Abstract
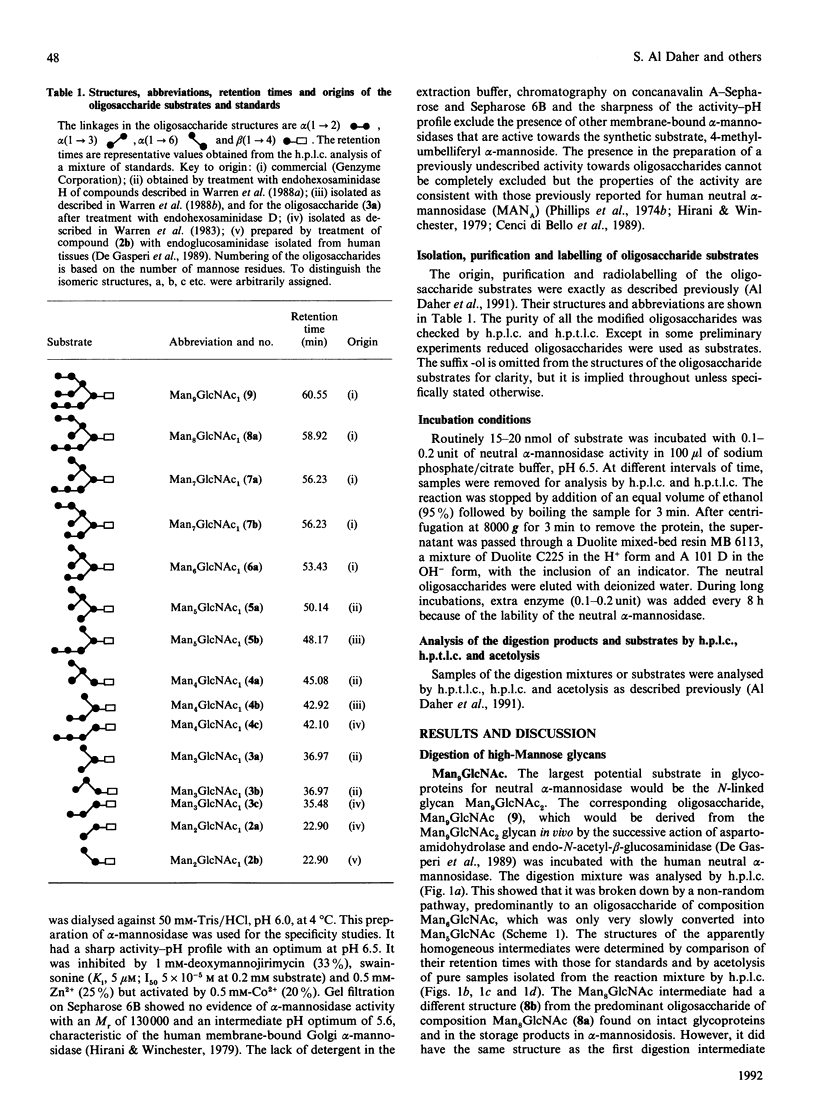
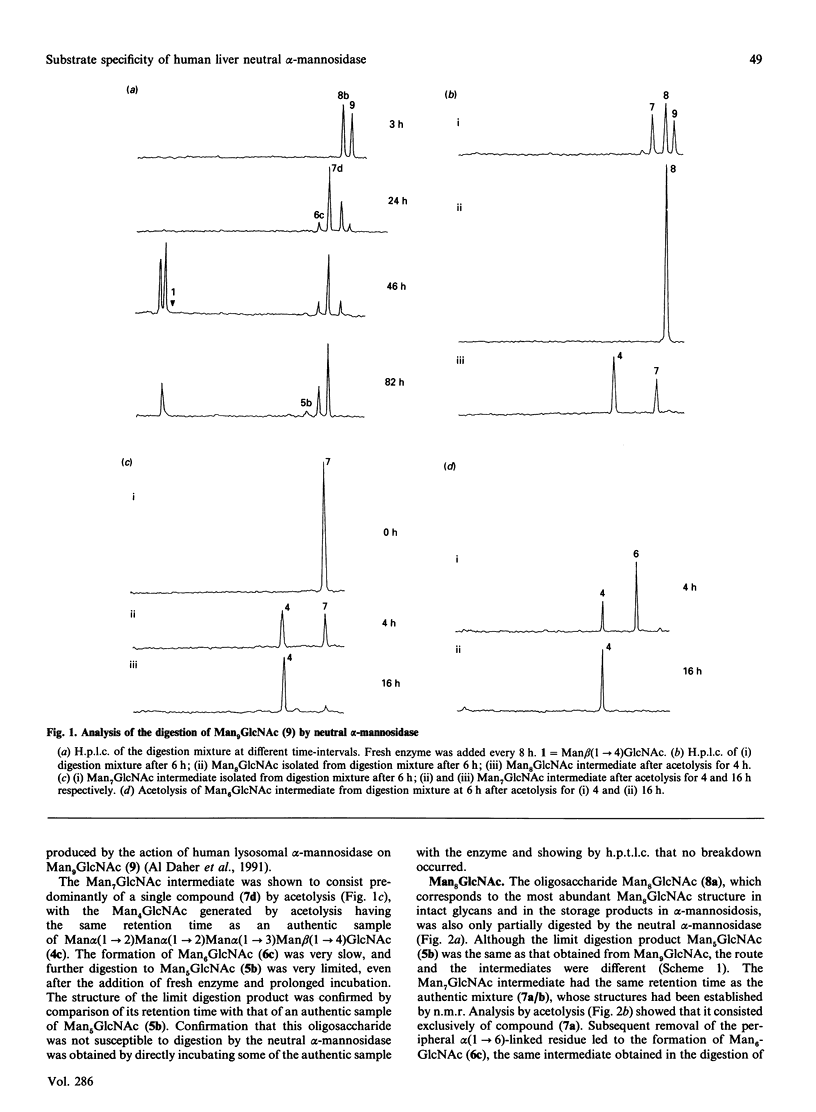
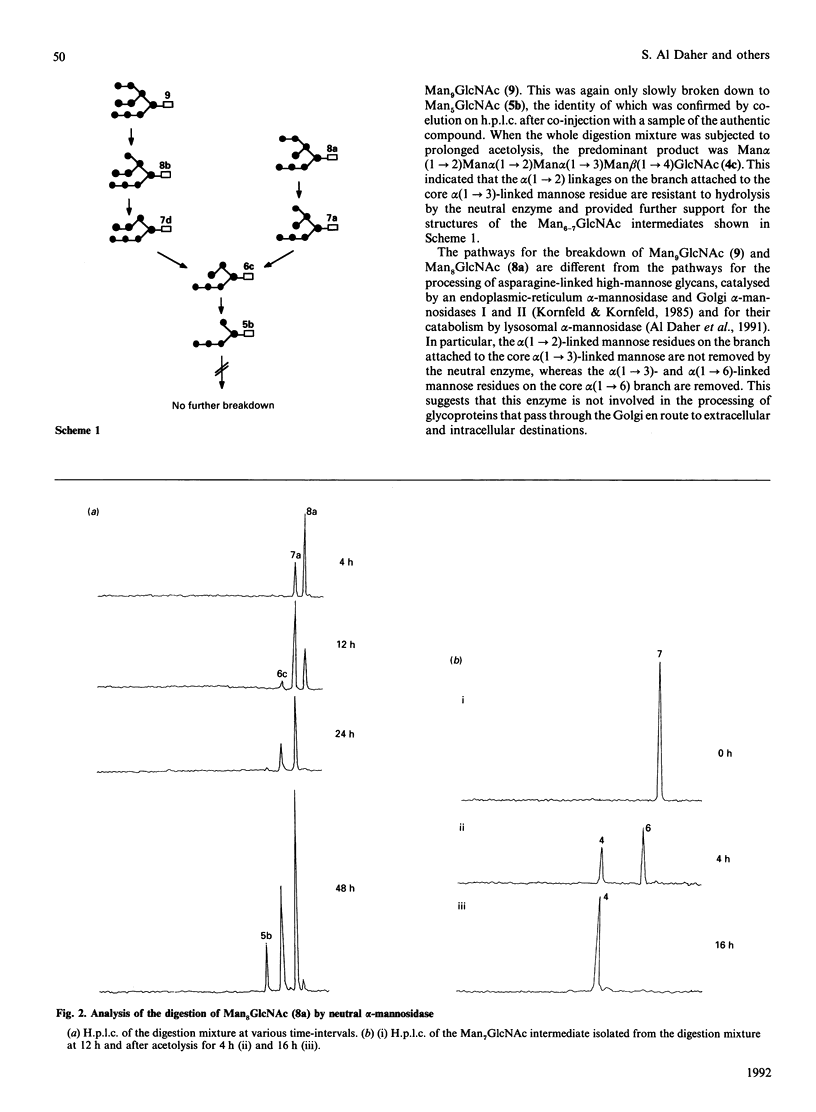
The digestion of radiolabelled natural oligosaccharide substrates by human liver neutral alpha-mannosidase has been studied by h.p.l.c. and h.p.t.l.c. The high-mannose oligosaccharides Man9GlcNAc and Man8GlcNAc are hydrolysed by the enzyme by two distinct non-random routes to a common product of composition Man6GlcNAc, which is then slowly converted into a unique Man5GlcNAc oligosaccharide, Man alpha(1----2)Man alpha(1----2)Man alpha(1----3)[Man alpha (1----6)] Man beta(1----4)GlcNAc. These pathways are different from the processing and lysosomal catabolic pathways for these structures. In particular, the alpha(1----2)-linked mannose residues attached to the core alpha(1----3)-linked mannose residue are resistant to hydrolysis. The key processing intermediate, Man alpha(1----3)[Man alpha(1----6)]Man alpha(1----6)[Man alpha(1----3)] Man beta(1----4)GlcNAc, is not produced in the digestion of high-mannose glycans by the neutral alpha-mannosidase, but it is hydrolysed by the enzyme by a non-random route to Man beta(1----4)GlcNAc via the core structure Man alpha(1----3)[Man alpha(1----6)]Man beta(1----4)GlcNAc. In contrast with its ready hydrolysis by lysosomal alpha-mannosidase, the core alpha(1----3)-mannosidic linkage is quite resistant to hydrolysis by neutral alpha-mannosidase. The precise specificity of neutral alpha-mannosidase towards high-mannose oligosaccharides suggests that it has a role in the modification of such structures in the cytosol.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff J., Kornfeld R. The soluble form of rat liver alpha-mannosidase is immunologically related to the endoplasmic reticulum membrane alpha-mannosidase. J Biol Chem. 1986 Apr 5;261(10):4758–4765. [PubMed] [Google Scholar]
- Bischoff J., Liscum L., Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986 Apr 5;261(10):4766–4774. [PubMed] [Google Scholar]
- Carroll M., Dance N., Masson P. K., Robinson D., Winchester B. G. Human mannosidosis--the enzyme defect. Biochem Biophys Res Commun. 1972 Oct 17;49(2):579–583. doi: 10.1016/0006-291x(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Cenci di Bello I., Fleet G., Namgoong S. K., Tadano K., Winchester B. Structure-activity relationship of swainsonine. Inhibition of human alpha-mannosidases by swainsonine analogues. Biochem J. 1989 May 1;259(3):855–861. doi: 10.1042/bj2590855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion M. J., Brown J. A., Shows T. B. Assignment of cytoplasmic alpha-mannosidase (MANA) and confirmation of mitochondrial isocitrate dehydrogenase (IDHM) to the q11 leads to qter region of chromosome 15 in man. Cytogenet Cell Genet. 1978;22(1-6):498–502. doi: 10.1159/000131007. [DOI] [PubMed] [Google Scholar]
- Champion M. J., Brown J. A., Shows T. B. Studies on the alpha-mannosidase (MANB), peptidase D (PEPD), and glucose phosphate isomerase (GPI) syntenic group on chromosome 19 in man. Cytogenet Cell Genet. 1978;22(1-6):186–189. doi: 10.1159/000130932. [DOI] [PubMed] [Google Scholar]
- DeGasperi R., Li Y. T., Li S. C. Presence of two endo-beta-N-acetylglucosaminidases in human kidney. J Biol Chem. 1989 Jun 5;264(16):9329–9334. [PubMed] [Google Scholar]
- DeGasperi R., al Daher S., Daniel P. F., Winchester B. G., Jeanloz R. W., Warren C. D. The substrate specificity of bovine and feline lysosomal alpha-D-mannosidases in relation to alpha-mannosidosis. J Biol Chem. 1991 Sep 5;266(25):16556–16563. [PubMed] [Google Scholar]
- Haeuw J. F., Strecker G., Wieruszeski J. M., Montreuil J., Michalski J. C. Etude comparée des catabolismes lysosomique et cytosolique des glycannes de type oligommannosidique. C R Acad Sci III. 1991;312(4):131–139. [PubMed] [Google Scholar]
- Hirani S., Winchester B. The multiple forms of alpha-D-mannosidase in human plasma. Biochem J. 1979 Jun 1;179(3):583–592. doi: 10.1042/bj1790583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Marsh C. A., Gourlay G. C. Evidence for a non-lysosomal alpha-mannosidase in rat liver homogenates. Biochim Biophys Acta. 1971 Apr 14;235(1):142–148. doi: 10.1016/0005-2744(71)90041-6. [DOI] [PubMed] [Google Scholar]
- Michalski J. C., Haeuw J. F., Wieruszeski J. M., Montreuil J., Strecker G. In vitro hydrolysis of oligomannosyl oligosaccharides by the lysosomal alpha-D-mannosidases. Eur J Biochem. 1990 Apr 30;189(2):369–379. doi: 10.1111/j.1432-1033.1990.tb15498.x. [DOI] [PubMed] [Google Scholar]
- Monis E., Bonay P., Hughes R. C. Characterization of a mannosidase acting on alpha 1----3- and alpha 1----6-linked mannose residues of oligomannosidic intermediates of glycoprotein processing. Eur J Biochem. 1987 Oct 15;168(2):287–294. doi: 10.1111/j.1432-1033.1987.tb13419.x. [DOI] [PubMed] [Google Scholar]
- Okumura T., Yamashina I. Purification of alpha-mannosidase from hog kidney and its action on glycopeptides. J Biochem. 1970 Oct;68(4):561–571. doi: 10.1093/oxfordjournals.jbchem.a129386. [DOI] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Characterization of human liver alpha-D-mannosidase purified by affinity chromatography. Biochem J. 1976 Mar 1;153(3):579–587. doi: 10.1042/bj1530579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G. Human liver alpha-D-mannosidase activity. Clin Chim Acta. 1974 Aug 30;55(1):11–19. doi: 10.1016/0009-8981(74)90328-3. [DOI] [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G., Jolly R. D. Mannosidosis in Angus cattle. The enzymic defect. Biochem J. 1974 Feb;137(2):363–371. doi: 10.1042/bj1370363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup V. A., Touster O. Purification and characterization of the alpha-D-mannosidase of rat liver cytosol. J Biol Chem. 1976 Jul 10;251(13):3845–3852. [PubMed] [Google Scholar]
- Suzuki I., Kushida H., Shida H. [Mammalian glycosidases. 2. Ph-dependence and heat tolerance of alpha-mannosidase alpha-glucosidase and alpha-galactosidase activities of the extracts of the various organs of rats and rabbits]. Seikagaku. 1969 Jul;41(7):334–341. [PubMed] [Google Scholar]
- Suzuki I., Kushida H. Studies on mammalian glycosidases. V. Effects of metal ions upon acidic and neutral alpha-glucosidases, beta-galactosidases, and alpha-mannosidases in liver extract of rabbit. J Biochem. 1973 Sep;74(3):627–629. doi: 10.1093/oxfordjournals.jbchem.a130285. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Substrate specificities of rat kidney lysosomal and cytosolic alpha-D-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J Biol Chem. 1987 May 15;262(14):6506–6514. [PubMed] [Google Scholar]
- Warren C. D., Azaroff L. S., Bugge B., Jeanloz R. W., Daniel P. F., Alroy J. The accumulation of oligosaccharides in tissues and body fluids of cats with alpha-mannosidosis. Carbohydr Res. 1988 Sep 15;180(2):325–338. doi: 10.1016/0008-6215(88)80089-2. [DOI] [PubMed] [Google Scholar]
- Warren C. D., Daniel P. F., Bugge B., Evans J. E., James L. F., Jeanloz R. W. The structures of oligosaccharides excreted by sheep with swainsonine toxicosis. J Biol Chem. 1988 Oct 15;263(29):15041–15049. [PubMed] [Google Scholar]
- Warren C. D., Schmit A. S., Jeanloz R. W. Chromatographic separation of oligosaccharides from mannosidosis urine. Carbohydr Res. 1983 Jun 1;116(2):171–182. doi: 10.1016/0008-6215(83)88107-5. [DOI] [PubMed] [Google Scholar]
- Winchester B. Role of alpha-D-mannosidases in the biosynthesis and catabolism of glycoproteins. Biochem Soc Trans. 1984 Jun;12(3):522–524. doi: 10.1042/bst0120522. [DOI] [PubMed] [Google Scholar]
- al Daher S., de Gasperi R., Daniel P., Hall N., Warren C. D., Winchester B. The substrate-specificity of human lysosomal alpha-D-mannosidase in relation to genetic alpha-mannosidosis. Biochem J. 1991 Aug 1;277(Pt 3):743–751. doi: 10.1042/bj2770743. [DOI] [PMC free article] [PubMed] [Google Scholar]