Abstract
The KidGen Collaborative's Policy Implementation Workshop 2023 celebrated the 10th anniversary of Australia's first kidney genetics clinic in Brisbane. This event marked the establishment of a national network now comprising 19 kidney genetics clinics across Australia, all dedicated to providing equitable access to genomic testing for families affected by genetic kidney diseases. The workshop reflected on past progress and outlined future objectives for kidney genetics in Australia, recognising the collaborative efforts of clinical teams, researchers, and patients. Key insights from the workshop are documented in the proceedings.
Keywords: KidGen, Genomic testing, Kidney disease, Personalised medicine, Creative problem-solving
Introduction
Over the past ten years, significant progress has been made in integrating genomics into clinical care, research, and policy, including in the complex area of genetic kidney diseases (GKD). The KidGen Collaborative offers state-of-the-art care through a network of kidney genetics clinics (KGCs) nationwide. These clinics provide patients access to cutting-edge kidney genomic medicine. Along with its clinical network, KidGen has a well-established network of diagnostic and research laboratories focused on genetic kidney disease. The KidGen Collaborative recently introduced a hybrid program featuring five Virtual Seminars addressing current issues, complemented by an in-person Workshop. The August 2023 KidGen Workshop, held on the traditional lands of the Wulgurukaba people in North Queensland, Australia, brought together collaborators from diverse disciplines to advance healthcare for individuals with GKD. This document outlines the priority topics guiding KidGen's work for at least the next half-decade.
The KidGen virtual seminars: exploring kidney genetics
Five virtual seminars complemented the KidGen Workshop 2023. These seminars offered participants a comprehensive exploration of the latest advancements and emerging trends in kidney genomics and laid the groundwork for the workshop's core content—clinical implementation of genomics. The seminars were well-received, with over 400 attendees. Two-thirds of the attendees were nephrologists and genetic counsellors. Other specialties included government, industry, consumer representatives, geneticists, nurses, scientists, junior doctors, and patients. Table 1 provides concise summaries of each seminar, and comprehensive details are available on the KidGen website (https://www.kidgen.org/kidgen-events).
Table 1.
Laying the Groundwork: Virtual Seminars at KidGen 2023
| Seminar | Topic | Content | Future progress |
|---|---|---|---|
| 1 | Kidney Panels in Genomic Testing: Benefits and Considerations | The seminar focused on understanding virtual kidney panels in genomic testing, emphasizing the importance of maximizing patient benefits and considering factors influencing result interpretation and the need for genetic counselling | Optimizing virtual kidney panels to ensure type-specifics of genetic kidney diseases |
| 2 | Kidney Genetics Multidisciplinary Team Models Across Australia | The kidney genetic clinic—multidisciplinary team (KGC-MDT) model is used to integrate genomic medicine into standard care for patients with GKD. Hospitals, however, must carefully plan and continuously educate to empower their renal teams in utilizing this technology | Widespread adoption, standardization, and knowledge sharing within a sustainable framework |
| 3 | Advancing Genomic Research Through Collaborative Partnerships | Kidney research needs teamwork! This seminar on research strategies stressed working across disciplines. Participants learned advanced techniques for future discoveries in kidney genomics | Building stronger research networks and translating research into clinical applications |
| 4 | Patient Voices: Patient Engagement and Education | Empowering families with knowledge about renal genomic medicine is central to engaging them in informed and shared decision-making. This seminar discussed communicating genetic testing results and diagnoses, supporting patients with undiagnosed conditions, and ensuring equitable access to genomic testing and genetic counselling services | To promote shared decision-making, we need tools and resources that empower patients, families, and healthcare professionals |
| 5 | Enhancing Patient Outcomes: Integrating Data and Clinical Trials in Healthcare | A national infrastructure is needed to improve patient outcomes by integrating genomic information into clinical decision-making and conducting large-scale genomic studies for kidney healthcare | Building the standardized national infrastructure with the focus on data security, privacy and interoperability |
The KidGen workshop 2023: innovations in kidney genetics
The KidGen Workshop 2023 was a dynamic platform for participants to engage in forward-thinking discussions and foster collaborative efforts. The workshop commenced with a retrospective look at the advancements in GKD clinical care and research over the past decade in Australia [1, 2]. This session acknowledged the significant contributions of individuals across various disciplines, including paediatric and adult nephrologists, clinical geneticists, genetic counsellors, researchers, and evaluators. Following this, the workshop offered a forum to envision the next decade and identify fresh opportunities for collaboration and innovation.
The workshop placed a particular emphasis on enhancing the diagnosis of GKD. This encompassed pre-symptomatic detection of kidney diseases in infants through genomic newborn screening [3] and extended to diagnosis to identify novel treatments and interventions. This included discussions on new clinical trials for delivering evidence-based therapies, emphasising the importance of involving genetic counsellors and families in the design of these trials.
A central theme of the workshop revolved around ensuring equitable access to high-quality kidney care for all Australians, regardless of their background or geographical location. The discussion shifted towards therapies for GKD, highlighting the need for a solid research-clinical link. This collaborative effort is essential for swiftly translating research breakthroughs into clinical practice. The following structured summary encapsulates the key highlights and outcomes of the workshop.
Advancing kidney genomics: exploring innovative clinic models
Over the last decade, Australia's kidney genetic model has evolved significantly, reshaping healthcare for patients with genetic kidney disease. A national network of 19 clinics now offers comprehensive paediatric and adult care [4]. Some clinics have merged over time, with many adopting virtual modalities to improve access for patients in regional areas. The referral base has expanded, reflecting increased awareness and demand for genetic diagnosis, indicating our impact.
The clinics now feature various combinations of nephrologists, clinical geneticists, and genetic counsellors, with some sites providing all three services in one location. This multidisciplinary team approach ensures comprehensive patient care, and the model successfully enables genetic diagnosis provision nationally and internationally [4–6].
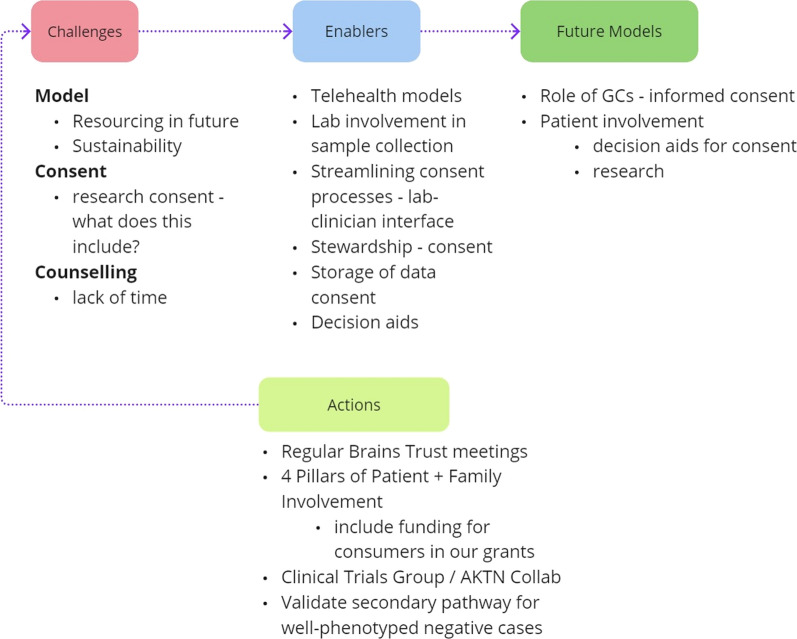
Persistent challenges, such as funding, infrastructure, workforce, and information technology (IT) issues [4], are not just hurdles but opportunities for us to excel (Fig. 1). Funding uncertainties directly impact workforce sustainability, while IT infrastructure deficiencies hinder clinic operations. Addressing these challenges is vital for sustained progress.
Fig. 1.
Navigating the Genomic Landscape in Nephrology: Advancing Precision Care. Informed by their real-world nephrology experiences, KidGen Workshop participants emphasised the need for greater involvement of genetic counsellors and patient representatives in shaping future healthcare models. While challenges like resourcing, consenting, and counselling exist, the participants also identified enablers such as telehealth, stewardship, and decision aids. They recommended organising regular brain trust meetings and creating secondary pathways for genetically undiagnosed but well-phenotyped patients as integral components of future models
Diagnostic laboratories conducting genetic testing face staffing and infrastructure challenges as testing volumes rise [7]. Sustainable solutions are needed for kidney genomic medicine, focusing on efficient services and patient needs. Informed genetic/genomic testing consent is crucial, with strategies like national consent forms and educational initiatives supporting consistent uptake [8, 9].
The Australian multidisciplinary team model is a 'care and learn' approach that supports training future medical professionals. Transitioning from multidisciplinary kidney genetic clinics to local nephrology clinics aims to mainstream genomics into patient care. This shift has already commenced, with genomic tests increasingly performed outside these specialised clinics. This will ensure equitable service delivery across Australia while adapting to local needs. Collaboration between regional and national clinics is essential for consistent quality of care and training.
Empowering patients: the vital role of consumers in kidney genomic medicine
Identifying and implementing patient values and priorities in research, practice, and policy is widely advocated to improve patient-centred care and outcomes [10]. The KidGen collaborative has been committed to enhancing family involvement in research to further our understanding of the unique consumer experiences in GKD, which shape the prioritisation of patient-reported outcomes. Family involvement in all stages of research has the benefits of improved participant recruitment and retention, enriched data analysis, and broader dissemination and translation of research findings to practice and policy [11–13]. Patient-centred research initiatives can shape the future of GKD care by addressing unmet needs, including improved consumer health literacy, specialised counselling, supportive care, and accessibility, especially in diverse communities. In Australia, culturally sensitive research and healthcare practices are being used, led by trained genetic counsellors, to improve access to specialised kidney care. This benefits urban communities and reaches rural areas where up to a sixth of Indigenous Australians live, according to data from the Australian Bureau of Statistics (ABS) 2021 Census. As a result, Indigenous patients are attending our research programs and renal genetic clinics in proportion to their population. The KidGen collaborative environment emphasises family impact, therapy options, health literacy, and patient involvement. Recommendations to ensure equal representation and successful consumer engagement include budget allocations for family participation and education and training opportunities for research involvement.
Advancements in kidney genomic medicine: exploring trials and innovative treatments
Progress in personalised medicine for genetic kidney diseases has revolutionised diagnostic methods, yet challenges persist due to the genetic heterogeneity of kidney conditions linked to over 350 genes [14]. Collaborative clinical trial experiences are crucial for developing effective therapies. While some conditions, like renal tubular acidosis (RTA), respond to standard treatments [15], others, such as autosomal dominant polycystic kidney disease (ADPKD), lack a definitive cure, highlighting the need for innovative therapeutic approaches. Tailoring clinical trials to factors like disease severity and patient population size is essential. Adaptive trials offer flexibility compared to traditional designs, enhancing patient care and collaborative efforts among nephrology professionals [16]. However, the overarching objective will be establishing a national cohort model for continuous GKD clinical trials, supported by a robust regulatory framework, appropriate funding, and comprehensive data accessibility. Learning from successful international models like the nephropathic cystinosis cohort is critical to advancing patient-centric care in Australia [17].
Exploring the benefits and drawbacks of whole genome/exome sequencing in nephrology
The balance of cost-effectiveness and desire to achieve the highest technical yield in the genetic investigation is an ongoing challenge, particularly in conditions with complex genomic regions like ADPKD. Efforts to improve diagnostic outcomes, lower costs, and increase efficiency continue. Genome sequencing, though having a demonstrated higher diagnostic yield than exome sequencing in ADPKD, requires more funding but is expected to match exome sequencing's cost in the future. Clinicians should focus on overlooked phenotypes and disease mechanisms and consider adjunct copy number variant (CNV) tests. Exploring genome sequencing for ADPKD in routine practice requires careful criteria [18]. Investment in genomic technologies and infrastructure is crucial for quality diagnostic services; ideally, offering whole genome sequencing as a test should be the first choice in the near future. Validation studies and multimodal approaches enhance accuracy but need funding. Personalised patient profiling tests could lessen the genomic testing burden. While Australia has made strides in diagnostic technologies, cost remains a concern despite Medicare subsidy—efforts like introducing new sequencing approaches aim to improve access [19]. Access to research pathways and funding via the Medical Research Future Fund can aid undiagnosed cases [20, 21].
Mastering medicare benefits schedule (MBS) item numbers: navigating today and anticipating future expansion
Genomic testing for kidney disease in Australia is a complex and evolving process enabled by publicly funded reimbursement pathways such as the Australian Government’s MBS [22]. The reassessment of MBS item numbers related to kidney conditions is ongoing, involving a complex review process that considers scientific evidence and stakeholder input. While updates occur, older item numbers may still be used alongside new ones to ensure patient care continuity.
Differences in testing practices exist across Australian states and territories [6], including geographical disparities in testing rates. These can impact patient outcomes, highlighting the value of improved awareness of testing options. Expanding operational funding for testing, raising awareness of testing alternatives, and mitigating out-of-pocket expenses are recommended to address these issues. Clinicians' understanding of public financing sources and efforts to harmonise testing access with legislation nationally are crucial. Achieving these goals is critical to optimising Australia's genomic testing system for kidney diseases. Healthcare providers need to master interpreting Medicare rebates for genomic testing. This involves understanding the criteria for specific MBS items for a particular kidney disease.
The national legislative landscape of kidney genomic medicine: examining policies and regulations
Ensuring a supportive legal framework for kidney genomics requires considering the latest scientific evidence and input from stakeholders to guarantee alignment and smooth adaptation. However, there are still disparities in genetic testing practices across Australian states and territories, mainly due to different funding approaches. These differences underscore the urgent need for solutions, with the most important step being to enhance clinicians' knowledge of relevant legislation through the learning healthcare system. This is a crucial and necessary move for a unified and equitable national system [23]. Challenges persist at the state and territory levels regarding genomic medicine policies, requiring collaborative platforms and approaches to Health Technology Assessment and continued reviews of legislative frameworks such as the Genetic Health Insurance Act of 1973.
Furthermore, healthcare professionals involved in genomic medicine and testing in Australia must navigate a complex legal and regulatory landscape. They must be familiar with several key areas to ensure safe and ethical care. The Privacy Act 1988 protects patient genetic information by classifying it as sensitive and requiring explicit consent for its collection and use. The Disability Discrimination Act 1992 prohibits discrimination based on genetic information, ensuring fair treatment in employment, insurance, and other areas of public life. Understanding the Medicare Benefits Schedule (MBS) is essential for healthcare professionals to secure reimbursement for genomic tests, and they must stay updated on which tests are covered as the list evolves with advancements in technology. State laws may also govern aspects of genomic medicine, emphasising the need for ongoing professional development and adherence to best practices.
Australian Genomics’ mission to advance genomic medicine through interdisciplinary collaboration signifies a significant step toward shaping the future of genomic interventions in Australia [24]. Legislative support is paramount in ensuring the equitable distribution of genomic medicine benefits across the population, emphasising the importance of a cohesive and inclusive approach to genomic healthcare delivery.
KidGen genomic health futures mission national kidney genomics program flagship
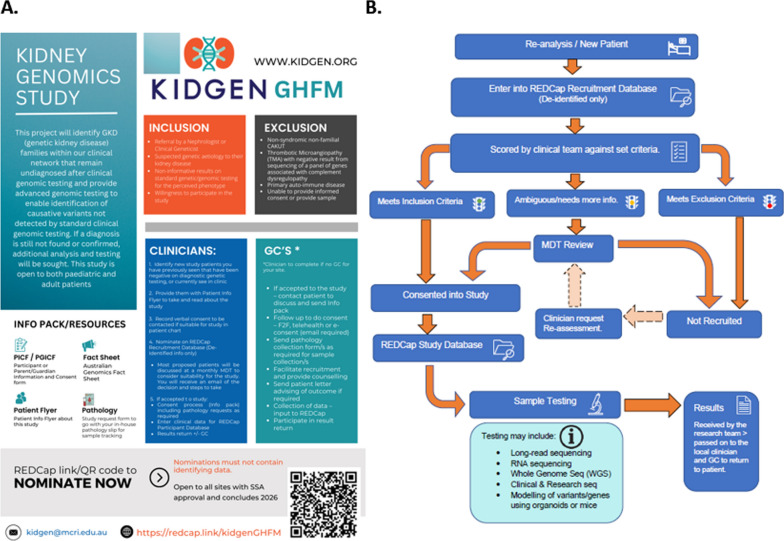
The KidGen Genomics Health Futures Mission (GHFM) National Kidney Genomics Program in Australia is a comprehensive study aiming to enhance patient outcomes for GKD (Fig. 2A). The project targets patients without a genetic diagnosis after clinical diagnostic testing by utilising advanced sequencing techniques not typically available in standard clinical settings [25]. The KidGen GHFM project actively involves patients through its hierarchical structure, which includes a Governance Committee with nine members, including consumer (patient) representatives. These representatives participate in key decision-making processes and ensure patient needs and priorities are central to the research design and execution. The project also offers educational resources and support programs to empower patients, allowing them to engage meaningfully in the research process. Since its launch in 2022, the project has made significant strides in establishing governance protocols, securing ethics approvals, and funding collaborating institutions. A research-based re-analysis of existing genomic datasets is in progress and has already surpassed the intended number of patients, showing promising results that could shape future clinical re-analysis approaches. Intending to recruit around 150 new patients, the progress lies within the expected numbers for a timeline where the national multidisciplinary team approves approximately 80% of cases nominated by 17 participating kidney genetic clinics across Australia. The patients’ research pathway towards genetic diagnosis is outlined in Fig. 2B.
Fig. 2.
Kidney Genomic Study: Overview and Study Flow. The study aims to use advanced genomic testing to deliver genetic diagnoses to previously undiagnosed individuals in clinical networks. A. It accepts both paediatric and adult patients. The diagram describes the inclusion criteria, such as referral by a nephrologist or clinical geneticist, and the exclusion criteria, including non-familial GKD. It also includes advice for doctors and genetic counsellors regarding patient inclusion in the study. The leaflet mentions several resources, including the Participation Information & Consent Form and the Australian Genomic Consent Fact Sheet. A QR code is provided for rapid access to nominations; however, nominations must not include identifiable information. B. The process begins with a new patient or re-analysing a de-identified recruitment database. The clinical team assesses patients based on predefined criteria, determining inclusion, exclusion, or the need for further information. Following a multidisciplinary team (MDT) review, patients are either enrolled in the study or not recruited. Enrolled patients' data are then transferred to a study database for sample testing, which may involve various sequencing methods. The researcher obtained and communicated the results to the local clinician and patient within 12 months
Problem-solving in kidney genetics: strategies and the power of frame innovation
Problem-solving in kidney genetics involves analysing the patient's genetic makeup, medical history, lifestyle choices, and environmental exposures. The field constantly evolves, requiring clinicians to stay updated with the latest research to develop tailored treatment strategies. Clinicians enhance problem-solving skills by mastering kidney genetics and understanding diagnostic pathways, including publicly funded testing and available treatments. Collaboration with colleagues and experts through the KGC-MDT (multidisciplinary team) model is crucial in tackling complex cases [4]. However, clinicians can explore innovative solutions beyond traditional boundaries of knowledge by applying critical and creative thinking [26]. The frame innovation method offers a powerful problem-solving approach for kidney genetics [27], potentially transforming how challenges are tackled. This technique involves redefining or reframing a problem to gain fresh perspectives and uncover novel solutions. It is beneficial for intricate problems, and clinicians can use several methods of reframing a problem, such as shifting the focus. i.e., instead of focusing on the disease, focus on the patient's strengths and capacities [27]. Embracing this method enables researchers and practitioners to explore new perspectives and solutions by reframing problems. Researchers can uncover innovative treatment avenues by focusing on patients' strengths rather than just the disease. Questioning assumptions can lead to breakthroughs in genetic understanding and treatment pathways. Considering broader societal and environmental factors allows for more holistic strategies to address health disparities [28]. Ultimately, applying frame innovation in kidney genetics can advance scientific knowledge, promote health equity, and improve outcomes for marginalised populations by developing targeted interventions.
Unveiling critical issues in kidney genomics in Australia: notes from a panel discussion
Invited experts discussed the future of genomic research and personalised care in kidney genomic medicine in Australia. The director of the Office of Research and Innovation at Queensland Health highlighted the challenges and opportunities ahead in kidney genomic medicine, emphasising the importance of innovation and sustainable research for the health service's viability. These recommendations are crucial for maximising the benefits of genomic medicine while managing costs effectively, including developing innovative care models to support kidney genomic medicine. It is essential to move away from outdated healthcare models and adopt progressive 21st-century concepts [29]. This involves prioritising efficient clinical data management, counselling, and resource allocation. An excellent illustration of this shift is the creation of a patient information registry in the mid-2010s, which exemplifies this change in perspective [30]. In nephrology, the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) plays a vital role as a comprehensive patient information registry, collecting and analysing data related to patients with end-stage kidney disease (ESKD) undergoing dialysis or kidney transplantation [31]. However, patient awareness of ANZDATA needs improvement, as only about half of them are aware of the registry [32]. Therefore, embracing population health strategies can address health challenges, particularly in vulnerable populations such as Aboriginal and Torres Strait Islander communities. Establishing collaborative health models with clear referral pathways is vital for comprehensive patient care.
The codesign concept involves patients and their representatives discussing their needs, which is critical to healthcare reforms. Active engagement with diverse voices, including patients and communities, is essential for shaping the future of genomic medicine through transparent communication and collaboration among stakeholders, which is crucial for meaningful change in the healthcare system.
Moving forward
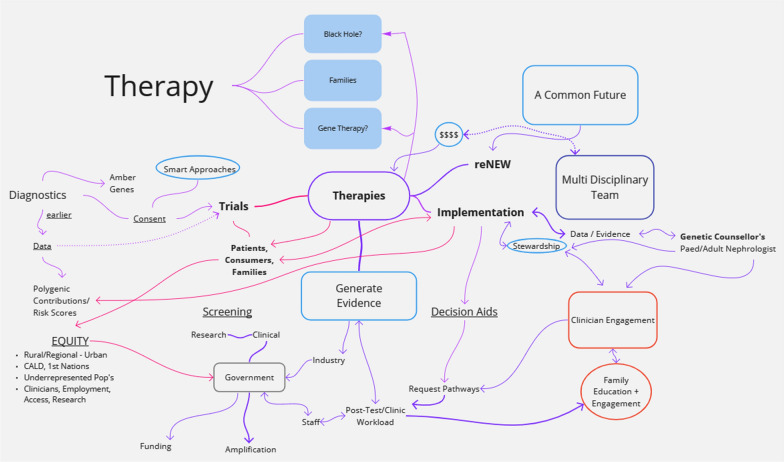
The workshop emphasised critical areas for advancing progress in kidney genomic medicine. The reasoning process is illustrated in Fig. 3, with curated results including:
National Cohort for Clinical Trials: Establish a national patient registry to streamline participation in ongoing and future clinical trials. This will improve access to novel treatments and expedite development processes.
Innovation in Research and Care: Encourage researchers/clinicians to apply "frame innovation"—a creative problem-solving method—to explore new perspectives and treatment options for GKD. This could lead to breakthroughs in understanding the disease and developing targeted interventions.
Patient Engagement and Education: Increase patient involvement in research design and decision-making through education and support programs. This will ensure a focus on patient needs and priorities.
Sustainable Funding Models: Explore innovative financing models, such as value-based payment for kidney genetic clinics, direct funding for genomic services within healthcare budgets, public–private partnerships, secure ongoing research grants, and advocate for equitable access to testing across all Australian jurisdictions, regions, and socioeconomic backgrounds.
Capacity Building: By developing comprehensive training programs for clinicians and genetic counsellors and fostering strong partnerships with health, community, and patient organisations, we will ensure equitable access and best practices for implementing genomic testing in kidney care across tertiary hospitals in Australia.
Fig. 3.
Visualizing Interactive Reasoning in Crafting the Future of Care for Genetic Kidney Diseases. This diagram, collaboratively developed during the KidGen Workshop, encapsulates discussions about the imperative for innovative therapies within well-structured clinical trials for patients facing genetic kidney diseases. Undertaking this intricate task involves the collective efforts of various stakeholders, such as government institutions, clinicians, families, educational activities, and funding bodies, working synergistically towards a shared goal: better lives for patients with genetic kidney diseases
Conclusion
The future of kidney genomics promises a transformative vision for nephrology. By embracing collaboration and innovation, we can unlock the full power of genomics for personalised care. However, building trust for the future requires responsible implementation that addresses ethics, data privacy, and financial sustainability. Population health strategies must be community-led and address social determinants of health. Learning from past mistakes, such as incorporating traditional knowledge, is essential for successful implementation. This path leads to improved patient outcomes and an equitable and transformed landscape of kidney care.
Acknowledgements
Not applicable
Abbreviations
- ADPKD
Autosomal dominant polycystic kidney disease
- CNV
Copy number variant
- GHFM
Genomics Health Futures Mission
- GKD
Genetic kidney disease
- KGC
Kidney genetics clinic
- MBS
Medicare Benefits Schedule
- MDT
Multidisciplinary team
- RTA
Renal tubular acidosis
Author contributions
Conceptualisation: Andrew J Mallett and Zornitza Stark; Writing—original draft: Amali Mallawaarachchi and Erik Biros; Writing—review and editing: Amali Mallawaarachchi, Erik Biros, Trudie Harris, Bruce Bennetts, Tiffany Boughtwood, Justine Elliott, Lindsay Fowles, Robert Gardos, Denisse Garza, Ilias Goranitis, Matilda Haas, Vanessa Huntley, Julia Jefferis, Karin Kassahn, Anna Leaver, Ben Lundie, Sebastian Lunke, Caitlin O'Connor, Greg Pratt, Catherine Quinlan, Dianne Shearman, Jacqueline Soraru, Madhivanan Sundaram, Michel Tchan, Giulia Valente, Julie White, Ella Wilkins, Steve I Alexander, Noa Amir, Stephanie Best, Hossai Gul, Kushani Jayasinghe, Hugh McCarthy, Chirag Patel, Zornitza Stark, Andrew J Mallett.
Funding
Grant ID 4-E8LEZOZ (KidGen CEDAR Program) from the Australian Government Department of Health and Aged Care supported this work.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amali Mallawaarachchi and Erik Biros contributed equally.
References
- 1.Jayasinghe K, et al. Implementation and evaluation of a national multidisciplinary kidney genetics clinic network over 10 years. Kidney International Reports. 2024. 10.1016/j.ekir.2024.04.068. 10.1016/j.ekir.2024.04.068 [DOI] [Google Scholar]
- 2.Mallawaarachchi AC, et al. Genomic testing in patients with kidney failure of an unknown cause: a national australian study. Clin J Am Soc Nephrol. 2024. 10.2215/CJN.0000000000000464. 10.2215/CJN.0000000000000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark Z, Scott RH. Genomic newborn screening for rare diseases. Nat Rev Genet. 2023;24(11):755–66. 10.1038/s41576-023-00621-w. 10.1038/s41576-023-00621-w [DOI] [PubMed] [Google Scholar]
- 4.Mallett A, et al. A multidisciplinary renal genetics clinic improves patient diagnosis. Med J Aust. 2016;204(2):58–9. 10.5694/mja15.01157. 10.5694/mja15.01157 [DOI] [PubMed] [Google Scholar]
- 5.Alkanderi S, et al. Lessons learned from a multidisciplinary renal genetics clinic. QJM. 2017;110(7):453–7. 10.1093/qjmed/hcx030. 10.1093/qjmed/hcx030 [DOI] [PubMed] [Google Scholar]
- 6.Jayasinghe K, et al. Renal genetics in Australia: kidney medicine in the genomic age. Nephrology. 2019;24(3):279–86. 10.1111/nep.13494. 10.1111/nep.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallett A, et al. Genomics in the renal clinic–translating nephrogenetics for clinical practice. Hum Genom. 2015;9(1):13. 10.1186/s40246-015-0035-1. 10.1186/s40246-015-0035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayasinghe K, et al. Comprehensive evaluation of a prospective Australian patient cohort with suspected genetic kidney disease undergoing clinical genomic testing: a study protocol. BMJ Open. 2019;9(8): e029541. 10.1136/bmjopen-2019-029541. 10.1136/bmjopen-2019-029541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas MA, et al. Evaluation of CTRL: a web application for dynamic consent and engagement with individuals involved in a cardiovascular genetic disorders cohort. Eur J Hum Genet. 2023. 10.1038/s41431-023-01454-1. 10.1038/s41431-023-01454-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amir N, McCarthy HJ, Tong A. Qualitative research in nephrology: an introduction to methods and critical appraisal. Kidney360. 2021;2(4):737–41. 10.34067/KID.0006302020. 10.34067/KID.0006302020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon SC, et al. Applying a community-based participatory research framework to patient and family engagement in the development of patient-centered outcomes research and practice. Transl Behav Med. 2018;8(5):683–91. 10.1093/tbm/ibx026. 10.1093/tbm/ibx026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsythe LP, et al. Patient engagement in research: early findings from the patient-centered outcomes research institute. Health Aff. 2019;38(3):359–67. 10.1377/hlthaff.2018.05067. 10.1377/hlthaff.2018.05067 [DOI] [PubMed] [Google Scholar]
- 13.Amir N, McCarthy HJ, Tong A. A working partnership: a review of shared decision-making in nephrology. Nephrology. 2021;26(11):851–7. 10.1111/nep.13902. 10.1111/nep.13902 [DOI] [PubMed] [Google Scholar]
- 14.Robertson AJ, et al. Evolution of virtual gene panels over time and implications for genomic data re-analysis. Genet Med Open. 2023;1(1): 100820. 10.1016/j.gimo.2023.100820. 10.1016/j.gimo.2023.100820 [DOI] [Google Scholar]
- 15.Palmer BF, Kelepouris E, Clegg DJ. Renal tubular acidosis and management strategies: a narrative review. Adv Ther. 2021;38(2):949–68. 10.1007/s12325-020-01587-5. 10.1007/s12325-020-01587-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorlund K, et al. Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ. 2018;360: k698. 10.1136/bmj.k698. 10.1136/bmj.k698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emma F, et al. An international cohort study spanning five decades assessed outcomes of nephropathic cystinosis. Kidney Int. 2021;100(5):1112–23. 10.1016/j.kint.2021.06.019. 10.1016/j.kint.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 18.de Wert G, et al. Opportunistic genomic screening. Recommendations of the European society of human genetics. Eur J Hum Genet. 2021;29(3):365–77. 10.1038/s41431-020-00758-w. 10.1038/s41431-020-00758-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhoads A, Au KF. PacBio sequencing and its applications. Genom Proteom Bioinform. 2015;13(5):278–89. 10.1016/j.gpb.2015.08.002. 10.1016/j.gpb.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert SE, et al. A comparison of the distribution of medical research future fund grants with disease burden in Australia. Med J Aust. 2021;214(3):111-113e1. 10.5694/mja2.50916. 10.5694/mja2.50916 [DOI] [PubMed] [Google Scholar]
- 21.Truty R, et al. Spectrum of splicing variants in disease genes and the ability of RNA analysis to reduce uncertainty in clinical interpretation. Am J Hum Genet. 2021;108(4):696–708. 10.1016/j.ajhg.2021.03.006. 10.1016/j.ajhg.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angeles MR, Crosland P, Hensher M. Challenges for Medicare and universal health care in Australia since 2000. Med J Aust. 2023;218(7):322–9. 10.5694/mja2.51844. 10.5694/mja2.51844 [DOI] [PubMed] [Google Scholar]
- 23.Casey JD, et al. What can a learning healthcare system teach us about improving outcomes? Curr Opin Crit Care. 2021;27(5):527–36. 10.1097/MCC.0000000000000857. 10.1097/MCC.0000000000000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark Z, et al. Australian genomics: outcomes of a 5-year national program to accelerate the integration of genomics in healthcare. Am J Hum Genet. 2023;110(3):419–26. 10.1016/j.ajhg.2023.01.018. 10.1016/j.ajhg.2023.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becherucci F, et al. A clinical workflow for cost-saving high-rate diagnosis of genetic kidney diseases. J Am Soc Nephrol. 2023;34(4):706–20. 10.1681/ASN.0000000000000076. 10.1681/ASN.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papp KK, et al. Milestones of critical thinking: a developmental model for medicine and nursing. Acad Med. 2014;89(5):715–20. 10.1097/ACM.0000000000000220. 10.1097/ACM.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 27.Dorst, K., Frame Innovation: Create New Thinking by Design. Frame Innovation: Create New Thinking by Design, 2015: p. 1–204 10.7551/mitpress/10096.001.0001.
- 28.Light DW. Addressing health care disparities: a radical perspective and proposal. Front Sociol. 2020;5:29. 10.3389/fsoc.2020.00029. 10.3389/fsoc.2020.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlke DC. From dispensaries to community health centers: health delivery change across the twentieth century. J Commun Health. 2018;43(3):625–7. 10.1007/s10900-018-0471-7. 10.1007/s10900-018-0471-7 [DOI] [PubMed] [Google Scholar]
- 30.Fencl JL. Guideline implementation: patient information management. AORN J. 2016;104(6):566–77. 10.1016/j.aorn.2016.09.020. 10.1016/j.aorn.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 31.McDonald SP. Australia and New Zealand dialysis and transplant registry. Kidney Int Suppl. 2011;5(1):39–44. 10.1038/kisup.2015.8. 10.1038/kisup.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncanson E, et al. Patient perspectives of center-specific reporting in kidney failure care: an Australian qualitative study. Kidney Int Rep. 2024;9(4):843–52. 10.1016/j.ekir.2024.01.001. 10.1016/j.ekir.2024.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.