Abstract
Background
The elevated blood pressure (BP) and lower cardiac autonomic modulation (CAM) are associated with higher morbidity mortality risk among older adults. Although exercise is an important intervention for cardiovascular promotion, it is unclear whether combat sports training could benefit cardiovascular outcomes as much as autonomic in this population. This study compared the effects of 12 weeks of Muay Thai (MT) training against functional training (FT) on CAM and hemodynamic parameters in older adults.
Methods
The sample consisted of 50 older adults (41 women; 66.0 ± 5.3 years old), who were equaly randomized into FT (n = 25) and MT (n = 25) intervention groups. CAM was measured by 30-min rest heart rate variability. Systolic blood pressure (SBP), diastolic blood pressure (DBP) and resting heart rate (RHR) were measured using an automatic oscillometric device. Pulse pressure (PP) and the double product (DP) were also calculated. The interventions were carried out three times a week, with 60-min length per session, during 12 consecutive weeks. The intensity of the interventions was measured using the subjective perception of exertion scale and by accelerometer. Two-factor repeated measures analysis of covariance was used for groups comparison, considering intervention group and body mass as factors. The 95% confidence interval of the difference (95%CIdif) was also calculated and the effect size was measured using partial eta squared (η2p).
Results
CAM indices did not show significant changes across moments and intervention groups. In hemodynamic parameters, only in DBP was there an effect of the moment (F1,39 = 8.206; P = 0.007; η2p = 0.174, large) and interaction effect between group*moment (F1,39 = 7.950; P = 0.008; η2p = 0.169, large). Specifically, the MT group at the post-training moment showed lower DBP (P = 0.010; 95%CIdif = -13.3; -1.89) in relation to the FT group. Furthermore, the MT group showed a decrease in DBP during training (P = 0.002; 95%CIdif = -10.3; -2.6). Also, an increase in training intensity was also found over the 12 weeks in FT, with no difference between the groups.
Conclusion
After 12 weeks of MT practice there was a reduction in DBP compared to FT in older adults.
Trial registration
NCT03919968 Registration date: 01/02/2019.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04096-3.
Keywords: Combat sports, Aging, Blood Pressure, Exercise
Introduction
The autonomic nervous system plays an important role in cardiac and vascular adaptations [1]. Its behavior can be tracked through the analysis of cardiac autonomic modulation, which reflects global, sympathetic or parasympathetic modulations [2]. This indicator is important, since low cardiac autonomic modulation has been associated with a higher prevalence of mortality [3]. One of the characteristics that can contribute to the decrease in cardiac autonomic modulation is aging. A study of South Africans over the age of fifty found that low cardiac autonomic modulation was associated with a greater likelihood of mortality [4]. Furthermore, aging has been associated with other cardiovascular risk factors such as high blood pressure [5, 6].
Low cardiac autonomic modulation and hypertension are also associated with worse quality of life, in addition to being associated with increased drug treatment [7–9]. In this sense, health promotion strategies that can increase cardiac autonomic modulation and reduce blood pressure values should be encouraged. Among the different types of strategies for this purpose, physical activity stands out. Some studies have shown positive effects of physical activity on cardiovascular parameters in older adults [10–12] and cardiac autonomic modulation is greater in active older adults when compared to insufficiently active older adults [13].
An alternative form of physical activity with this objective may be combat sports, which are intermittent and can help improve cardiorespiratory fitness [14, 15]. Furthermore, combat sports, as long as they are adapted to the characteristics of older adults, following basic training principles (for example, progressive overload), with moderate to vigorous intensity and a frequency of two or three weekly 60-min sessions, improve physical health, functional, physiological and psycho-emotional of older adults [16]
Among the combat sports modalities is Muay Thai (MT), a percussion modality with an intermittent or interval character [17], which has already been shown to be beneficial in the pediatric population on body composition [18], aerobic fitness [19] and cardiovascular parameters, both acutely [20] and after 16 weeks of training [21, 22]. Better physical fitness of healthy women practicing MT compared to non-practitioners was also observed [23]. This type of training is an alternative for practicing physical activity in older adults that should be tested, as they are generally carried out in groups and the exercises are divided into pairs, which can promote an increase in older adults’ adherence to exercise, since only 22% of them have participated in regular activities twice a week [24]. Even so, interval or intermittent training has been a tool that promotes improvement in the control of cardiac autonomic modulation [25], especially those of higher intensity [26].
Another training modality which has this intervallic character, and has also been developed with older adults is Functional Training (FT). FT is similar to the daily activities of the older adults, seeking adaptations that are more comfortable and effective to improve physical capabilities and health functionality in general [27–31]. FT appears to promote beneficial effects on cardiac autonomic modulation, characterized by increased parasympathetic modulation, in postmenopausal women [32]. Choi et al. [33] analyzed the effects of 12 weeks of FT on blood pressure and pulse pressure in healthy older adults, and observed a reduction in these variables in the group that practiced FT when compared to the control group.
A systematic review [34] indicated that studies on combat sports and the cardiovascular health of older adults mostly used a cross-sectional design. In turn, longitudinal studies on this topic compared an intervention group with a non-intervention group (control) [34]. Therefore, there is a need for studies in which intervention using combat sports modalities is compared with more traditional approaches. Another review [35] highlights that, despite the important findings of some studies on the effects of combat sports on cardiac autonomic modulation in older adults, the lack of randomization may be a limiting factor related to the composition of the sample, indicating the need for studies with this care in their designs. A short review presented the great difficulty of measuring training intensity with combat sports modalities due mainly to physical confrontation, intervals and plyometrics [36] therefore, studies are needed to better determine the external (e.g., via accelerometry) and internal (e.g., via subjective perception of effort) intensity of combat sports sessions.
Therefore, the present study aimed to explore these gaps of: i) not using just a control group; ii) low methodological rigor when detailing the intervention, not randomizing the sample, and the majority with cross-sectional studies; iii) more modalities and practice possibilities for the elderly to increase adherence to physical activity; iv) without controlling the intensity; using a longitudinal and randomized design, comparison of a combat sport modality with another type of physical exercise program more traditional, and measuring the external (e.g., via accelerometry/ physiological) and internal loads (e.g., via subjective perception of effort/ sense) of the sessions. Thus, the objective of this clinical trial was to analyze the effects of MT compared to FT on cardiac autonomic modulation and hemodynamic parameters in older adults. The hypothesis was that the groups would have similar results throughout the program, with improvements in cardiovascular health parameters for both groups, indicating that MT could be as good as FT on the variables studied; Therefore, it is an alternative in exercise programs for this population.
Methods
Participants
Participants were recruited in the Presidente Prudente region through advertisements on social networks and local media, flyers in health centers, squares, churches or by appearing to seek care at the study locations. To be included, participants must: 1) Be between 60 and 89 years old; 2) Present a medical certificate confirming that the older adult was able to practice physical exercise. As exclusion criteria, participants could not: 1) Perform any other systematic exercise practice, in addition to regular physical activity, such as walking; 2) Having serious cardiovascular diseases such as myocardial infarction, or procedures such as saphenous bypass and pacemaker; 4) Use crutches, canes, walkers, wheelchairs or other similar utensils; 5) Present a self-report of mobility difficulties to carry out the intervention practice; 6) Present an error greater than 5% in the cardiac autonomic modulation tracing. Participants signed the informed consent form before data collection and the study was conducted in the second half of 2019.
After collecting data at the initial moment, the older adult who participated in this study were randomly distributed into one of two groups: practicing MT or FT. The randomization process was carried out by a researcher who was not part of this project (with the aim of blinding the sample allocation process) through a sequence of numbers generated on the website http://www.randomization.com, randomization was done by groups, and according to sex.
The sample allocation process was carried out secretly with opaque, sealed envelopes and following the numerical allocation sequence. In the first practice session, the envelope was opened in front of the older adult who participated in the project, informing them which group they would be included in. This entire process is in line with the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) for randomized clinical trials [37].
The present study was approved by the FCT/UNESP Research Ethics Committee (CAAE: 91923318.7.0000.5402), and was registered and presented publicly on the clinical trials platform clinicaltrials.gov/ with protocol number NCT03919968 in 01/02/2019 with the name “Effect of Muay Thai vs. Circuit Training on the Cardiovascular Health of Elderly”, following the standards proposed by CONSORT.
Sample calculation
Due to the lack of research in the literature involving MT and cardiovascular parameters in older adults with the necessary information to carry out the sample calculation, we opted for studies that investigated Tai Chi Chuan. The sample calculation was carried out based on the study of Tsai et al. [38] which compared the blood pressure of elderly people practicing Tai Chi Chuan with a control group. The variable diastolic blood pressure was used with a difference of 8 mmHg to be detected and a standard deviation of 8 mmHg, a test power of 80% and a significance of 5%. Adding 20% of possible sample losses, the sample size was at least 36 older adults (18 older adults for each group).
Primary outcomes
Cardiac autonomic modulation
To evaluate cardiac autonomic modulation, the older adults were instructed that at least 12 h before carrying out the experimental protocol they should not use alcoholic and/or stimulant drinks/foods, such as coffee, tea and chocolates, so that there would be no direct influence on cardiac autonomic behavior at the time of collection [39]. Cardiac autonomic modulation was assessed using heart rate variability. For this purpose, the heart rate was recorded beat by beat for 30 min using a heart rate monitor (Polar Electro Oy, Kempele, Finland—model V800) positioned in the distal third of the sternum. The intervals between heartbeats (RR intervals) obtained by the heart rate monitor with a sampling frequency of 1000 Hz53 were exported by the Polar Flow Web Service (https://flow.polar.com/) and saved in text format for later analysis by Kubios HRV® software (Biosignal Analysis and Medical Image Group, Department of Physics, University of Kuopio, Finland).
For analysis, the RR interval time series was initially subjected to preprocessing to eliminate premature ectopic beats and artifacts, with moderate filtering and interval correction with the cubic spline interpolation method. After pre-processing, the data were analyzed and subsequently visually screened to verify the absence of artifacts or cardiac arrhythmias that could interfere with the analyses [40]. Only RR interval series with more than 95% sinus beat will be included in the study [41]. 1000 consecutive RR intervals were used for analysis, taken from the stretch of greatest signal stability.
For data analysis, the HRV software Kubios HRV 2.0 Analysis Software for Windows (Analysis Group, Department of Applied Physics, University of Kuopio, Finland) was used [42] and the mean heart rate (HR mean), the average RR intervals (RR mean) and the linear indices of the time and frequency domains were extracted. In the time domain, the following were calculated: standard deviation of the RR intervals (SDNN), the square root of the mean of the square of the differences between adjacent normal RR intervals (rMSSD) and the percentage of adjacent RR intervals with a difference in duration greater than 50 ms (pNN50). In the frequency domain, the indices of: high frequency (HF), low frequency (LF) and the LF/HF ratio were calculated. Quantitative analysis of the Poincaré plot was also performed, obtaining the indices: SD1 (standard deviation of instantaneous beat-to-beat variability), SD2 (long-term standard deviation of continuous RR intervals), and the SD1/ SD2. Furthermore, a qualitative analysis of the plot was carried out, through the analysis of the figures formed by the attractor, as proposed by Tulppo et al. [43].
Blood pressure and heart rate
Systolic blood pressure, diastolic blood pressure and heart rate were measured using an Omron automatic oscillometric device (Model HEM-742, Japan). For these measurements, the individuals remained lying down at rest for five minutes, with their legs uncrossed, and were asked not to talk during this time. These procedures are in line with the American Society of Cardiology [44]. These measurements were taken again 10 to 15 min after the initial measurement. For analysis, the average of these two measurements was used.
Secondary outcomes
Pulse pressure and double product
Pulse pressure was calculated as the difference between mean systolic blood pressure and mean diastolic blood pressure [45]. The double product is an estimated measure of myocardial oxygen consumption. Its value was obtained by multiplying the average heart rate by the average systolic blood pressure [46].
Anthropometric measurements
Body mass was measured using a digital scale (Plenna, Brazil) and height using a fixed stadiometer (Sanny, Brazil). Using these values, body mass index was calculated by dividing body mass by the square of height [47]. The older adults were evaluated barefoot and wearing light clothing.
Intervention
For intervention, participants were randomly divided into two groups: MT group and FT group. In both groups, the intervention lasted 12 weeks, carried out three times a week, on non-consecutive days, lasting approximately 60 min. The initial two weeks were dedicated to adaptation and familiarization, both with the exercise protocol and with the subjective perceived exertion scale, used to determine exercise intensity. The 60 min for both groups were divided into 10 min of general exercises, 40 min of specific exercises and 10 min of playful activities and simulations of what was learned during the session. Each day of the week focused on a specific part of the body. The first day of the week was focused on upper limb exercises, the second day on lower limbs and the last for the whole body. All activities respected the biological individuality of each older adult person and were always carried out in pairs with older adult of similar size and weight, in both groups. Furthermore, throughout the intervention period there was an increase in monthly overload, through an increase in the time spent performing the exercises, a reduction in active rest and passive rest, as well as an increase in the difficulty of the exercises (mainly in the case of MT) and increased weights (mainly in the case of FT). Furthermore, these variables were considered in the interventions [48]:
Organization of sessions (periodized): adaptation (2 weeks);
Types and order of exercises: first session of the week for upper limbs; second session for lower limbs and third session for full body. Size of the exercises was it broader first and more localized later;
Resistance options used in FT: stick, dumbells, elastic, medicine ball, string, body weight;
Types of contraction: all exercises were isotonic;
Contraction speed (cadence): it was moderate so that the participant could correctly execute the movement with the degree of intensity within the expected range;
Range of motion: was complete or adapted according to the participant’s joint conditions;
Rest between sets: 1 min to 30 s in MT and 1 min to 50 s in FT according to the training protocol in the supplementary figures;
Strategies for controlling intensity: use of the Borg scale, accelerometer and participants' self-report;
Volume: number of exercises, number of sets, and number of repetitions as described in training protocol in the supplementary figures.
Intensity
Intensity was controlled using the subjective perception of exertion scale by Borg et al. [49] from six (minimum effort) to 20 (maximum effort) at the end of every training session, however, to standardize with the accelerometer, we only present one week (the three days of training) at the end of each month of training. The intensity was maintained between 12 and 13, which is classified as moderate. Furthermore, training intensity was measured using an Actigraph GT3X accelerometer (ActiGraph, LLC, Pensacola, FL, USA) for one week (the three days of training) at the end of each month of training, strengthening intensity control. The accelerometer data was filtered, digitized and adjusted to a frequency of 30Hz with an interval (epoch) of 60 s. The accelerometer was analyzed using ActiLife 6 software (ActiGraph, LLC, Pensacola, FL, USA).
Muay Thai training
The MT group, in the initial 10 min of general exercises, performed stretching and physical warm-ups specific to the MT modality. In turn, throughout the 40 min of specific exercises, seven exercises were given per session, adapted for the elderly, according to the part of the body in focus that day. On Monday, exercises were carried out with punches (jab, straight, cross, hook and elbows), dodges and defenses. On Wednesday there were kicks (circular thigh and frontal height), knees and defenses, both stationary and in movement. On Friday, exercises were performed combined with upper and lower limb movements in the same exercise. Protections such as shin guards, gloves, Thai pads, gauntlets, rib protectors and head protectors were always used, prioritizing the safety of the elderly when carrying out activities. In the first month, the seven exercises per day were performed for two minutes, with one older adults always receiving the blows, while the other struck. There was a recovery time of one minute when changing exercises, making it easy to change equipment (Supplementary Figure S1) and after that time acting as a partner, receiving the blows for two minutes. In the second month, there were two and a half minutes of execution, with 30 s of recovery, two and a half minutes acting as a partner and maintaining the seven exercises. In the last month, it was the same protocol as the second month, but with eight exercises and an increase in their difficulty and complexity. The final 10 min of playful activities and simulations of the exercises learned were through activities that included some developed skills, as well as fighting simulation, through the sparing technique (a person who is not an opponent, but who helps to improve the technique practitioner).
Functional training
The FT group, during the 10 min of general exercises, performed stretching and physical warm-ups. In turn, throughout the 40 min of specific exercises, 13 exercises were given per day adapted for the elderly, according to the part of the body in focus that day. On Monday they performed the following exercises: barbell curls with a stick, lateral raises with dumbbells, triceps curls on an elastic band, shoulder presses with dumbbells, front and diagonal raises with a stick, high rows with a stick, front raises with elbow flexion with a medicine ball on the chair, open push-up on the wall, barbell curl with dumbbells, shoulder coordination with medicine ball (raising the ball above the shoulder), crucifix with elastic, inverted curl with elastic and abdominal standing on the side with dumbbells. On Wednesday the exercises were: squats sitting on a chair, marching on the agility ladder, supporting sumo squats, climbing on the step, walking on the line, lateral leg raise supporting, seated knee extension with shin guards, trunk semi-flexion (good day) with a stick, unilateral hip extension, maintained single-leg support exercise (balance), plantar flexion sitting with weight on your lap, sit-ups while standing, raising the leg and coordinating with the arm stationary. And on Friday: marching on the line with holding objects, climbing the step with trunk rotation, getting into the hula hoop, squatting, picking up and lifting above the shoulder, squats on the chair with front pole raise, marching with high knees and arm coordination, touching the cones with the hand, sitting on the chair and going around the cone (agility), trunk rotation with a medicine ball (passing the ball to the partner), touching the feet with a medicine ball (tying shoelaces), arm coordination with standing running and abdominal with squats.
In the first month there were two series (the older adult performed the same exercise twice), of 40 s of execution for each exercise with 20 s of active rest between them (to change exercises) and one minute of passive rest between series (Supplementary Figure S2). In the first month there were two series (the older adult performed the same exercise twice), of 40 s of execution for each exercise with 20 s of active rest between them (to change exercises) and one minute of passive rest between series. In the second month, there were three series (the older adult performed the same exercise three times), of 50 s of execution for each exercise with 10 s of active rest between them (to change exercises) and 50 s of passive rest between series. In the last month, the same protocol as in the second month was maintained, however the exercises had an increase in difficulty, complexity, dual task insertion and those with dumbbells, elastic bands, shin guards, among others, increased weight/resistance. The older adults were in pairs at each station (due to the number of older adults, to be similar to the MT who was in pairs and maintain interaction), but they performed the same exercises together, often facilitating the execution, such as trunk rotation. with medicine ball (passing the ball to the partner). The final 10 min of playful activities and simulations of the exercises learned were through activities that included some developed skills, such as “Dead Alive” in the lower limbs room or “Your master ordered” making movements that worked the entire body.
Statistical analysis
The Kolmogorov–Smirnov test was performed to verify data normality. The descriptive analysis was presented as mean and standard deviation or frequency (%). To check possible differences in the randomized groups at the beginning of training, the t-test for independent samples and Chi-square for frequencies were performed. Mauchly's test of sphericity with Greenhouse–Geisser correction when necessary. Analysis of variance (ANOVA; groups: MT vs. FT; moments: session of four, eight and 12 weeks) with repeated measures on the second factor was used to analyze and compare the intensity of a training session for each muscle group (upper limbs, lower limbs and global) at the end of each month. Furthermore, to analyze and compare the effects of MT and FT training on the older adults, analysis of covariance (ANCOVA; groups: MT vs. FT; moments: pre and post 12 weeks of training) was performed with repeated measures on the second factor adjusted by body mass that showed a difference between the groups at the initial moment. When differences in ANCOVA or ANOVA were detected, correction was applied using the Bonferroni post-roc test. The 95% confidence interval of the difference (95%CIdif) was also calculated for variables in which there was a significant effect of at least one factor. The effect size was measured using partial eta squared (η2p) in which 0.01 was considered small, 0.06 medium and 0.14 large. [50]. The Statistical Package for the Social Sciences (SPSS) software version 25.0 was used for the analyses, GraphPad Prism version 8.0 for creating the graphs, Microsoft Excel version 16.0 for creating the plot graph and the significance established was p-value < 0.05.
Results
The initial sample consisted of 50 older adults (41 women and 9 men) aged 66.04 ± 5.32 years, who were randomly divided into two groups: MT (n = 25) and FT (n = 25). At the initial moment, the groups were compared and no differences were observed between them, which can be considered a homogeneous sample. Among the older adults in MT, 60% (n = 15) had high blood pressure, 4% (n = 1) heart disease, 28% (n = 7) diabetes, none smoked, 32% (n = 8) consumed alcohol, 16% (n = 4) were single, 44% (n = 11) married, 16% (n = 4) divorced/separated, 24% (n = 6) widowed, 64% (n = 16) of white ethnicity, none black, 8% (n = 2) oriental and 28% (n = 7) mixed race. In turn, the older adults in the FT were 56% (n = 14) hypertensive (chi-square comparing the groups P = 0.774), 16% (n = 4) diabetic (P = 0.306), 12% ( n = 3) had heart disease (P = 0.297), 4% (n = 1) smoked (P = 0.312), 32% (n = 8) consumed alcoholic beverages (P = 1.000), 8% (n = 2) were single (P = 0.384), 64% (n = 16) married (P = 0.156), 8% (n = 2) divorced/separated (P = 0.384), 20% (n = 5) widowed (P = 0.733), 68% (n = 17) white (P = 0.765), 4% (n = 1) black (P = 0.312), 4% (n = 1) oriental (P = 0.552) and 24% (n = 6) mixed race (P = 0.747).
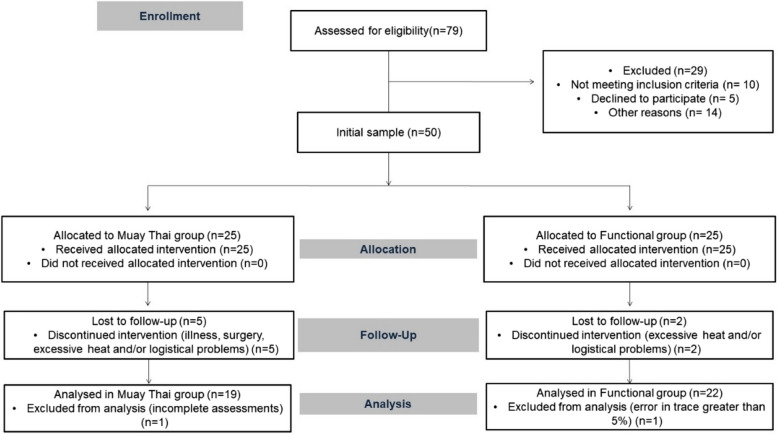
During the intervention period, seven older adults left the study due to illness, surgery, excessive heat and/or logistical problems and two were excluded from the analyses, one for not having complete assessments and the other due to an error in the cardiac autonomic modulation tracing greater than 5% (18% sample loss). Thus, the final sample was composed of 41 older adults (9 men and 33 women), 19 in MT (5 men) and 22 in FT (4 men) (Fig. 1).
Fig. 1.
Sample flowchart based on CONSORT
The MT and FT groups were compared at the initial moment, considering only those who completed the study and a difference was observed only in the body mass variable (t = -2.138; F40 = 2.814; P = 0.039) (Table 1).
Table 1.
Characteristics of older adults in the Muay Thai and Functional groups at the beginning of the study
|
Muay Thai (n = 19) Mean (SD) |
Functional (n = 22) Mean (SD) |
p-value | |
|---|---|---|---|
| Age (years) | 65.9 (4.9) | 64.9 (4.6) | 0.509 |
| Body mass (kg) | 65.8 (9.7) | 75.2 (16.9) | 0.039 |
| Height (cm) | 154.5 (6.8) | 156.2 (7.1) | 0.447 |
| Body mass index (kg/m2) | 27.1 (3.4) | 30.3 (6.8) | 0.064 |
| Systolic blood pressure (mmHg) | 123 (15) | 126 (16) | 0.540 |
| Diastolic blood pressure (mmHg) | 73 (10) | 74 (10) | 0.666 |
| Pulse pressure (mmHg) | 49 (12) | 52 (10) | 0.438 |
| Heart rate (bpm) | 64 (10) | 67 (8) | 0.298 |
| Double product (bpm*mmHg) | 7934 (1631) | 8503 (1592) | 0.260 |
| HR mean (1/min) | 68.0 (10.0) | 68.3 (10.1) | 0.912 |
| RR mean (1/min) | 902.7 (134.9) | 872.7 (106.8) | 0.427 |
| SDNN (ms) | 22.1 (10.6) | 20.4 (8.5) | 0.578 |
| rMSSD (ms) | 19.8 (10.5) | 21.2 (14.9) | 0.742 |
| pNN50 (%) | 3.2 (4.2) | 5.2 (10.1) | 0.402 |
| LF (n.u) | 66.5 (12.6) | 58.2 (22.3) | 0.136 |
| HF (n.u) | 33.2 (12.5) | 41.5 (21.9) | 0.138 |
| LF/HF | 2.4 (1.3) | 2.5 (2.9) | 0.839 |
| SD1 (ms) | 14.0 (7.4) | 15.0 (10.5) | 0.739 |
| SD2 (ms) | 27.7 (12.8) | 24.0 (8.4) | 0.267 |
| SD1/SD2 (ms2) | 2.0 (0.4) | 1.9 (0.8) | 0.758 |
Captions: SD standard deviation, HR heart rate, kg kilograms, cm centimeters, mmHg millimeters of mercury, bpm beats per minute, min minute, m2 meters squared, % percentage, RR RR intervals, SDNN standard deviation of RR intervals, ms millisecond, rMSSD square root of the mean square of the differences between adjacent normal RR intervals, pNN50 percentage of adjacent RR intervals with a difference in duration greater than 50 ms, LF low frequency, HF high frequency, n.u. normalized unit, ms2 millisecond squared, SD1 standard deviation of instantaneous beat-to-beat variability, SD2 long-term standard deviation of continuous RR intervals
Table 2 presents the effects of MT and FT on cardiac autonomic modulation indexes adjusted by body mass at the initial moment. In the mean HR, no group effect was shown (F1,39 = 1.009; p = 0.321; η2p = 0.025, small), nor the interaction (F1,39 = 2.583; p = 0.116; η2p = 0.128, medium), but there was an effect of the moment (F1,39 = 5.701; p = 0.022; η2p = 0.062, medium). However, when Bonferroni correction was applied, this difference was not confirmed. No statistical differences were observed in the other indices of cardiac autonomic modulation.
Table 2.
Effect of Muay Thai training compared to functional training on cardiac autonomic modulation indices in older adults, through analysis of covariance adjusted by body mass
|
Muay Thai (n = 19) Mean (SD) |
Functional (n = 22) Mean (SD) |
Effect | F | p-value | η2p | |||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||
| HR mean (1/min) | 68.0 (10.0) | 67.8 (10.6) | 68.1 (9.9) | 71.1 (11.3) | Group | 1.009 | 0.321 | 0.025 |
| Moment | 5.701 | 0.022 | 0.128 | |||||
| Interaction | 2.583 | 0.116 | 0.062 | |||||
| RR mean (1/min) | 902.7 (134.9) | 904.9 (136.4) | 872.7 (106.8) | 861.4 (124.8) | Group | 2.280 | 0.139 | 0.055 |
| Moment | 1.755 | 0.193 | 0.043 | |||||
| Interaction | 0.648 | 0.426 | 0.016 | |||||
| SDNN (ms) | 2 2.1 (10.6) | 22.4 (8.9) | 20.4 (8.5) | 22.0 (19.8) | Group | 0.318 | 0.576 | 0.080 |
| Moment | 0.848 | 0.363 | 0.022 | |||||
| Interaction | 0.267 | 0.609 | 0.007 | |||||
| rMSSD (ms) | 19.8 (10.5) | 23.3 (13.2) | 21.2 (14.9) | 26.1 (34.5) | Group | 0.001 | 0.977 | < 0.001 |
| Moment | 0.802 | 0.376 | 0.020 | |||||
| Interaction | 0.166 | 0.686 | 0.004 | |||||
| pNN50 (%) | 3.2 (4.2) | 5.4 (7.1) | 5.2 (10.1) | 8.2 (18.7) | Group | 0.205 | 0.653 | 0.005 |
| Moment | 0.800 | 0.377 | 0.020 | |||||
| Interaction | 0.159 | 0.692 | 0.004 | |||||
| LF (n.u.) | 66.5 (12.6) | 60.2 (19.4) | 58.2 (22.3) | 59.2 (23.2) | Group | 0.032 | 0.859 | 0.001 |
| Moment | 0.170 | 0.682 | 0.004 | |||||
| Interaction | 1.865 | 0.180 | 0.046 | |||||
| HF ( n.u.) | 33.2 (12.5) | 39.6 (19.3) | 41.5 (21.9) | 40.5 (23.0) | Group | 0.030 | 0.863 | 0.001 |
| Moment | 0.162 | 0.689 | 0.004 | |||||
| Interaction | 1.888 | 0.177 | 0.046 | |||||
| LF/HF | 2.4 (1.3) | 2.0 (1.2) | 2.5 (2.3) | 2.3 (1.9) | Group | 0.476 | 0.494 | 0.012 |
| Moment | 1.534 | 0.223 | 0.038 | |||||
| Interaction | 0.634 | 0.431 | 0.016 | |||||
| SD1 (ms) | 14.0 (7.4) | 16.4 (9.3) | 15.0 (10.5) | 18.4 (24.4) | Group | 0.001 | 0.979 | < 0.001 |
| Moment | 0.799 | 0.377 | 0.020 | |||||
| Interaction | 0.160 | 0.591 | 0.004 | |||||
| SD2 (ms) | 27.7 (12.8) | 26.7 (9.9) | 24.0 (8.4) | 23.8 (15.9) | Group | 1.457 | 0.235 | 0.036 |
| Moment | 0.833 | 0.367 | 0.021 | |||||
| Interaction | 0.237 | 0.629 | 0.006 | |||||
| SD1/SD2 (ms2) | 2.0 (0.4) | 1.8 (0.6) | 1.9 (0.8) | 1.9 (0.7) | Group | 0.306 | 0.583 | 0.008 |
| Moment | 0.171 | 0.682 | 0.004 | |||||
| Interaction | 0.543 | 0.466 | 0.014 | |||||
Captions: SD standard deviation, η2p partial eta squared, HR heart rate, min minute, % percentage, RR RR intervals, SDNN standard deviation of RR intervals, ms millisecond, rMSSD square root of the mean square of the differences between adjacent normal RR intervals, pNN50 percentage of adjacent RR intervals with a difference in duration greater than 50 ms, LF low frequency, HF high frequency, n.u. normalized unit, ms2 millisecond squared, SD1 standard deviation of instantaneous beat-to-beat variability, SD2 long-term standard deviation of continuous RR intervals
The intensity of FT and MT was analyzed at the end of each month using the subjective perception of effort scale according to the days of the week and the muscle group in focus, Monday upper limbs, Wednesday lower limbs and Friday -day global exercises working the whole body are presented in Fig. 2. It can be seen that the intensity of the groups was similar and that there was an increase and/or maintenance of training intensity each month. In the upper limbs there was no effect of group (F1,10 = 1.179; p = 0.303; η2p = 0.105, medium), nor the interaction (F2,20 = 1.255; p = 0.307; η2p = 0.112, medium), but there was an effect of the moment (F2,20 = 8.521; p = 0.002; η2p = 0.460, large). The CT showed an increase in intensity due to the subjective perception of effort in the upper limbs compared to the moment four weeks with 12 weeks (P = 0.030; 95%CIdif = -4.1; -0.2). Globally there was no group effect (F1,9 = 0.609; p = 0.455; η2p = 0.063, medium), nor the interaction (F2,18 = 0.562; p = 0.580; η2p = 0.059, small), but there was an effect of the moment (F2,18 = 3.608; p = 0.048; η2p = 0.286, large), however, it was not confirmed in the Bonferroni correction. There was also reinforcement of intensity control using the accelerometer and it can be observed that throughout the intervention, in both groups, the time spent in light physical activity decreased, moderate physical activity increased and, at times, vigorous and very intense physical activity vigorous (Supplementary Figure S3).
Fig. 2.
Description and comparison of the intensity of Functional Training and Muay Thai measured by subjective perception of effort according to the days of the week. Note: MT = Muay Thai; FT = functional training; SPE = subjective perception of effort; * indicates difference between moments
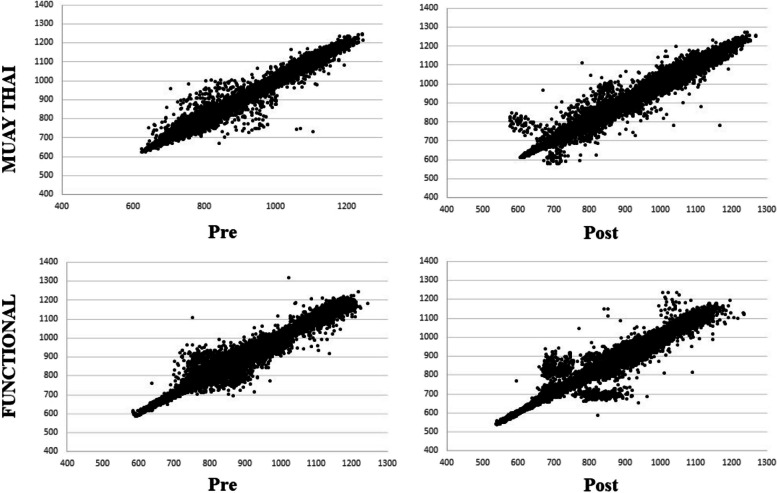
In the visual analysis of the Poincaré plot, although both groups showed a little more dispersion after 12 weeks of training, it was observed that the amplitude of the dispersion of the points in the RR intervals was similar in the FT and MT groups (Fig. 3).
Fig. 3.
Poincaré plot graph observed in the Muay Thai training group and Functional Training group. Note: Pre = initial moment; Post = moment after 12 weeks of intervention
The cardiovascular effects after 12 weeks of MT training intervention compared to FT were analyzed in the variables of systolic and diastolic blood pressure, pulse pressure, heart rate and double product. For diastolic blood pressure, there was no group effect (F1,39 = 1.876; P = 0.179; η2p = 0.046, small), but there was an effect of the moment (F1,39 = 8.206; P = 0.007; η2p = 0.174, large), as well as interaction effect between group and moment (F1,39 = 7.950; P = 0.008; η2p = 0.169, large). Specifically, the MT group at the post-training moment had lower diastolic blood pressure (P = 0.010; 95%CIdif = -13.3; -1.89) in relation to the FT group. Furthermore, the MT group showed a decrease in diastolic blood pressure during training (P = 0.002; 95%CIdif = -10.3; -2.6). The double product showed no group effect (F1,39 = 3.959; p = 0.054; η2p = 0.092, medium) and interaction (F1,39 = 2.620; p = 0.114; η2p = 0.063, medium), but it had a moment effect (F1,39 = 6.230; p = 0.017; η2p = 0.138, medium) Therefore, the MT group at the post-training moment presented a lower double product (P = 0.029; 95%CIdif = -2647.0; -151.7) in relation to the FT group. No statistical differences were evident for the other hemodynamic parameters (Fig. 4).
Fig. 4.
Effect of Functional Training compared to Muay Thai training on systolic, diastolic and pulse blood pressure, resting heart rate and double product in older adults. Note: F = strength; η2p = partial eta squared; mmHg = millimeters of mercury; bpm = beats per minute; Pre = initial moment; Post = moment after 12 weeks of intervention; * indicates difference between moments; # indicates difference between groups
Discussion
Among the main findings of the present study, a significant reduction in diastolic blood pressure was observed in the MT group and lower values post-training compared to FT. Furthermore, the double product of the MT post-intervention moment was smaller than that of the FT. An increase in training intensity was also found over the 12 weeks in FT, with no difference between the groups.
The World Health Organization has highlighted the importance of increasing intensities in weekly physical activity [51]. Previous studies have shown that moderate to vigorous physical intensity is linked to greater cardiac autonomic modulation [52–54]. Oliveira-Dantas et al. [55] in a study with elderly hypertensive women reported that the practice of exercise through resistance training, compared to control, after 10 weeks, contributed to increases in cardiac autonomic modulation. Another study with adult sedentary workers also showed improvements in cardiac autonomic modulation after 12 weeks of FT [28].
Despite presenting themselves as similar, previous studies with older adults [56–59] present an adaptation of dance movements that are used in traditional Muay Thai rituals, called Wai Kru, some present them as Thai boxing or Thai dance, but they are not fighting movements, they are dance. These showed benefits in balance and functional fitness parameters [56–59]. Considering our knowledge, only one study analyzed the effects of the Muay Thai combat sport modality with older adults and after 8 weeks of training it promoted benefits on balance and flexibility [60].
The present study did not find differences for other indexes indicative of autonomic modulation, which does not corroborate previous studies [32, 61]. It is noteworthy that our study compared two different types of training, while the comparative group in other studies was the control, in which participants did not practice any type of physical exercise [32, 61], which certainly contributed to the observed discrepancies. Furthermore, in addition to the difference in intervention time, the study of Rezende Barbosa et al. [62] was carried out only with postmenopausal women and, combined with FT, included aerobic exercises in the intervention, which may also have contributed to the difference in results in relation to those of the present study. Still, a determining factor in promoting benefits on cardiac autonomic modulation is training intensity [26, 63]. Therefore, aging can reduce the performance of practitioners, which is why controlling the intensity of training is essential. As we can see, in general, the older adults did not reach high intensity in training, which may also have influenced our results.
Another important result of the present study is the maintenance of the Poincaré plot indices in the FT and MT groups. The SD1 index and the SD1/SD2 ratio have parasympathetic autonomic influence, while SD2 represents global variability [64–66]. Unlike the present study, Rezende Barbosa et al. [32], after 18 weeks of FT, they observed an increase in SD1, SD2 and SD1/SD2 ratios, with the increase in SD1 being significant when compared to the group. Perhaps, it is possible can attribute this lack of positive results to the time of intervention, since for autonomic modulation it may have been an insufficient time, given that the cardiovascular condition of elderly people is more compromised than younger individuals and, therefore, of slower progression and response. However, the analysis of the Poincaré plot in the present study allowed us to observe the maintenance of cardiac autonomic modulation in the MT and FT groups. This is an important result of reducing the harmful effects of aging on cardiac autonomic modulation.
In young populations, the practice of MT has already demonstrated benefits on cardiovascular parameters [20–22], however, there is no study on the practice of MT and cardiovascular parameters in older adults. The intermittent characteristic of MT may explain the significant reduction observed in diastolic blood pressure in present study. Despite different populations, middle-aged hypertensive adults, previous studies with 12-week intermittent exercise interventions improved myocardial function and reduced systolic and diastolic blood pressure [67]. The present study corroborates previous studies in adults with hypertension, in which the practice of Tai Chi resulted in a reduction in blood pressure compared to brisk walking, after three months of intervention [68]. Additionally, a meta-analysis indicated that Tai Chi significantly reduced systolic and diastolic blood pressure compared to the control group in adults with essential hypertension [69]. However, Tai Chi Chuan is considered a soft modality, with lighter movements, close to Yoga, so they are different modalities. Another aspect is that approximately 60% of the volunteers in the present study had high blood pressure, but their values were controlled and this may be one of the reasons why there was no reduction in SBP, only in diastolic blood pressure, since it is more difficult to reduce blood pressure values of normotensive individuals. Finally, because they are considered hard modalities (FT and MT), with movements of moderate to vigorous intensity, the older adults who signed up to participate in the interventions may have better cardiovascular health, whereas those with greater heart problems may be afraid of the characteristic of the modality, unlike if they were modalities like Tai Chi and Yoga, for example. However, it is a characteristic of the sample that could not be controlled, and could be considered a sample selection bias.
In the literature, the effects of FT on blood pressure are still controversial. Rezende Barbosa et al. [70] in a 12-week FT study with healthy women, no significant differences were observed in systolic and diastolic blood pressure. Nonetheless Choi et al. [33], after 12 weeks of FT, they observed a reduction in systolic and diastolic blood pressure in healthy older adults, while the control group increased these variables. It is important to highlight that in the aging process, the maintenance of cardiovascular parameters or a slight improvement are already considered beneficial effects, since in the older adults the tendency is to increase blood pressure due to changes resulting from the decrease in arterial compliance [71]. As mentioned previously, most previous studies compared the intervention with a sedentary control group (or one that did not receive any intervention), which increases the tendency for positive results in the intervention group (result bias), while ours compared two modalities in that (at least hypothetically) both would bring benefit, although they are different, both modalities showed similar results in this population.
Pulse pressure is a viable tool for measuring vascular aging and a good marker of cardiovascular risk in the elderly, as the higher the pulse pressure values, the greater the cardiovascular risk [72]. Furthermore, pulse pressure is an independent indicator of arterial stiffness and is more associated with cardiovascular events than systolic or diastolic blood pressure alone [73]. Previous studies such as Choi et al. [33] showed a reduction in pulse pressure after 12 weeks of FT when compared to the control group, which was not observed in the present study. This result may be due to the groups having similar results in this variable, not implying a difference between them. However, the absence of differences between the groups does not mean that both groups improved as expected, as the comparative analyzes did not show a significant effect of time (even if there was no group effect). Therefore, in some aspects it did not improve significantly pre vs. post (regardless of the intervention, which is different from expectations).
In our study, the double product was also analyzed, which is an index that indicates myocardial oxygen consumption or cardiac workload demand [74, 75]. The double product of the MT post-intervention moment was smaller than the FT. The decrease in MT double product may originate from the reduction in myocardial oxygen demand and improvement in myocardial performance [74, 75]. Therefore, practicing MT can improve the effectiveness of cardiac function with less energy use. Additionally, the effect of nitric oxide on reducing systolic blood pressure and improving heart rate function may also have contributed to this finding. In general, MT showed a tendency to improve the hemodynamic parameters of practitioners. This may have occurred due to the characteristic of the modality in which there are peaks of higher intensities within the session. Consequently, the use of pulsometers to control intensity during the session is suggested in future studies.
Combat sports modalities have specificities such as physical confrontation, frequent intervals and plyometrics, which limit the ways of monitoring the intensity of this type of training [36]. The present study advances in using accelerometry to complement the subjective perception of effort, since ratings of perceived effort are one of the most used and valid ways of monitoring and/or controlling the intensity of combat sports training [36, 76]. However, it does not always have a high relationship with the physiological changes produced during physical exercise [77]. Studies have shown significant correlations between subjective perception of exertion, blood lactate and heart rate [36]. Even though these studies did not use an accelerometer, this equipment can provide physical activity intensity values (light, moderate, vigorous and very vigorous), with greater accuracy and precision than self-report measures or pedometers, for example [78].
Despite the relevance of the study, it is necessary to mention some limitations such as the lack of control of habitual physical activity and food intake, which may influence the variables analyzed. Another point is that it was not possible to blind the evaluators and volunteers, as this is a study that uses physical exercise programs as an intervention. Finally, the lack of use of pulsometers to control intensity during training sessions what could have caused older adults have not reached and maintained high intensity in training. As strengths, the study design stands out, which was a randomized clinical trial using two types of training, and intensity monitoring using the subjective perception of exertion scale and accelerometer. As practical applications of this study, healthcare professionals often provide recommendations for patients to exercise regularly to promote a healthy lifestyle and reduce the risk of chronic diseases. In this sense, MT can be recommended by health professionals for the older adults due to its ease of application, as long as it is adapted, as it is a dynamic physical activity and can be worked on in groups.
Thus, it is possible to conclude that 12 weeks of MT practice was able to reduce diastolic blood pressure post-training in older adults. Furthermore, MT showed lower values of diastolic blood pressure and double product in the post-training period of older adults compared to FT. An increase in training intensity was also found over the 12 weeks in FT, with no difference between the groups. However, it is recommended that future randomized clinical trials use and compare these training models, including a control group, to confirm these promising results.
Supplementary Information
Acknowledgements
Thanks to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 for financing part of the study.
Authors’ contributions
BTCS, EF, ASR, LAG, MAC, LCMV, GF, WRT, and DGDC: the conception and design of the study, or acquisition of data, or analysis and interpretation of data; BTCS, EF, ASR, LAG, MAC, and LCMV: drafting the article or revising it critically for important intellectual content; BTCS, EF, ASR, LAG, MAC, LCMV, GF, WRT, and DGDC: final approval of the version to be submitted.
Funding
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Availability of data and materials
The data that supports the findings of this study are available from Bruna T. C. Saraiva (corresponding author) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from Bruna T. C. Saraiva.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibbons CH. Basics of autonomic nervous system function. Handb Clin Neurol. 2019;160:407–18. 10.1016/B978-0-444-64032-1.00027-8 [DOI] [PubMed] [Google Scholar]
- 2.Catai AM, Pastre CM, de Godoy MF, da Silva E, de Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Braz J Phys Ther. 2020;24:91–102. 10.1016/j.bjpt.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hämmerle P, Eick C, Blum S, Schlageter V, Bauer A, Rizas KD, et al. Heart rate variability triangular index as a predictor of cardiovascular mortality in patients with atrial fibrillation. J Am Heart Assoc. 2020;9:e016075. 10.1161/JAHA.120.016075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopman JJE, van Bodegom D, Maan AC, Li Z, Ziem JB, Westendorp RGJ, et al. Heart rate variability, but not heart rate, is associated with handgrip strength and mortality in older Africans at very low cardiovascular risk: A population-based study. Int J Cardiol. 2015;187:559–61. 10.1016/j.ijcard.2015.03.383 [DOI] [PubMed] [Google Scholar]
- 5.Kohler IV, Sudharsanan N, Bandawe C, Kohler H-P. Aging and hypertension among the global poor—Panel data evidence from Malawi. PLOS Global Public Health. 2022;2:e0000600. 10.1371/journal.pgph.0000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JPH, Beilharz JE, Vollmer-Conna U, Cvejic E. Heart rate variability as a marker of healthy ageing. Int J Cardiol. 2019;275:101–3. 10.1016/j.ijcard.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 7.Djärv T, Wikman A, Lagergren P. Number and burden of cardiovascular diseases in relation to health-related quality of life in a cross-sectional population-based cohort study. BMJ Open. 2012;2:e001554. 10.1136/bmjopen-2012-001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peacock E, Joyce C, Craig LS, Lenane Z, Holt EW, Muntner P, et al. Low medication adherence is associated with decline in health-related quality of life: results of a longitudinal analysis among older women and men with hypertension. J Hypertens. 2021;39:153–61. 10.1097/HJH.0000000000002590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanbara K, Morita Y, Hasuo H, Abe T. The association between heart rate variability and quality of life in patients with functional somatic syndrome and healthy controls. Appl Psychophysiol Biofeedback. 2021;46:279–85. 10.1007/s10484-021-09515-1 [DOI] [PubMed] [Google Scholar]
- 10.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5:e003231. 10.1161/JAHA.116.003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira IC, de Santos ZMSA, Mont’ Alverne DGB, Martins ABT, de Magalhães CBA. Efeitos do exercício físico no controle da hipertensão arterial em idosos: uma revisão sistemática. Revista Brasileira de Geriatria e Gerontologia. 2012;15:587–601. 10.1590/S1809-98232012000300019 [DOI] [Google Scholar]
- 12.Park S, Han K, Lee S, Kim Y, Lee Y, Kang MW, et al. Cardiovascular or mortality risk of controlled hypertension and importance of physical activity. Heart. 2021;107:1472–9. 10.1136/heartjnl-2020-318193 [DOI] [PubMed] [Google Scholar]
- 13.Raffin J, Barthélémy J-C, Dupré C, Pichot V, Berger M, Féasson L, et al. Exercise frequency determines heart rate variability gains in older people: a meta-analysis and meta-regression. Sports Med. 2019;49:719–29. 10.1007/s40279-019-01097-7 [DOI] [PubMed] [Google Scholar]
- 14.Ravier G, Dugué B, Grappe F, Rouillon J-D. Maximal accumulated oxygen deficit and blood responses of ammonia, lactate and pH after anaerobic test: a comparison between International and National Elite Karate Athletes. Int J Sports Med. 2006;27:810–7. 10.1055/s-2005-872965 [DOI] [PubMed] [Google Scholar]
- 15.Franchini E, Cormack S, Takito MY. Effects of high-intensity interval training on olympic combat sports athletes’ performance and physiological adaptation: a systematic review. J Strength Cond Res. 2019;33:242–52. 10.1519/JSC.0000000000002957 [DOI] [PubMed] [Google Scholar]
- 16.Valdés-Badilla P, Herrera-Valenzuela T, Ramirez-Campillo R, Aedo-Muñoz E, Báez-San Martín E, Ojeda-Aravena A, et al. Effects of olympic combat sports on older adults’ health status: a systematic review. Int J Environ Res Public Health. 2021;18:7381. 10.3390/ijerph18147381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crisafulli A, Vitelli S, Cappai I, Milia R, Tocco F, Melis F, et al. Physiological responses and energy cost during a simulation of a Muay Thai boxing match. Appl Physiol Nutr Metab. 2009;34:143–50. 10.1139/H09-002 [DOI] [PubMed] [Google Scholar]
- 18.Saraiva BTC, Scarabottolo CC, Christofaro DGD, Silva GCR, Freitas Junior IF, Vanderlei LCM, et al. Effects of 16 weeks of Muay Thai training on the body composition of overweight/obese adolescents Ido movement for culture. J Martial Arts Anthropol. 2021;21:35–44. [Google Scholar]
- 19.Saraiva BTC, Ritti-Dias RM, Scarabottolo CC, da Silva ALF, Tebar WR, Christofaro DGD. Effects of nine months practice of martial arts on aerobic fitness in children and adolescentes. Sci Sports. 2023;38:394–400. 10.1016/j.scispo.2022.02.007 [DOI] [Google Scholar]
- 20.Saraiva BTC, do Prado WL, Vanderlei LCM, Milanez VF, de Damato TMM, dos Santos AB, et al. Acute effects of Muay Thai on blood pressure and heart rate in adolescents with overweight/obesity. Obesities. 2022;2:94–102. 10.3390/obesities2010009 [DOI] [Google Scholar]
- 21.Saraiva BTC, Ritti-Dias RM, Farah BQ, Suetake VYB, Diniz TA, Costa Júnior P, et al. Cardiovascular effects of 16 weeks of martial arts training in adolescents. Revista Brasileira de Medicina do Esporte. 2018;24:212–5. 10.1590/1517-869220182403179093 [DOI] [Google Scholar]
- 22.Saraiva BTC, Franchini E, Vanderlei LCM, Milanez VF, Tebar WR, Beretta VS, et al. Effects of 16-week Muay Thai practice on cardiovascular parameters in children and adolescents with overweight/obesity. Sport Sci Health. 2024;20:647–57. 10.1007/s11332-023-01158-5 [DOI] [Google Scholar]
- 23.Rapkiewicz JA, Nunes JP, Mayhew JL, Ribeiro AS, Nabuco HC, Fávero MT, et al. Effects of Muay Thai training frequency on body composition and physical fitness in healthy untrained women. J Sports Med Phys Fitness. 2018;58:1808. 10.23736/S0022-4707.17.07969-5 [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Adult participation in aerobic and muscle-strengthening physical activities–United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:326–30. [PMC free article] [PubMed] [Google Scholar]
- 25.de Abreu RM, Rehder-Santos P, Simões RP, Catai AM. Can high-intensity interval training change cardiac autonomic control? A systematic review. Braz J Phys Ther. 2019;23:279–89. 10.1016/j.bjpt.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granero-Gallegos A, González-Quílez A, Plews D, Carrasco-Poyatos M. HRV-based training for improving VO2max in endurance athletes. A systematic review with meta-analysis. Int J Environ Res Public Health. 2020;17:7999. 10.3390/ijerph17217999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venturelli M, Cè E, Limonta E, Schena F, Caimi B, Carugo S, et al. Effects of endurance, circuit, and relaxing training on cardiovascular risk factors in hypertensive elderly patients. Age (Omaha). 2015;37:101. 10.1007/s11357-015-9835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira-Junior SA, Boullosa D, Mendonça MLM, Vieira LFC, Mattos WW, Amaral BOC, et al. Effects of circuit weight-interval training on physical fitness, cardiac autonomic control, and quality of life in sedentary workers. Int J Environ Res Public Health. 2021;18:4606. 10.3390/ijerph18094606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcos-Pardo PJ, Orquin-Castrillón FJ, Gea-García GM, Menayo-Antúnez R, González-Gálvez N, de Vale RGS, et al. Effects of a moderate-to-high intensity resistance circuit training on fat mass, functional capacity, muscular strength, and quality of life in elderly: a randomized controlled trial. Sci Rep. 2019;9:7830. 10.1038/s41598-019-44329-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinrich KM, Crawford DA, Langford CR, Kehler A, Andrews V. High-intensity functional training shows promise for improving physical functioning and activity in community-dwelling older adults: a pilot study. J Geriatr Phys Ther. 2021;44:9–17. 10.1519/JPT.0000000000000251 [DOI] [PubMed] [Google Scholar]
- 31.De Matos DG, Mazini Filho ML, Moreira OC, De Oliveira CE, De Oliveira Venturini GR, Da Silva-Grigoletto ME, et al. Effects of eight weeks of functional training in the functional autonomy of elderly women: a pilot study. J Sports Med Phys Fitness. 2017;57:272. 10.23736/S0022-4707.16.06514-2 [DOI] [PubMed] [Google Scholar]
- 32.Rezende Barbosa MP, Vanderlei LC, Neves LM, Takahashi C, Torquato PR, Silva AK, et al. Functional training in postmenopause: Cardiac autonomic modulation and cardiorespiratory parameters, a randomized trial. Geriatr Gerontol Int. 2019;19:823–8. 10.1111/ggi.13690 [DOI] [PubMed] [Google Scholar]
- 33.Choi H-M, Hurr C, Kim S. Effects of elastic band exercise on functional fitness and blood pressure response in the healthy elderly. Int J Environ Res Public Health. 2020;17:7144. 10.3390/ijerph17197144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Origua Rios S, Marks J, Estevan I, Barnett LM. Health benefits of hard martial arts in adults: a systematic review. J Sports Sci. 2018;36:1614–22. 10.1080/02640414.2017.1406297 [DOI] [PubMed] [Google Scholar]
- 35.Zou L, Sasaki J, Wei G-X, Huang T, Yeung A, Neto O, et al. Effects of mind-body exercises (Tai Chi/Yoga) on heart rate variability parameters and perceived stress: a systematic review with meta-analysis of randomized controlled trials. J Clin Med. 2018;7:404. 10.3390/jcm7110404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slimani M, Davis P, Franchini E, Moalla W. Rating of perceived exertion for quantification of training and combat loads during combat sport-specific activities: a short review. J Strength Cond Res. 2017;31:2889–902. 10.1519/JSC.0000000000002047 [DOI] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332–c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai J-C, Wang W-H, Chan P, Lin L-J, Wang C-H, Tomlinson B, et al. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. J Altern Complement Med. 2003;9:747–54. 10.1089/107555303322524599 [DOI] [PubMed] [Google Scholar]
- 39.Ramos E, Vanderlei L, Ramos D, Teixeira L, Pitta F, Veloso M. Influence of pursed-lip breathing on heart rate variability and cardiorespiratory parameters in subjects with chronic obstructive pulmonary disease (COPD). Braz J Phys Ther. 2009;13:288–93. 10.1590/S1413-35552009005000035 [DOI] [Google Scholar]
- 40.Quintana DS, Heathers JAJ. Considerations in the assessment of heart rate variability in biobehavioral research. Front Psychol. 2014;5:805. 10.3389/fpsyg.2014.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radespiel-Tröger M, Rauh R, Mahlke C, Gottschalk T, Mück-Weymann M. Agreement of two different methods for measurement of heart rate variability. Clin Auton Res. 2003;13:99–102. 10.1007/s10286-003-0085-7 [DOI] [PubMed] [Google Scholar]
- 42.Niskanen J-P, Tarvainen MP, Ranta-aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73–81. 10.1016/j.cmpb.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 43.Tulppo MP, Mäkikallio TH, Seppänen T, Laukkanen RT, Huikuri HV. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am J Physiol-Heart Circul Physiol. 1998;274:H424–9. 10.1152/ajpheart.1998.274.2.H424 [DOI] [PubMed] [Google Scholar]
- 44.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals. Circulation. 2005;111:697–716. 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 45.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. 10.1161/01.HYP.13.4.392 [DOI] [PubMed] [Google Scholar]
- 46.Rafie AHS, Sungar GW, Dewey FE, Hadley D, Myers J, Froelicher VF. Prognostic value of double product reserve. Eur J Cardiovasc Prev Rehabil. 2008;15:541–7. 10.1097/HJR.0b013e328305deef [DOI] [PubMed] [Google Scholar]
- 47.Freitas Júnior IF. Padronização de medidas antropométricas e avaliação da composição corporal. São Paulo: Selo Literário 20 anos da Regulamentação da Profissão de Educação Física; 2018. [Google Scholar]
- 48.Bavaresco Gambassi B, Lopes dos Santos MD, de Furtado Almeida FJ. Basic guide for the application of the main variables of resistance training in elderly. Aging Clin Exp Res. 2019;31:1019–20. 10.1007/s40520-019-01181-y [DOI] [PubMed] [Google Scholar]
- 49.Borg G, Hassmén P, Lagerström M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur J Appl Physiol Occup Physiol. 1987;56:679–85. 10.1007/BF00424810 [DOI] [PubMed] [Google Scholar]
- 50.Maher JM, Markey JC, Ebert-May D. The other half of the story: effect size analysis in quantitative research. CBE-Life Sci Educ. 2013;12:345–51. 10.1187/cbe.13-04-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulli G, Cevese A, Cappelletto P, Gasparini G, Schena F. Moderate aerobic training improves autonomic cardiovascular control in older women. Clin Auton Res. 2003;13:196–202. 10.1007/s10286-003-0090-x [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues JAL, Ferrari GD, Trapé ÁA, de Moraes VN, Gonçalves TCP, Tavares SS, et al. β2 adrenergic interaction and cardiac autonomic function: effects of aerobic training in overweight/obese individuals. Eur J Appl Physiol. 2020;120:613–24. 10.1007/s00421-020-04301-z [DOI] [PubMed] [Google Scholar]
- 54.Christofaro DGD, Tebar WR, Vanderlei LCM, Fernandes RA, Mota J, Mielke GI, et al. Association of cardiac autonomic modulation with different intensities of physical activity in a small Brazilian inner city: a gender analysis. Eur J Sport Sci. 2023;23:649–55. 10.1080/17461391.2022.2044913 [DOI] [PubMed] [Google Scholar]
- 55.Oliveira-Dantas FF, do Brasileiro-Santos MS, Thomas SG, Silva AS, Silva DC, Browne RAV, et al. Short-term resistance training improves cardiac autonomic modulation and blood pressure in hypertensive older women: a randomized controlled trial. J Strength Cond Res. 2020;34:37–45. 10.1519/JSC.0000000000003182 [DOI] [PubMed] [Google Scholar]
- 56.Areeudomwong P, Saysalum S, Phuttanurattana N, Sripoom P, Buttagat V, Keawduangdee P. Balance and functional fitness benefits of a Thai boxing dance program among community-dwelling older adults at risk of falling: a randomized controlled study. Arch Gerontol Geriatr. 2019;83:231–8. 10.1016/j.archger.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 57.Noopud P, Suputtitada A, Khongprasert S, Kanungsukkasem V. Effects of Thai traditional dance on balance performance in daily life among older women. Aging Clin Exp Res. 2019;31:961–7. 10.1007/s40520-018-1040-8 [DOI] [PubMed] [Google Scholar]
- 58.Kaewjoho C, Mato L, Thaweewannakij T, Nakmareong S, Phadungkit S, Gaogasigam C, et al. Thai dance exercises benefited functional mobility and fall rates among community-dwelling older individuals. Hong Kong Physiother J. 2020;40:19–27. 10.1142/S1013702520500031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janyacharoen T, Srisamai T, Sawanyawisuth K. An ancient boxing exercise improves physical functions, balance, and quality of life in healthy elderly persons. Evid-Based Complement Altern Med. 2018;2018:1–4. 10.1155/2018/6594730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sukmee K, Khongprasert S, Laohapakdee R. The effect of Muay Thai exercise on balance and flexibility in Thai elderly. J Sports Sci Health. 2020;21:432–45. [Google Scholar]
- 61.Yeh GY, Wayne PM, Phillips RS. T’ai Chi Exercise in Patients with Chronic Heart Failure. In: Tai Chi Chuan. Basel: KARGER; 2008. p. 195–208. [DOI] [PubMed] [Google Scholar]
- 62.da de Rezende Barbosa MPC, Oliveira VC, da Silva AKF, Pérez-Riera AR, Vanderlei LC. Effectiveness of functional training on cardiorespiratory parameters: a systematic review and meta-analysis of randomized controlled trials. Clin Physiol Funct Imaging. 2018;38:539–46. 10.1111/cpf.12445 [DOI] [PubMed] [Google Scholar]
- 63.Carrasco-Poyatos M, González-Quílez A, Altini M, Granero-Gallegos A. Heart rate variability-guided training in professional runners: effects on performance and vagal modulation. Physiol Behav. 2022;244:113654. 10.1016/j.physbeh.2021.113654 [DOI] [PubMed] [Google Scholar]
- 64.da Silva S, Guida HL, dos SantosAntônio AM, Vanderlei L, Ferreira LL, de Abreu L, et al. Auditory stimulation with music influences the geometric indices of heart rate variability in men. Int Arch Med. 2014;7:27. 10.1186/1755-7682-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santana MD, de Souza AC, de Abreu L, Valenti VE. Association between oral variables and heart rate variability. Int Arch Med. 2013;6:49. 10.1186/1755-7682-6-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderlei LCM, Pastre CM, Hoshi RA, de Carvalho TD, de Godoy MF. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Rev Bras Cir Cardiovasc. 2009;24:205–17. 10.1590/S0102-76382009000200018 [DOI] [PubMed] [Google Scholar]
- 67.Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–60. 10.1177/1741826711400512 [DOI] [PubMed] [Google Scholar]
- 68.Chan AWK, Chair SY, Lee DTF, Leung DYP, Sit JWH, Cheng HY, et al. Tai Chi exercise is more effective than brisk walking in reducing cardiovascular disease risk factors among adults with hypertension: a randomised controlled trial. Int J Nurs Stud. 2018;88:44–52. 10.1016/j.ijnurstu.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 69.Liang H, Luo S, Chen X, Lu Y, Liu Z, Wei L. Effects of Tai Chi exercise on cardiovascular disease risk factors and quality of life in adults with essential hypertension: a meta-analysis. Heart Lung. 2020;49:353–63. 10.1016/j.hrtlng.2020.02.041 [DOI] [PubMed] [Google Scholar]
- 70.da de Rezende Barbosa MPC, Netto Júnior J, Cassemiro BM, de Souza NM, Bernardo AFB, da Silva AKF, et al. Impact of functional training on cardiac autonomic modulation, cardiopulmonary parameters and quality of life in healthy women. Clin Physiol Funct Imaging. 2016;36:318–25. 10.1111/cpf.12235 [DOI] [PubMed] [Google Scholar]
- 71.La SC. hipertensión arterial en el anciano. Hipertens Riesgo Vasc. 2017;34:26–9. 10.1016/S1889-1837(18)30072-2 [DOI] [PubMed] [Google Scholar]
- 72.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–8. 10.1016/S0735-1097(00)00687-2 [DOI] [PubMed] [Google Scholar]
- 73.O’Rourke M, Frohlich ED. Pulse Pressure. Hypertension. 1999;34:372–4. 10.1161/01.HYP.34.3.372 [DOI] [PubMed] [Google Scholar]
- 74.Fornitano LD, de Godoy MF. Duplo produto elevado como preditor de ausência de coronariopatia obstrutiva de grau importante em pacientes com teste ergométrico positivo. Arq Bras Cardiol. 2006;86:138. 10.1590/S0066-782X2006000200010 [DOI] [PubMed] [Google Scholar]
- 75.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–56. 10.1161/01.CIR.57.3.549 [DOI] [PubMed] [Google Scholar]
- 76.Kirk C, Langan-Evans C, Clark DR, Morton JP. Quantification of training load distribution in mixed martial arts athletes: a lack of periodisation and load management. PLoS One. 2021;16:e0251266. 10.1371/journal.pone.0251266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873–99. 10.1080/026404102320761787 [DOI] [PubMed] [Google Scholar]
- 78.Bailey DP, Fairclough SJ, Savory LA, Denton SJ, Pang D, Deane CS, et al. Accelerometry-assessed sedentary behaviour and physical activity levels during the segmented school day in 10–14-year-old children: the HAPPY study. Eur J Pediatr. 2012;171:1805–13. 10.1007/s00431-012-1827-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from Bruna T. C. Saraiva (corresponding author) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission from Bruna T. C. Saraiva.