Abstract
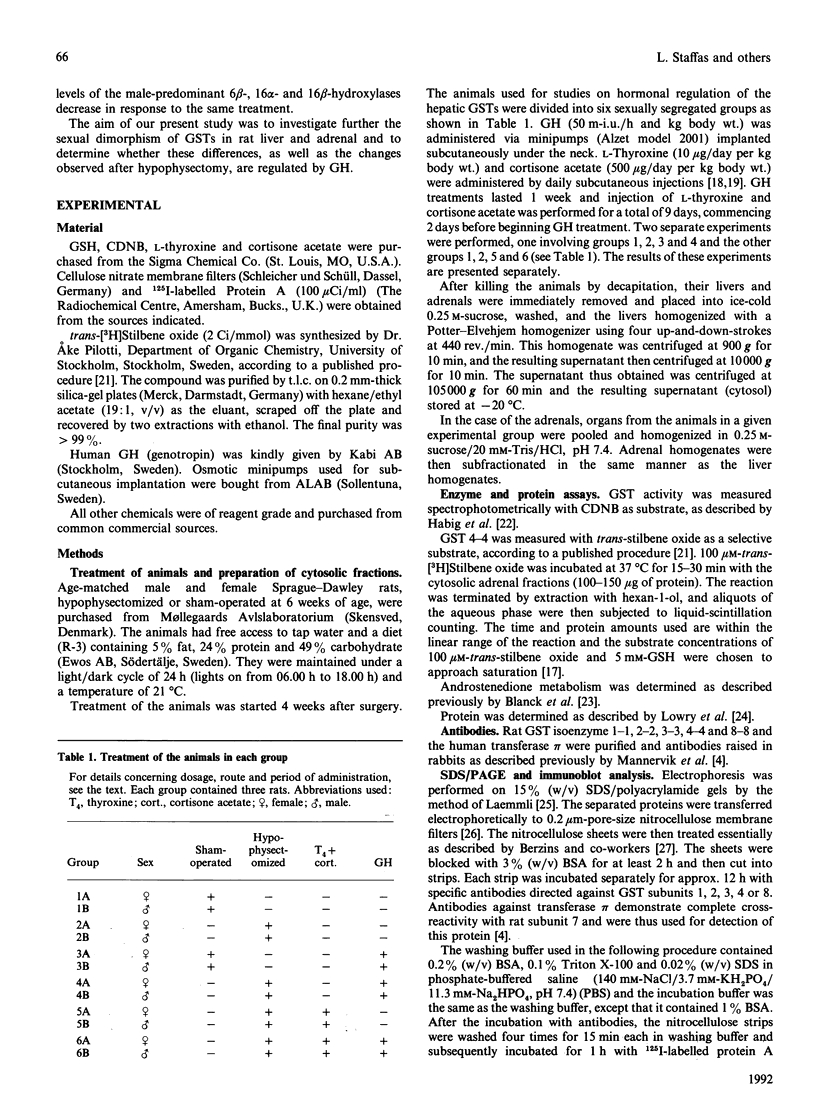
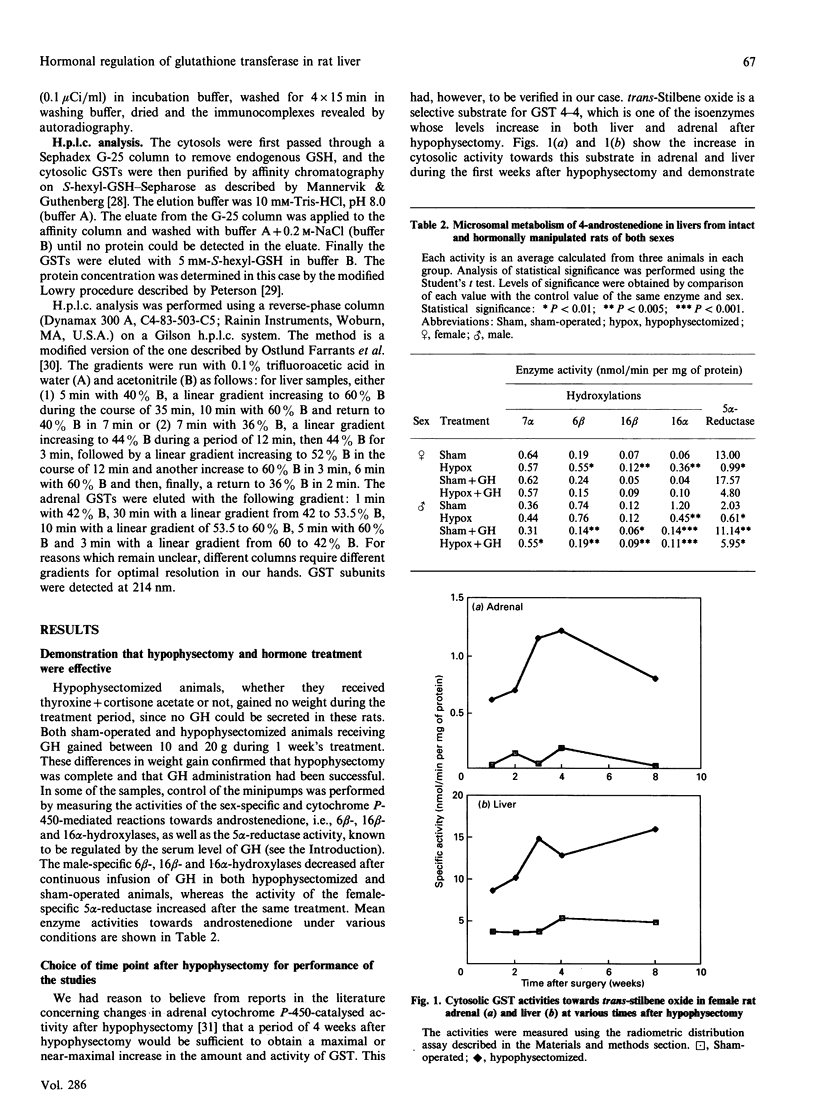
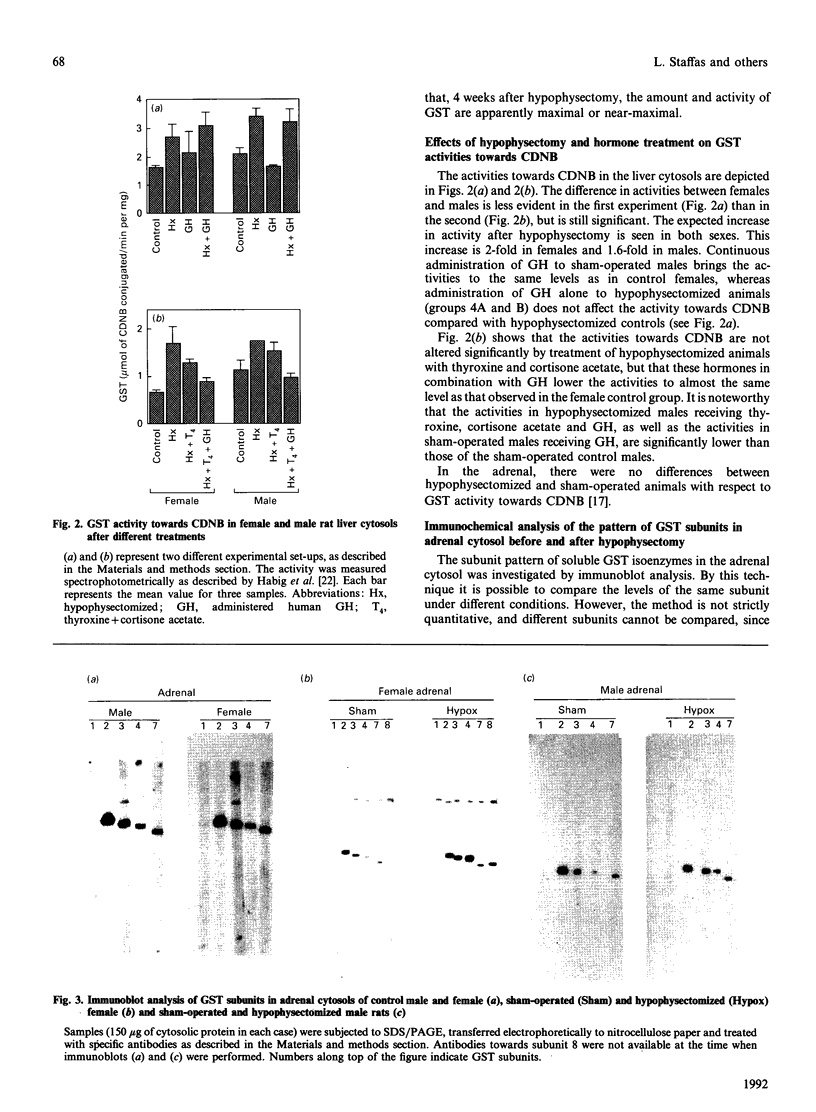
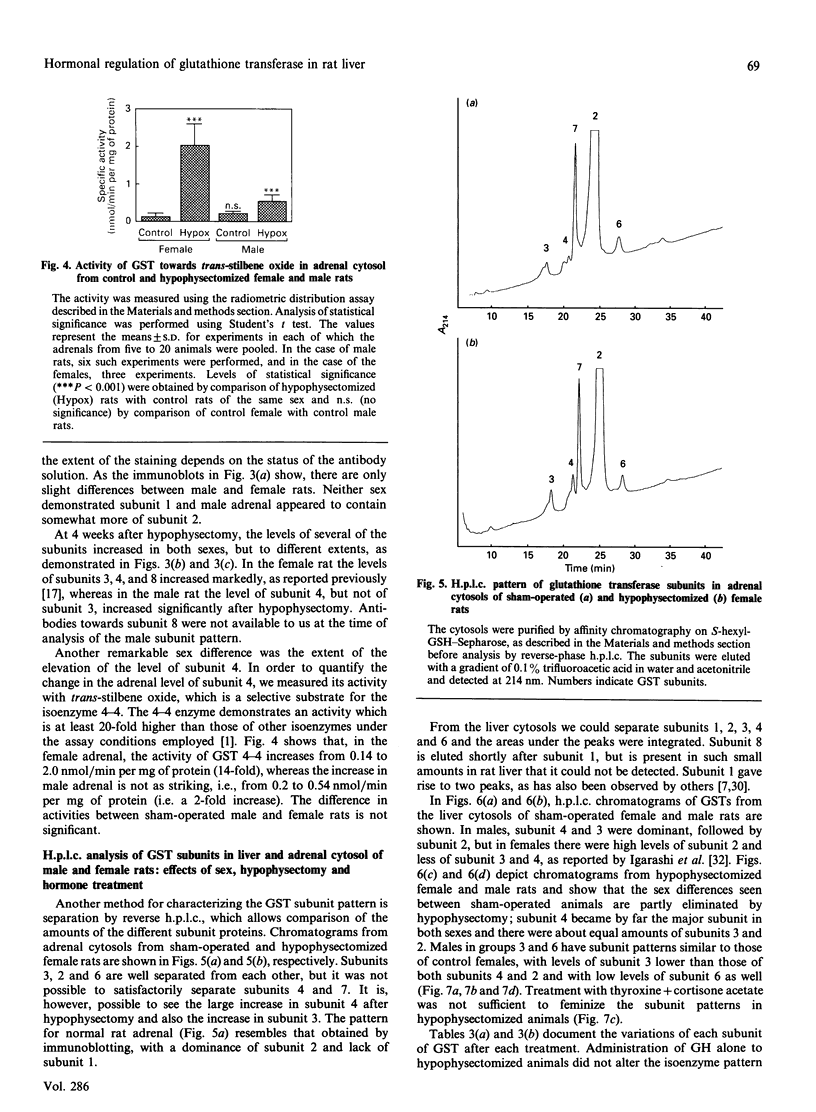
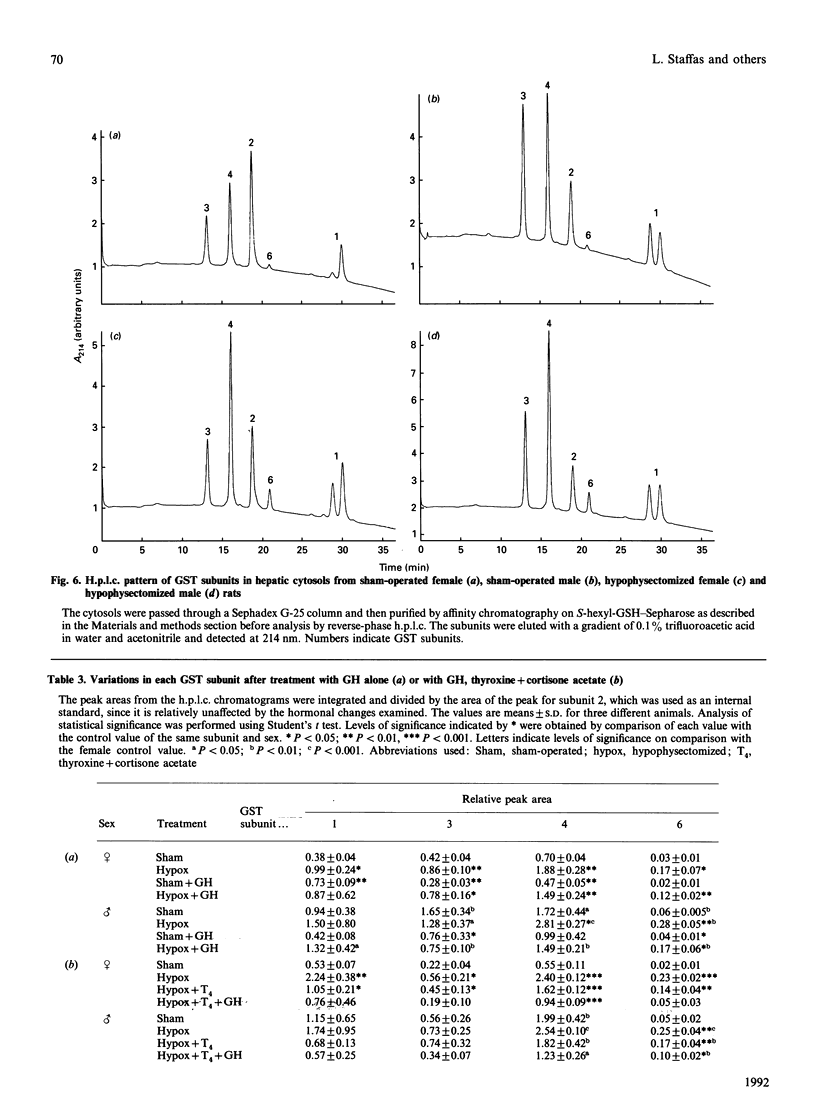
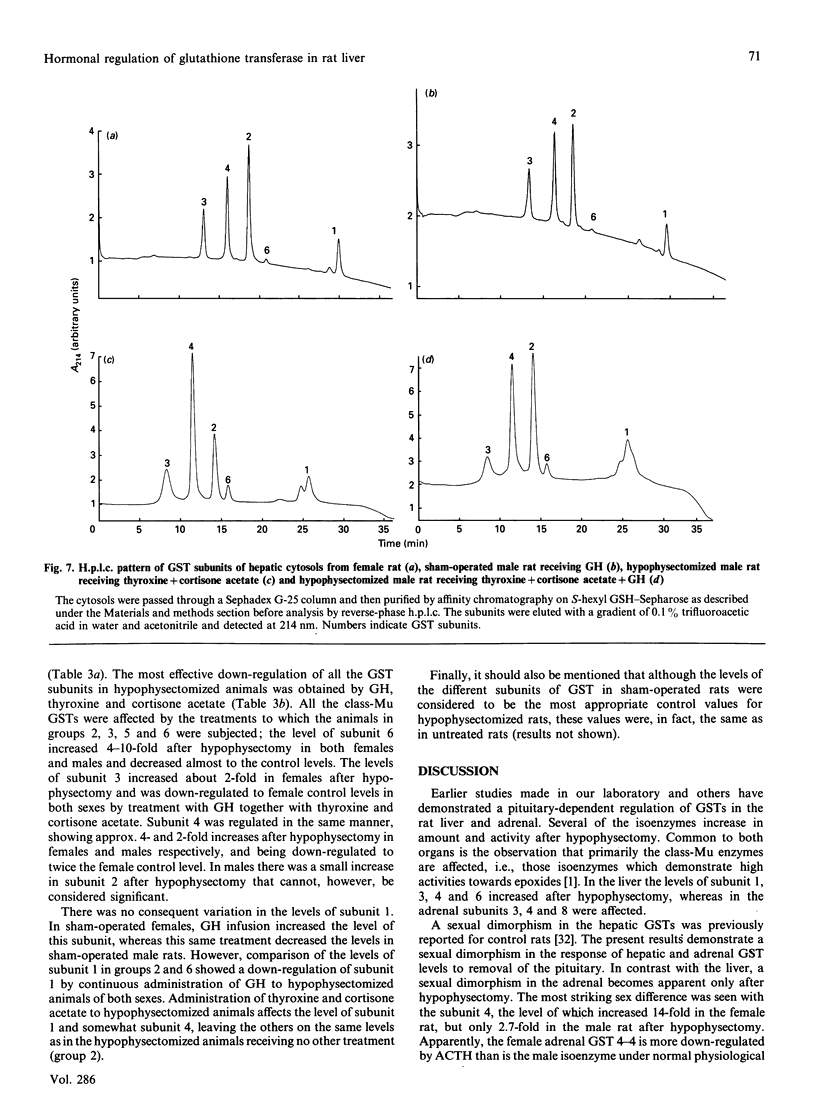
Immunoblot experiments and reverse-phase h.p.l.c. were used to study the levels of glutathione transferase subunits 1, 2, 3, 4, 6, 7 and 8 in the liver and adrenal of intact and hypophysectomized male and female Sprague-Dawley rats. A sexual dimorphism in the levels of several of these isoenzymes and in their responses to hypophysectomy was demonstrated. In the liver of sham-operated females and males there are differences in glutathione transferase activities and isoenzyme pattern. H.p.l.c. analysis showed higher levels of subunits 1, 3 and 4 in male rats compared with females. In contrast with the pronounced sex differences in sham-operated rats, the isoenzyme patterns of hypophysectomized males and females were very similar. In the adrenal glands, however, a sexual dimorphism became apparent only after hypophysectomy, when the level of subunit 4 was increased 14-fold in the female, whereas the corresponding increase in the male rat was only 2.7-fold. The hepatic pattern of glutathione transferase subunits could be altered by continuous infusion of growth hormone to both sham-operated and hypophysectomized rats of both sexes. This treatment feminized the isoenzyme pattern in sham-operated males and a similar effect was obtained upon treating hypophysectomized rats with thyroxine, cortisone acetate and a continuous infusion of growth hormone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson M., Montelius J., Mankowitz L., Rydström J. Metabolism of polycyclic aromatic hydrocarbons in the rat ovary. Comparison with metabolism in adrenal and liver tissues. Biochem Pharmacol. 1983 Jan 1;32(1):129–136. doi: 10.1016/0006-2952(83)90664-0. [DOI] [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Blanck A., Hansson T., Eriksson L. C., Gustafsson J. A. On mechanisms of sex differences in chemical carcinogenesis: effects of implantation of ectopic pituitary grafts on the early stages of liver carcinogenesis in the rat. Carcinogenesis. 1984 Oct;5(10):1257–1262. doi: 10.1093/carcin/5.10.1257. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chengelis C. P. Age- and sex-related changes in epoxide hydrolase, UDP-glucuronosyl transferase, glutathione S-transferase, and PAPS sulphotransferase in Sprague-Dawley rats. Xenobiotica. 1988 Nov;18(11):1225–1237. doi: 10.3109/00498258809042246. [DOI] [PubMed] [Google Scholar]
- Edén S. Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology. 1979 Aug;105(2):555–560. doi: 10.1210/endo-105-2-555. [DOI] [PubMed] [Google Scholar]
- Gill S. S., Ota K., Hammock B. D. Radiometric assays for mammalian epoxide hydrolases and glutathione S-transferase. Anal Biochem. 1983 May;131(1):273–282. doi: 10.1016/0003-2697(83)90166-5. [DOI] [PubMed] [Google Scholar]
- Guenthner T. M., Nebert D. W., Menard R. H. Microsomal aryl hydrocarbon hydroxylase in rat adrenal: regulation by ACTH but not by polycyclic hydrocarbons. Mol Pharmacol. 1979 May;15(3):719–728. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Harris J. M., Meyer D. J., Coles B., Ketterer B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991 Aug 15;278(Pt 1):137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama I., Satoh K., Sato K. Developmental and hormonal regulation of the major form of hepatic glutathione S-transferase in male mice. Biochem Biophys Res Commun. 1986 Oct 30;140(2):581–588. doi: 10.1016/0006-291x(86)90771-0. [DOI] [PubMed] [Google Scholar]
- Hiratsuka A., Sebata N., Kawashima K., Okuda H., Ogura K., Watabe T., Satoh K., Hatayama I., Tsuchida S., Ishikawa T. A new class of rat glutathione S-transferase Yrs-Yrs inactivating reactive sulfate esters as metabolites of carcinogenic arylmethanols. J Biol Chem. 1990 Jul 15;265(20):11973–11981. [PubMed] [Google Scholar]
- Igarashi T., Satoh T., Iwashita K., Ono S., Ueno K., Kitagawa H. Sex difference in subunit composition of hepatic glutathione S-transferase in rats. J Biochem. 1985 Jul;98(1):117–123. doi: 10.1093/oxfordjournals.jbchem.a135249. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Satoh T., Ono S., Iwashita K., Hosokawa M., Ueno K., Kitagawa H. Effect of steroidal sex hormones on the sex-related differences in the hepatic activities of gamma-glutamyltranspeptidase, glutathione S-transferase and glutathione peroxidase in rats. Res Commun Chem Pathol Pharmacol. 1984 Aug;45(2):225–232. [PubMed] [Google Scholar]
- Igarashi T., Satoh T. Sex and species differences in glutathione S-transferase activities. Drug Metabol Drug Interact. 1989;7(2-3):191–212. doi: 10.1515/dmdi.1989.7.2-3.191. [DOI] [PubMed] [Google Scholar]
- Ketterer B. Detoxication reactions of glutathione and glutathione transferases. Xenobiotica. 1986 Oct-Nov;16(10-11):957–973. doi: 10.3109/00498258609038976. [DOI] [PubMed] [Google Scholar]
- Kispert A., Meyer D. J., Lalor E., Coles B., Ketterer B. Purification and characterization of a labile rat glutathione transferase of the Mu class. Biochem J. 1989 Jun 15;260(3):789–793. doi: 10.1042/bj2600789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamartiniere C. A. The hypothalamic--hypophyseal--gonadal regulation of hepatic glutathione S-transferases in the rat. Biochem J. 1981 Jul 15;198(1):211–217. doi: 10.1042/bj1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listowsky I., Abramovitz M., Homma H., Niitsu Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab Rev. 1988;19(3-4):305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- Lund J., Faucher D. J., Ford S. P., Porter J. C., Waterman M. R., Mason J. I. Developmental expression of bovine adrenocortical steroid hydroxylases. Regulation of P-450(17 alpha) expression leads to episodic fetal cortisol production. J Biol Chem. 1988 Nov 5;263(31):16195–16201. [PubMed] [Google Scholar]
- Mankowitz L., Castro V. M., Mannervik B., Rydström J., DePierre J. W. Increase in the amount of glutathione transferase 4-4 in the rat adrenal gland after hypophysectomy and down-regulation by subsequent treatment with adrenocorticotrophic hormone. Biochem J. 1990 Jan 1;265(1):147–154. doi: 10.1042/bj2650147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mode A., Norstedt G., Simic B., Eneroth P., Gustafsson J. A. Continuous infusion of growth hormone feminizes hepatic steroid metabolism in the rat. Endocrinology. 1981 Jun;108(6):2103–2108. doi: 10.1210/endo-108-6-2103. [DOI] [PubMed] [Google Scholar]
- Mode A., Wiersma-Larsson E., Ström A., Zaphiropoulos P. G., Gustafsson J. A. A dual role of growth hormone as a feminizing and masculinizing factor in the control of sex-specific cytochrome P-450 isozymes in rat liver. J Endocrinol. 1989 Feb;120(2):311–317. doi: 10.1677/joe.0.1200311. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Vos R. M., Snoek M. C., van Berkel W. J., Müller F., van Bladeren P. J. Differential induction of rat hepatic glutathione S-transferase isoenzymes by hexachlorobenzene and benzyl isothiocyanate. Comparison with induction by phenobarbital and 3-methylcholanthrene. Biochem Pharmacol. 1988 Mar 15;37(6):1077–1082. doi: 10.1016/0006-2952(88)90513-8. [DOI] [PubMed] [Google Scholar]
- Vos R. M., Van Bladeren P. J. Glutathione S-transferases in relation to their role in the biotransformation of xenobiotics. Chem Biol Interact. 1990;75(3):241–265. doi: 10.1016/0009-2797(90)90069-y. [DOI] [PubMed] [Google Scholar]



