieve health equity. Hastings Cent Rep. 2022;52(Suppl 1):S39-s41. doi: 10.1002/hast.1368 [DOI] [PubMed] [Google Scholar]
- 71.Regnante JM, Richie NA, Fashoyin-Aje L, et al. US cancer centers of excellence strategies for increased inclusion of racial and ethnic minorities in clinical trials. J Oncol Pract. 2019;15(4):e289-e299. doi: 10.1200/jop.18.00638 [DOI] [PubMed] [Google Scholar]