Abstract
Background:
Testicular cancer (TC) is a relatively rare type of cancer in men. Early diagnosis of TC remains challenging. Metabolomics holds promise in offering valuable insights in this regard. In this study, a metabolic fingerprinting approach was employed to identify potential biomarkers in both serum and seminal plasma of TC patients.
Methods:
A total of 9 patients with testicular cancer and 10 controls were included in the study. The metabolic fingerprinting approach was utilized as a rapid diagnostic tool to analyze the metabolome in serum and seminal plasma of TC patients in comparison to fertile men. Raman spectroscopy was applied for the analysis of metabolites in these biological samples.
Results:
Principal component analysis (PCA) and functional group analysis showed that the differentiation between serum samples from healthy men and TC patients was not possible. However, when analyzing seminal plasma, a significant difference was found between the two groups (p<0.05). Functional group analysis of serum only showed an increase in tryptophan concentration ratio in TC patients as compared to healthy men (p=0.03). In contrast, in seminal plasma of TC patients, this increase was observed in all analyzed compounds, including phenylalanine, tyrosine, lipids, proteins, phenols (p<0.001).
Conclusion:
Our study highlights the potential of metabolic fingerprinting as a fast diagnostic tool for screening TC patients, with seminal plasma serving as a valuable biological sample. Furthermore, several potential biomarkers, particularly phenylalanine, were identified in seminal plasma. This research contributes to our understanding of TC pathogenesis and has the potential to pave the way for early detection and personalized treatment approaches.
Keywords: Metabolic fingerprinting, Raman spectroscopy, Seminal plasma, Serum, Testicular cancer
Introduction
Testicular cancer (TC) is a relatively rare cancer (1%) diagnosed in young and adult men aged 14 to 44 (1, 2). It has been reported that its prevalence is increasing worldwide in the past two decades specifically in Western countries (3, 4). TC is classified into seminoma and non-semi-nomatous germ cell tumors (5). Seminomas account for half of TC, while non-seminomatous germ cell tumors comprise approximately 40% of TC. Nearly 10% of TC consists of a combination of both seminoma and non-seminoma components (6). The differentiation between seminoma and non-seminoma is pivotal in determining the course of treatment and predicting the prognosis (6).
Germ cell tumors constitute the predominant type of testicular cancer, making up 95% of cases, while other forms of testicular neoplasms are extremely rare (7). Germ cell tumors may also appear in extragonadal regions including the retroperitoneum and the mediastinum (8). Nearly 70% of patients are routinely diagnosed with stage I testicular cancer, while 30% of them are identified with metastatic cancer (9). Due to the high recurrence rate of 50%, TC patients are treated with chemotherapy. The 5-year survival rate of testicular carcinomas is 95%. Furthermore, for patients diagnosed with stage I of the disease, the cancer-specific survival rate surpasses 99% even after 15 years (10–12).
To date, numerous biomarkers have been introduced for the diagnosis of TC. They could be considered as potential targets for treatment of cancer (5); however, their efficiency and validity need to be confirmed. Currently, three biomarkers including β-subunit of human chorionic gonadotropin (β-hCG), lactate dehydrogenase (LDH), and α-fetoprotein (AFP) are used for the diagnosis and subsequent monitoring of TC. However, while AFP and β-hCG have a high specificity (90%), the sensitivity is often very low (∼50%). It is important to note that in approximately 40% of men with recurrent testicular cancer, the levels of these biomarkers are typically within the normal range, indicating their limited effectiveness in detecting many recurrences (13). Moreover, considering the limited diagnostic accuracy of LDH (3), there is an undeniable need for the identification of specific biomarkers that can effectively diagnose this type of cancer (14, 15).
Currently, cancer diagnosis is based on immunohistochemical methods that use an antigen for detection. Therefore, the selection of the appropriate antigen is of utmost importance. In this regard, systems biology approaches with a focus on genomics, transcriptomics, proteomics, and metabolomics have been introduced and employed in cancer research. Today, metabolome analysis is frequently used to gain insights into disease progression and identify new biomarkers. The term metabolomics refers to studying metabolites (<2 kDa) in biological samples (16–18). During the last two decades, metabolomics has become more popular, primarily driven by advancements in instruments and bioinformatics (16, 17, 19, 20).
Raman spectroscopy, with its extensive history in metabolomics, is a powerful analytical technique. It not only reveals the molecular fingerprint of the biological sample through the Raman spectrum (21, 22), but also provides quantitative data regarding its chemical composition. Moreover, Raman spectra can detect biochemical changes associated with diseases (23, 24). Prior research has demonstrated the potential of fingerprinting as a highly effective diagnostic tool in men experiencing infertility (2, 14, 25).
The purpose of the current study was to explore the feasibility and reliability of Raman spectroscopy for conducting metabolic fingerprinting of serum and seminal plasma in TC patients. Additionally, the potential use of Raman spectroscopy to predict TC development in very early stages of the cancer onset was explored. By harnessing the capabilities of this high-throughput tool, an attempt was made to identify a novel and reliable marker for the early diagnosis of testicular cancer. To the best of our knowledge, it is the first time that metabolomics analysis has been performed on TC patients.
Methods
Sample selection: This research received approval from the Ethics Committee of Iran University of Medical Sciences under reference number IR. IUMS.REC1396.32223. This case–control research included 9 patients with testicular cancer who were referred to Avicenna Infertility Clinic for sperm cryopreservation before undergoing orchiectomy. The control group consisted of 10 confirmed fertile normozoospermic men. The control group were men who had at least one child and had visited Avicenna Infertility Clinic for sex selection. The serum and seminal plasma of TC patients were collected during 2016–2022 prior to undergoing orchiectomy. Informed consent was obtained from all participants involved in the study. Fresh semen samples were collected through masturbation after a period of sexual abstinence lasting 3 to 4 days. A portion of each sample was allocated for standard semen analysis, encompassing the evaluation of normal sperm morphology, sperm concentration, volume, pH, and motility, in accordance with the established procedures detailed in the WHO laboratory manual for human semen examination and processing (WHO 2010: 5th Edition; WHO 2021: 6th Edition). Briefly, the sperm of semen sample were enumerated by the Neubauer hemocytometer chamber. Sperm morphology analysis was conducted using Papanicolaou staining with a minimum evaluation of 400 spermatozoa with 1000x magnification in two replicates. Normozoospermic samples from fertile men met the following criteria: sperm concentration >15×10 6/ml, normal morphology >4%, progressive motility >32%, and progressive and non-progressive motility ≥40%. The demographic information and semen parameters of the patients are shown in table 1.
Table 1.
Demographic information and standard semen parameters of analyzed fertile and testicular cancer patients
| Variables | Control (n=10) | Testicular cancer (n=9) |
|---|---|---|
| Age (years) | 37.2±5.4 | 35.1±5.6 |
| Semen parameters | ||
| Volume (ml) | 4.2±1.8 | 7.60±9.21 |
| Sperm concentration (106 per ml) | 43.58±12.76 | 21.56±13.51 |
| Total count (106 per ejaculate ) | 175.92±84.71 | 67±45.07 |
| Normal morphology (%) | 5.75±2.14 | 1±0.92 |
| Total motile PR+NP (%) | 37.16±2.6 | 51.67±26.22 |
Values are reported as means±standard deviations
Metabolome extraction: One hundred microliter of serum and seminal plasma were purified from cell debris and proteome by adding 200 μl methanol (Merck, Germany) to H2O (v/v) in a ratio of 2:1. The mixture was then centrifuged at 9300 g for 10 min at 4 oC (26, 27). The resulting supernatant, which contained the metabolome, was transferred to a new Eppendorf vial and stored at −20°C until analysis by Raman spectroscopy (Thermo Fisher Scientific, USA).
Raman spectroscopy: Raman spectroscopy serves as a robust analytical technique for identifying and characterization of molecules and materials (28). It is based on the interaction between laser light and the sample where the scattered light (Raman scattering) carries information about the molecular vibrational modes within the sample. When laser light interacts with the specimen, a small fraction of photons undergoes energy loss, resulting in generation of lower-energy scattered light called Raman scattered light. This light is then detected and analyzed to reveal the vibrational frequencies of the chemical bonds in the molecules present in the sample. The Raman spectrum is generated by plotting the intensity of the scattered light as a function of its energy shift (frequency difference) from the incident laser light.
The Raman spectrum serves as a unique fingerprint of the molecular composition of a sample, allowing for the identification of different chemical compounds and functional groups. Raman spectroscopy is non-destructive and requires minimal sample preparation, making it suitable for analyzing a broad spectrum of samples, spanning from solids and liquids to gases. One of the key advantages of Raman spectroscopy is its high selectivity and specificity, enabling the detection of trace amounts of compounds in complex mixtures. Additionally, Raman spectroscopy finds valuable applications for in situ and in real-time monitoring, making it valuable in various fields such as materials science, pharmaceutical sciences, forensic analysis, and environmental monitoring.
In this research, Raman spectra were acquired using an Almega dispersive spectrometer (Thermo Nicolet, France) under the following settings to ensure sample safety: spectral range: 100-4, 200 cm−1; laser source: second harmonic at 532 nm from a Nd: YLF laser; resolution: 4 cm−1; and laser power: 30 mW. A total of forty-four scans were conducted, and the intensity of Raman spectra was utilized for classification purposes.
Statistical analysis: The Raman spectra were processed using Spectroscopy Ninja version 1.2.15, (Obsertdorf, Germany). Subsequently, the data matrix was analyzed through multivariate pattern recognition procedures, such as principal component analysis (PCA) and discriminant analysis. Additionally, Prism version 6.01 (GraphPad software Inc., USA) was employed for further analysis. These methodologies played a crucial role in elucidating the data structure and contributed to the development of a well-grounded hypothesis. Statistical significance was established using a threshold of p<0.05.
Results
The results obtained from Raman spectra, which captured differences in patterns and concentrations of various metabolites based on their functional groups, were compared between the two groups: men with testicular cancer and fertile men serving as the control group. This comparison was conducted for both serum and seminal plasma samples.
Serum: The first step of analysis of the Raman spectra was comparison of TC serum with normal serum. Figure 1 shows the results of this analysis, employing principal component analysis (PCA) and functional group analysis for serum samples from TC patients (6 samples) compared to a control group of fertile men (6 samples). As depicted in figure 1A, the serum of healthy men could not be reliably distinguished from that of testicular cancer patients (p>0.05). Furthermore, figure 1B presents a more comprehensive examination of functional group analysis, with a specific focus on the spectra of -SH groups (2400–2600 cm−1) and – CH groups (2,800–3,000 cm−1). These spectral regions provide insights into oxidative changes, offering valuable information for analysis. Notably, no significant alterations were observed in functional group analysis (p=0.1).
Figure 1.
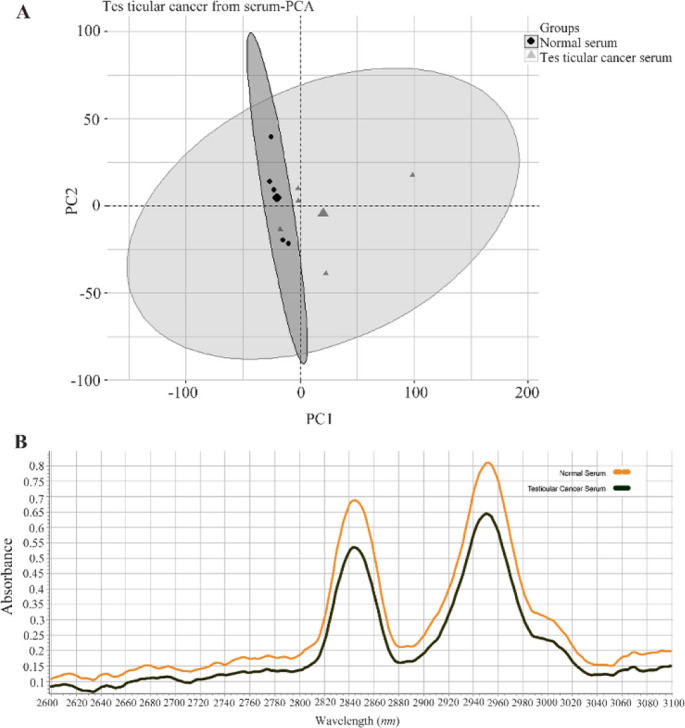
A) Results of principal component analysis (PCA) of the data matrix from the serum of testicular cancer (CS) patients (--▲--) versus healthy men (NS) (--◼--) as control group. B) Average Raman spectra for serum of testicular cancer (―) patients and healthy groups (--). 2,800-3,000 cm−1 corresponds to the -CH group while 2,400–2,600 cm−1 relates to the -SH group (p-value >0.05)
Raman spectroscopy enables identification of functional groups based on the vibration of chemical bonds. Table 2 provides an overview of the assigned chemical groups corresponding to different vibrational modes. As shown in the table, the majority of chemical groups did not exhibit significant changes. However, there was a significant increase of tryptophan (p=0.03) in serum. Additionally, table 2 shows an increase of tyrosine, lipids, and fatty acids in serum of testicular cancer patients, although these changes were not statistically significant (p>0.05).
Table 2.
Chemical assignments of vibrational modes for the Raman spectra acquired from serum
| Wavelength (cm−1) | Tentative band assignments | Fold change (TCS/NS)* | p-value |
|---|---|---|---|
| 3300–3500 | N-H vibration of proteins | 1.17 | 0.1 |
| 3200–3300 | CH, lipids, fatty acids | 1.35 | 0.05 |
| 2900–3000 | CH3- proteins | 0.8 | 0.83 |
| 2800–2900 | CH2- lipids | 0.83 | 0.16 |
| 2100–2200 | CN | 0.6 | 0.14 |
| 1700–1800 | C=O | 0.45 | 0.1 |
| 1400–1500 | CH2-lipids | 0.7 | 0.1 |
| 1350–1360 | Trp | 0.55 | 0.12 |
| 1100–1150 | CN | 0.57 | 0.12 |
| 1002–1020 | Phe | 1.24 | 0.14 |
| 850–900 | Tyr | 1.6 | 0.1 |
| 750–800 | Trp | 1.8 | 0.03 |
TCS: Testicular cancer serum, NS: Normal serum, Phe, Tyr, and Trp refer to the phenylalanine, tyrosine, and tryptophan residues, respectively
Seminal plasma: The Raman spectra obtained from the seminal plasma of both TC (9 samples) and normal (10 samples) groups were subjected to multivariate analysis. Figure 2A shows the metabolic fingerprinting of seminal plasma using the principal component analysis, where the samples from healthy individuals and TC patients can be distinguished.
Figure 2.
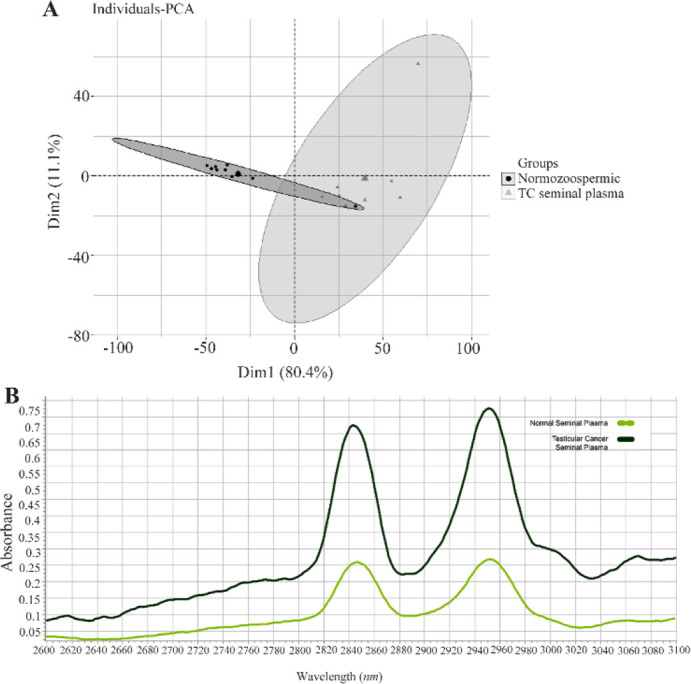
A) The principal component analysis (PCA) results of the data matrix of seminal plasma of testicular cancer (TC seminal plasma) patients (--▲--) versus healthy men (normozoospermic men) (--◼--). B) The Raman spectra mean for seminal plasma of testicular cancer (―) and healthy groups (--). The region of 2,800–3,000 cm−1 corresponds to the -CH group
A Raman spectrum provides valuable information about various functional groups from biological samples, including seminal plasma. Table 3 displays the fold changes of metabolites between TC and normal groups. The table reveals the substantial up-regulation of phenylalanine and represents the most significantly altered metabolite in the seminal plasma compared to the normal group (p<0.001). Additionally, statistical analysis reveals a significant increase of lipids and proteins (p<0.05).
Table 3.
Chemical assignments of vibrational modes for the Raman spectra acquired from human seminal plasma
| Wavelength (cm−1) | Group and class | Fold change (TC/N) | p-value |
|---|---|---|---|
| 800–900 | Tyr | 2.5 | <0.001 |
| 1000–1020 | Phe | 3.2 | <0.001 |
| 1400–1500 | Aromatic-CH2- | 2.8 | <0.001 |
| 1700–1800 | Ketone C=O | 2.8 | <0.001 |
| 2000–2200 | Thiocyanate ion (SCN−) | 2.5 | <0.001 |
| 2800–2900 | Lipids | 2.4 | <0.001 |
| 2900–3000 | Proteins | 2.6 | <0.001 |
| 3300–3400 | Phenols | 3 | <0.001 |
| 3400–3500 | H-band | 2.5 | <0.001 |
TC: Testicular cancer seminal plasma, NS: Normal seminal plasma. Phe and Tyr refer to the phenylalanine and tyrosine residues, respectively
Discussion
Testicular cancer (TC) is a relatively rare type of cancer that primarily affects young and adult men (1). The etiology of TC is currently unknown, but there has been a global increase in reported cases (4). Given its significance as a health concern, early detection plays a pivotal role in achieving favorable treatment outcomes (29). Current serum tumor markers, such as AFP, βhCG, and LDH, have limitations in effectively detecting TC due to their restricted accuracy and sensitivity when employed as diagnostic, prognostic, and predictive indicators (30). Spalt-like transcription factor 4 (SALL4) is a new potential biomarker for detection of testicular germ cell cancers (31). Our recent study demonstrated that the newly-developed SALL4-A monoclonal antibody effectively detected SALL4-A using either immunohistochemistry or ELISA (32). Although there were promising initial findings, the application of most pre-clinical biomarkers in clinical practice currently lacks validation (14). Thus, researchers aimed to identify potential biomarkers in liquid biopsies to improve early diagnosis (33). A liquid biopsy offers the benefits of being non-invasive, rapid, accurate, and notably capable of providing real-time information (34). Additionally, it has the potential to address the heterogeneity of tumors and could potentially replace conventional tissue biopsies in the future. Presently, liquid biopsy primarily encompasses the analysis of circulating tumor cells, exosomes, circulating tumor DNA, and metabolites, which can be obtained from bodily fluids including blood, saliva, urine, and seminal plasma (35). In this regard, the study of the metabolome, which refers to the comprehensive collection of small molecules found in biological samples such as body fluids, offers a potential diagnostic approach. Exploring metabolism at the ‘omics’ level is a quickly expanding field with significant potential to make a profound impact on the field of medicine (36). At the core of metabolomics is the concept of personalized medicine which takes into account an individual’s metabolic status along with the potential influence of gene expression, environmental factors, diet, and gut microbiome. Thus far, clinicians have been able to gather only a restricted amount of information from the metabolome, often relying on the measurement of a limited number of chemistry analytes present in the blood, to evaluate an individual’s health and determine disease status (8, 19, 37).
There are different approaches to study the metabolome (38). The fastest methods for clinical diagnosis involve pattern recognition techniques, which encompass optical spectrometry and nuclear magnetic resonance spectroscopy (NMR). In this study, a combination of optical spectroscopy and multivariable analysis was utilized on both serum and seminal plasma samples to differentiate between healthy individuals and patients with testicular carcinoma (TC).
In the first step of analysis of serum from TC patients, metabolic fingerprinting combined with unsupervised machine learning was utilized to classify the groups. Our results show that serum is not able to differentiate healthy fertile men from TC patients. Furthermore, based on serum data (Figure 1B), it was demonstrated that free radicals do not play a significant role. Additionally, potential biomarkers were investigated through chemical assignments of vibrational modes in the Raman spectra. A comparison of the spectrum of serum between TC patients and healthy men revealed alterations in the levels of lipids and fatty acids (3200–3300 cm−1) in TC patients (Table 2). Lipids are crucial components in the progression and metastasis of malignant tumors, as they are absolutely necessary for tumor growth and spread (38). Moreover, it was demonstrated that tryptophan (750–800 cm−1) is increase in TC patients. Tryptophan, being an essential amino acid, plays a significant role in a major metabolic pathway. This pathway not only enhances the intrinsic malignant properties of tumor cells but also hinders antitumor immunity. Therefore, it has become a scientifically robust focal point for drug development in the realm of cancer immunotherapy (39).
Seminal plasma is a complex fluid that consists of contributions from the seminiferous tubules in the testicles as well as secretions from all organs or tubules of the seminal tract. These include the prostate, bulbourethral glands, vas deferens, seminal vesicles, and epididymis. Seminal plasma serves as an outstanding resource for enhancing our understanding of male reproductive system disorders and exploring potential biomarkers associated with these conditions (39, 40). Secretions of the seminal vesicles contain ascorbic acid, fructose, and prostaglandins. Furthermore, neutral α-glucosidase and L-carnitine are abundant in epididymal secretions. To the best of our knowledge, no studies have been performed on seminal plasma from TC patients. In this study, metabolic fingerprinting and unsupervised machine learning were employed to examine the role of seminal plasma in TC patients.
Our results clearly show that seminal plasma can be used to differentiate healthy men from TC patients and can be used as a biological resource for diagnosis of TC. Furthermore, our findings indicate a substantial increase of free radicals, as evidenced by the increased levels of lipids and proteins. These free radicals serve as notable biomarkers in the context of our study. The involvement of free radicals in testicular cancer has garnered considerable attention as a crucial aspect of disease progression that warrants further investigation. Free radicals, including various reactive oxygen species (ROS), are generated as part of normal cellular oxidative processes (41). The increased concentration of free radicals can induce cellular damage and influence neurocellular and genetic processes. These alterations may disrupt the regulation of genes involved in cell growth and division, ultimately contributing to the enhanced growth and dissemination of TC (42–44). As shown in table 3, the Raman spectra demonstrate a notable up-regulation of the chemical assignments for vibrational modes.
This is an interesting and new finding, indicating that seminal plasma holds the potential as an alternative and specific source of biomarkers for TC. The aromatic amino acid pathway had been implicated in many disorders including cancer (45) (Figure 3). Our results show that phenylalanine and tyrosine are increase in seminal plasma of TC patients. An increase of these two amino acids in seminal plasma has not been previously reported in any type of cancer, including testicular cancer. However, the increase in aromatic amino acid concentrations in blood has been observed as a significant phenomenon in certain cancers such as gynecological and breast cancer (46).
Figure 3.
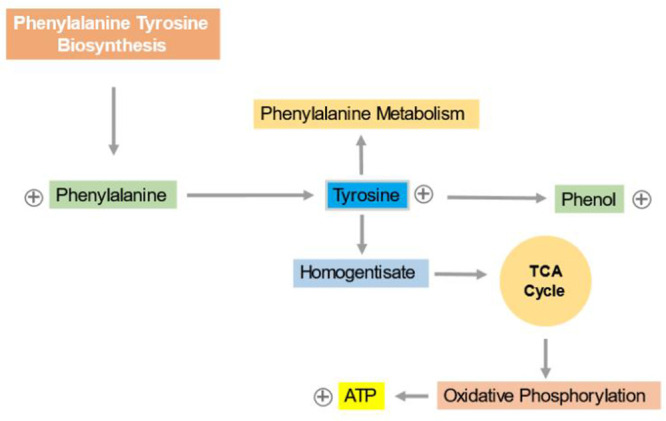
Phenylalanine and tyrosine biosynthesis pathway; the increase of phenylalanine and tyrosine and also the phenol pathway. ⊕ Indicates the up-regulation of metabolites
The reason behind the increased levels of aromatic amino acids in these cancers could be attributed to the increase activity of proteins associated with aromatic compounds or alterations in the metabolic pathways of these amino acids in cancer cells, leading to their accumulation in the blood. For example, chronic immune activation and inflammation can lead to oxidative stress, which may negatively affect the activity of phenylalanine (4)-hydroxylase (PAH), resulting in elevated phenylalanine concentrations (47). Therefore, this rationale may hold true in our study as well, suggesting that the observed increase in phenylalanine concentrations in seminal plasma could be attributed to the presence of oxidative stress. Moreover, our data suggest an increase of the phenol pathway, indicating that phenolic compounds may possess anti-cancer and anti-meta-static properties (48). Phenolic compounds demonstrate the potential to impede the beginning and progression of multiple signaling pathways in different cancer types.
Conclusion
This study, demonstrated that metabolic fingerprinting approach has the potential to be used as a rapid diagnostic tool for the screening of TC patients, with seminal plasma serving as the biological sample. In addition, phenylalanine in seminal plasma exhibited the highest concentration compared to other elevated markers in men diagnosed with testicular cancer. Therefore, it is essential to investigate this amino acid in seminal plasma of TC patients compared to healthy fertile men in a larger cohort of patients. If consistent results are obtained, phenylalanine would be a potentially suitable diagnostic marker for testicular cancer, particularly in clinical settings.
Acknowledgement
This research is part of a PhD thesis (Number: 32223). We would like to express our appreciation to Oncopathology Research Center at Iran University of Medical Sciences and Avicenna Research Institute for their invaluable support and collaboration.
Funding: This work was supported by Onco-pathology Research Center of Iran University of Medical Sciences (Grant No.: 32223) and Avicenna Research Institute (Grant No :960112-038).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4(1):29. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1. 0, cancer incidence and mortality worldwide. IARC. Cancer Base. 2013. 11. [DOI] [PubMed] [Google Scholar]
- 5.Milardi D, Grande G, Vincenzoni F, Pierconti F, Pontecorvi A. Proteomics for the identification of biomarkers in testicular cancer-review. Front Endocrinol (Lausanne). 2019;10:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leman ES, Gonzalgo ML. Prognostic features and markers for testicular cancer management. Indian J Urol. 2010;26(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol. 2009;5(9): 1389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adra N, Einhorn LH. Testicular cancer update. Clin Adv Hematol Oncol. 2017;15(5):386–96. [PubMed] [Google Scholar]
- 9.Koši Kunac A, Gnjidić M, Antunac Golubić Z, Gamulin M. Treatment of germ cell testicular cancer. Acta Clin Croat. 2020;59(3):496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugaard G, Gundgaard MG, Mortensen MS, Agerbæk M, Holm NV, Rørth M, et al. Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. J Clin Oncol. 2014;32(34): 3817–23. [DOI] [PubMed] [Google Scholar]
- 11.Mortensen MS, Lauritsen J, Gundgaard MG, Agerbæk M, Holm NV, Christensen IJ, et al. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur Urol. 2014;66(6):1172–8. [DOI] [PubMed] [Google Scholar]
- 12.Kier MG, Lauritsen J, Mortensen MS, Bandak M, Andersen KK, Hansen MK, et al. Prognostic factors and treatment results after bleomycin, etoposide, and cisplatin in germ cell cancer: a population-based study. Eur Urol. 2017;71(2):290–8. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson BD, Jones NR, Protheroe A, Joseph J, Roberts NW, Van den Bruel A, et al. The diagnostic performance of current tumour markers in surveillance for recurrent testicular cancer: a diagnostic test accuracy systematic review. Cancer Epidemiol. 2019;59:15–21. [DOI] [PubMed] [Google Scholar]
- 14.Lakpour N, Saliminejad K, Ghods R, Reza Sadeghi M, Pilatz A, Khosravi F, et al. Potential biomarkers for testicular germ cell tumour: risk assessment, diagnostic, prognostic and monitoring of recurrence. Andrologia. 2021;53(4):e13998. [DOI] [PubMed] [Google Scholar]
- 15.Lakpour N, Ghods R, Sadeghi MR, Ranjbar MM, Abolhasani M, Kiani J, et al. Production and characterization of a new specific monoclonal antibody against A-isoform of SALL4: a novel emerging testicular cancer marker. Andrologia. 2022;54(1):e14608. [DOI] [PubMed] [Google Scholar]
- 16.Fiehn O. Metabolomics--the link between genotypes and phenotypes. Plant Mol Biol. 2002;48(1–2):155–71. [PubMed] [Google Scholar]
- 17.Weckwerth W. Metabolomics in systems biology. Ann Rev Plant Biol. 2003;54:669–89. [DOI] [PubMed] [Google Scholar]
- 18.Goodacre R. Metabolomics of a superorganism. J Nutr. 2007;137(1 Suppl):259S–66S. [DOI] [PubMed] [Google Scholar]
- 19.Shulaev V. Metabolomics technology and bioinformatics. Brief Bioinform. 2006;7(2):128–39. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Locasale JW. Metabolomics: a primer. Trends Biochem Sci. 2017;42(4):274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumamoto Y, Harada Y, Takamatsu T, Tanaka H. Label-free molecularimaging and analysis by raman spectroscopy. Acta Histochem Cytochem. 2018;51(3):101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Qi Y, Tan SP, Bi R, Olivo M. Molecular fingerprint detection using raman and infrared spectroscopy technologies for cancer detection: a progress review. Biosensors (Basel). 2023;13(5): 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong K, Kendall C, Stone N, Notingher I. Raman spectroscopy for medical diagnostics--from in-vitro biofluid assays to in-vivo cancer detection. Adv Drug Deliv Rev. 2015;89:121–34. [DOI] [PubMed] [Google Scholar]
- 24.Jones RR, Hooper DC, Zhang L, Wolverson D, Valev VK. Raman techniques: fundamentals and frontiers. Nanoscale Res Lett. 2019;14(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Gilany K, Mani-Varnosfaderani A, Minai-Tehrani A, Mirzajani F, Ghassempour A, Sadeghi MR, et al. Untargeted metabolomic profiling of seminal plasma in nonobstructive azoospermia men: A noninvasive detection of spermatogenesis. Biomed Chromatogr. 2017;31(8). [DOI] [PubMed] [Google Scholar]
- 27.Gilany K, Moazeni-Pourasil RS, Jafarzadeh N, Savadi-Shiraz E. Metabolomics fingerprinting of the human seminal plasma of asthenozoospermic patients. Mol Reprod Dev. 2014;81(1): 84–6. [DOI] [PubMed] [Google Scholar]
- 28.Yu B, Ge M, Li P, Xie Q, Yang L. Development of surface-enhanced Raman spectroscopy application for determination of illicit drugs: towards a practical sensor. Talanta. 2019;191:1–10. [DOI] [PubMed] [Google Scholar]
- 29.Ugboma HA, Aburoma HL. Public awareness of testicular cancer and testicular self-examination in academic environments: a lost opportunity. Clinics (Sao Paulo). 2011;66(7):1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garolla A, De Giorgi U, Milardi D. Editorial: testicular cancer: new insights on the origin, genetics, treatment, fertility, general health, quality of life and sexual function. Front Endocrinol (Lausanne). 2020;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol. 2009;33(7): 1065–77. [DOI] [PubMed] [Google Scholar]
- 32.Lakpour N, Ghods R, Sadeghi MR, Ranjbar MM, Abolhasani M, Kiani J, et al. Production and characterization of a new specific monoclonal antibody against A-isoform of SALL4: a novel emerging testicular cancer marker. Andrologia. 2022;54(11):e14608. [DOI] [PubMed] [Google Scholar]
- 33.Jiang S, Liu Y, Xu Y, Sang X, Lu X. Research on liquid biopsy for cancer: a bibliometric analysis. Heliyon. 2023;9(3):e14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández-Lázaro D, García Hernández JL, García AC, Córdova Martínez A, Mielgo-Ayuso J, Cruz-Hernández JJ. Liquid biopsy as novel tool in precision medicine: origins, properties, identification and clinical perspective of cancer’s biomarkers. Diagnostics (Basel). 2020;10(4):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531–48. [DOI] [PubMed] [Google Scholar]
- 36.Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, et al. Metabolomics enables precision medicine: “a white paper, community perspective”. Metabolomics. 2016;12(10):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013;1(1):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasseur S, Guillaumond F. Lipids in cancer: a global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis. 2022;11(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platten M, Nollen EA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401. [DOI] [PubMed] [Google Scholar]
- 40.Drabovich AP, Saraon P, Jarvi K, Diamandis EP. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat Rev Urol. 2014;11(5):278–88. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Saleh RA, Bedaiwy MA. Bedaiwy, Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–43. [DOI] [PubMed] [Google Scholar]
- 42.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta-Elera G, Garrett AR, Robison RA, O’Neill KL. The role of oxidative stress in prostate cancer. Eur J Cancer Prev. 2012;21(2):155–62. [DOI] [PubMed] [Google Scholar]
- 44.Trachootham D, Lu W, Ogasawara MA, Valle NR, Huang P. Redox regulation of cell survival. Anti-oxid Redox Signal. 2008;10(8):1343–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbari Z, Dijojin RT, Zamani Z, Hosseini RH, Arjmand M. Aromatic amino acids play a harmonizing role in prostate cancer: a metabolomics-based cross-sectional study. Int J Reprod Biomed. 2021;19(8):741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietkiewicz D, Klupczynska-Gabryszak A, Plewa S, Misiura M, Horala A, Miltyk W, et al. Free amino acid alterations in patients with gynecological and breast cancer: a review. Pharmaceuticals (Basel). 2021;14(8):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neurauter G, Grahmann AV, Klieber M, Zeimet A, Ledochowski M, Sperner-Unterweger B, et al. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. 2008;272(1):141–7. [DOI] [PubMed] [Google Scholar]
- 48.Sharifi-Rad J, Seidel V, Izabela M, Monserrat-Mequida M, Sureda A, Ormazabal V, et al. Phenolic compounds as Nrf2 inhibitors: potential applications in cancer therapy. Cell Commun Signal. 2023;21(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]


