Abstract
Objective
To reveal the clinical features and assess risk factors linked to brain fog and its societal implications, including labor productivity, providing valuable insights for the future care of individuals who have experienced coronavirus disease 2019 (COVID‐19).
Methods
We analyzed a comprehensive cohort dataset comprising 1,009 patients with COVID‐19 admitted to Japanese hospitals. To assess brain fog, we analyzed patients who responded to a questionnaire indicating symptoms such as memory impairment and poor concentration.
Results
The prevalence of brain fog symptoms decreased 3 months posthospitalization but remained stable up to 12 months. Neurological symptoms such as taste and smell disorders and numbness at hospitalization correlated with a higher frequency of identifying brain fog as a long COVID manifestation. Our findings indicated that advanced age, female sex, a high body mass index, oxygen required during hospitalization, chronic obstructive pulmonary disease, asthma, and elevated C‐reactive protein and elevated D‐dimer levels were risk factors in patients exhibiting brain fog. Additionally, we demonstrated the negative impact of brain fog on labor productivity by presenteeism scores.
Interpretations
This study clarified the clinical characteristics of patients experiencing brain fog as a long COVID manifestation, specifically emphasizing neurological symptoms during hospitalization and their correlation with brain fog. Additionally, the study identified associated risk factors for its onset and revealed that the emergence of brain fog was linked to a decline in labor productivity.
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic, originated in Wuhan, China, in December 2019, has led to infections and deaths worldwide, including in Japan 1 . Long‐lasting effects of COVID‐19, commonly referred to as long COVID, manifest in a range of symptoms including persistent fatigue, breathing difficulty, muscle pain, headache, anxiety, depression, and taste and smell disorders 2 , 3 . Such systemic symptoms may persist even in mild outpatient cases for 3–24 months 4 , 5 , 6 and can decrease patients' quality of life and contribute to health and social burdens 7 . According to the World Health Organization (WHO), the term “post‐COVID condition” describes symptoms that continue for >12 weeks postrecovery from infection and that cannot be attributed to other medical conditions. Reports suggest that 10–30% of individuals who have recovered from an infection continue to experience long COVID symptoms 8 . “Brain fog,” a neurological symptom of long COVID, has particularly gathered attention.
Although lacking precise diagnostic criteria and definitions, brain fog refers to symptoms like memory impairment, poor concentration, dissociative phenomena, cognitive “slowness” and excessive effort, and communication difficulties 9 , 10 , 11 , 12 , 13 . Factors such as age 14 , 15 , 16 , 17 , 18 , COVID‐19 severity 16 , 18 , 19 , 20 , prolonged hospitalization 18 , and preexisting conditions (depression, anxiety, hyperlipidemia, cardiovascular disease, chronic obstructive pulmonary disease, and asthma) 15 , 16 , 18 , 20 , 21 are reported as potential risk factors for brain fog. Moreover, patients with brain fog often exhibit concomitant respiratory and gastrointestinal symptoms 21 , 22 . Hypotheses for the pathogenesis of the condition include direct neural damage by the virus, the presence of residual viral fragments, autoantibody production, inflammation, blood–brain barrier disruption, microvascular inflammation, and hypoxia 20 , 23 . Medical professionals increasingly recognize postinfectious brain fog symptoms, often associated with chronic fatigue syndrome 24 , 25 . In clinical practice, patients with symptoms such as memory impairment, poor concentration, and impaired executive function experience difficulties in their daily lives, including work 26 . Identifying individuals at a higher risk of developing brain fog is considered useful for appropriately allocating healthcare and administrative resources, particularly to provide medical care for patients with COVID‐19.
Some risk factors, such as the severity of the COVID‐19 in the acute phase and female, have consistently been reported in previous studies 15 , 27 . However, several other risk factors remain controversial. Firstly, the factors considered in the analyses, including background information, additional symptoms, and test results during hospitalization, varied among the reports. Second, although the impact of brain fog on employment is significant, detailed reports, including quantitative scoring, are limited. Third, research on brain fog is scarce in Japan, and whether the findings of overseas studies apply to the Japanese population remains uncertain. To address these three items, clarifying the societal impact of brain fog is crucial, as it aids in identifying patient groups in need of support.
To elucidate these points, we utilized a large cohort dataset of longitudinal questionnaire responses collected >12 months from patients with COVID‐19 in Japan 28 , 29 . We analyzed the dataset to determine factors that may increase patients' brain fog risk based on their background information. Clinically, we have observed that patients with specific combinations of symptoms during hospitalization, particularly those involving neurological symptoms, have tended to experience brain fog as part of long COVID. Furthermore, we assessed the impact of brain fog on labor productivity and revealed its negative effect using the WHO's presenteeism score 30 , 31 .
Methods
Patients
We utilized data from a prospective nationwide observational study conducted in Japan 28 , 29 . The detailed protocol for this study has been previously reported 28 . The study was conducted from January 2020 to the end of February 2021, encompassing 26 participating medical institutions and targeting confirmed cases of COVID‐19 (diagnosed via SARS‐CoV‐2 polymerase chain reaction [PCR] or antigen testing). The 26 participating facilities that received ethical approval and research authorization sent research invitations via mail to patients aged ≥18 years postdischarge. Those who consented were asked to complete a questionnaire regarding the presence or absence of symptoms, socioeconomic background, and to complete a survey based on a scoring system, including the World Health Organization Health and Work Performance Questionnaire (WHO‐HPQ), on paper or via a smartphone application at 3, 6, and 12 months postdiagnosis. Additionally, symptoms during hospitalization were simultaneously assessed in a survey conducted at 3 months postdiagnosis. Comprehensive medical information covering 168 clinical survey items at each facility was collected from each medical institution through an electronic data system. Data concerning cognitive impairment or psychiatric disease prior to COVID‐19 were not collected uniformly, but information concerning these conditions was collected from the open‐ended fields in relation to medical history.
We obtained consent from 1,200 individuals to participate in the survey, with 1066 cases having matched partial survey responses and medical information 29 . In this study, we analyzed 1,009 cases in which information regarding “memory impairment” and “poor concentration” was available from patients at least once (Fig. S1).
Questionnaire
This study assessed 24 representative symptoms and other symptoms, if present, following COVID‐19 diagnosis, including cough; sputum production; shortness of breath (dyspnea); sensitivity to sound, light, and smell (sensory hypersensitivity); fatigue and malaise; hair loss; joint pain; muscle pain; muscle weakness; headache; sore throat; ear ringing; altered consciousness; abdominal pain; diarrhea; rash; numbness in hands or feet; eye‐related symptoms (eye pain, itching, foreign body sensation, redness, and watery eyes); memory impairment (forgetfulness and difficulty finding words); and poor concentration (decreased thinking and concentration abilities) 29 .
Notably, the term “brain fog” was not explicitly included among the 24 symptoms and specific diagnostic criteria or definitions for “brain fog” were not established 10 , 11 , 13 . Although its definition remains incomplete, the term “brain fog” is widely used. Previous reports have commonly encompassed a spectrum of brain fog experiences, including patients exhibiting symptoms such as “memory impairment” and “poor concentration.” 13 In line with previous studies, we defined the experience of brain fog as the presence of either symptom.
WHO‐HPQ
The globally recognized WHO‐HPQ serves as an indicator to assess current work performance and was utilized to conduct evaluations. We employed the Japanese version of the translated WHO‐HCP criteria to assess presenteeism, evaluating brain fog's effect on work 30 , 31 , 32 . We administered the questionnaire following the report by Suzuki and colleagues, in which patients were asked two questions to assess their presenteeism: Question 1, on a scale from 0 to 10, where 0 is the worst job performance anyone could achieve in your job, and 10 is the performance of a top worker, how would you rate the usual performance of most workers in a job similar to your job?; and Question 2, using the same 0–10 scale, how would you rate your overall job performance on the days you worked during the past 4 weeks (28 days)?
Based on these two questions, we calculated relative and absolute presenteeism, in which relative presenteeism comprised Questions 2 and 1 and absolute presenteeism equaled Questions 2 × 10. For relative presenteeism, values <0.25 were set to 0.25, and values >2.0 were set to 2.0. A score of 0.25 represented the lowest relative performance, signifying ≤25% compared to the performance of other workers, whereas a score of 2.0 indicated the highest level of performance, signifying ≥200%. While for absolute presenteeism, the score ranged from 0 to 100, in which 0 represented complete performance loss, and 100 represented the absence of any performance deficit.
Definition of variables
Continuous variables included age, body mass index (BMI), D‐dimer, fibrinogen, and C‐reactive protein (CRP) levels, and absolute and relative presenteeism. Patient admission data concerning D‐dimer, fibrinogen, and CRP levels were collected from each participating facility. However, upon summarizing patient characteristics in stratified groups, we categorized age into three groups: young (18–40 years), middle‐aged (41–64 years), and older adults (≥65 years). Similarly, BMI was classified in four groups: underweight (<18.5 kg/m2), normal range (18.5–24.99 kg/m2), pre‐obese (25–29.99 kg/m2), and obese (≥30 kg/m2). Educational level was categorized as having a Bachelor's degree or higher, or lower than a Bachelor's degree, while work types were divided into regular and nonregular employment.
Binary variables included sex, comorbidities (asthma, immunodeficiency, chronic obstructive pulmonary disease [COPD], cardiovascular disease [CVD]), intensive care unit [ICU] admission, oxygen requirement during hospitalization, and long COVID symptoms during hospitalization (memory impairments, poor concentration, and brain fog). We categorized oxygen requirements during hospitalization into two groups: patients without the need for oxygen and those with oxygen requirements. For the longitudinal outcome measures, we included memory impairments, poor concentration, and brain fog during hospitalization and at 3, 6, and 12 months posthospitalization. The four time points for “visits” were specified to be during hospitalization, and at 3, 6, and 12 months.
Statistical analysis
Patient characteristics were summarized using means and standard deviations for continuous variables and numbers and percentages for categorical variables. The association between candidate risk factors and the presence of brain fog was evaluated using a generalized linear mixed‐effects model (GLMM) with a logit‐link function for memory impairment or poor concentration. We included visits, age, BMI, D‐dimer, fibrinogen, and CRP levels, sex, asthma, immunodeficiency, COPD, CVD, ICU admission, and oxygen requirement as covariates in the GLMM. These covariates were selected based on potential risk factors identified from previous studies (Table S1) 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 27 , 33 , 34 , 35 , 36 , 37 . We specified an unstructured covariance matrix for the GLMM. If convergence was not achieved, we then used a first‐order autoregressive structure, and if that also failed, a compound symmetry structure.
Mixed‐effects models for repeated measures (MMRM) were employed to examine the association between absolute or relative presenteeism at 3, 6, and 12 months and brain fog, memory impairment, or poor concentration during hospitalization. For the MMRM, we incorporated covariates identical to those in the GLMM, adding an interaction term between visits and brain fog during hospitalization. The variance–covariance structure of the MMRMs was specified as unstructured. As a sensitivity analysis, we also conducted a complete case analysis using the same model. All analyses were performed using SAS (v.9.4; SAS Institute, Cary, NC, USA) at a two‐sided 5% significance level.
Results
Patient characteristics
The number of study respondents decreased over time (1,009 patients during hospitalization, 924 at 3 months, 865 at 6 months, and 724 at 12 months). Patient characteristics were compared based on the presence or absence of brain fog, memory impairment, or poor concentration at 3 months postdiagnosis (Table 1). The respective patient characteristics are summarized for the overall group and for each brain fog, memory impairment, and poor concentration subgroup. Owing to missing data at various time points, only patient background data concerning 690 patients who completed the questionnaire at all time points are summarized. Study patients comprised young (19.0%), middle‐aged (45.7%), and older (35.1%) adults, and patients of unknown age (0.2%). In total, 11.9% of patients required oxygen support, and 63.3% of patients were male. The highest level of education attained was a bachelor's degree or higher, with percentages of 51.7% (522/1009) or 54.3% (522/962). In terms of employment, the proportion of regular employees was 50.3% (508/1009) or 68.6% (508/740) (Table 1). Among patients with brain fog, 11.9% required oxygen support, and 63.3% were male. The prevalence of specific conditions included CVD at 6.4%, autoimmune disorders at 1.9%, COPD at 3.2%, asthma at 5.4%, and ICU admission at 9.7%. Laboratory findings showed the mean value of CRP levels at 4.24 mg/dL, D‐dimer at 1.44 μg/mL, and fibrinogen at 485.75 mg/dL. Of 119 patients with brain fog, two had a history of psychiatric disease (one with panic disorder and one with insomnia), and no patients had a history of dementia. Of 890 patients without brain fog, nine had a history of psychiatric disease (five with depression, two with schizophrenia, one with anxiety disorder, and one with unspecified psychiatric disease), and six had a history of dementia.
Table 1.
Baseline characteristics of all patients.
| All (n = 1009) | Brain fog (−) at 3 months (n = 816) | Brain fog (+) at 3 months (n = 119) | Memory impairment (n = 68) | Poor concentrarion (n = 102) | Answer at all time point (n = 690) | |
|---|---|---|---|---|---|---|
| Severity (n (%)) | ||||||
| Oxygen not required | 881 (87.3) | 715 (87.6) | 94 (79.0) | 50 (73.5) | 80 (78.4) | 599 (86.8) |
| Oxygen required | 120 (11.9) | 94 (11.5) | 24 (20.2) | 18 (26.5) | 21 (20.6) | 86 (12.5) |
| Missed | 8 (0.8) | 7 (0.9) | 1 (0.8) | 0 (0) | 1 (1.0) | 5 (0.7) |
| Age group (n (%)) | ||||||
| Young (18–40) | 192 (19.0) | 153 (18.8) | 21 (17.6) | 9 (13.2) | 21 (20.6) | 108 (15.7) |
| Middle (41–64) | 461 (45.7) | 377 (46.2) | 56 (47.1) | 29 (42.7) | 48 (47.0) | 323 (46.8) |
| Eldery (64<) | 354 (35.1) | 285 (34.9) | 42 (35.3) | 30 (44.1) | 33 (32.4) | 259 (37.5) |
| NA | 2 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| BMI (n (%)) | ||||||
| Underweight (<18.5) | 49 (4.9) | 36 (4.4) | 8 (6.7) | 4 (5.9) | 8 (7.8) | 29 (4.2) |
| Normal (18.5–24.99) | 538 (53.3) | 437 (53.6) | 60 (50.4) | 35 (51.5) | 53 (52.1) | 374 (54.2) |
| Pre‐obese (25–29.99) | 284 (28.1) | 235 (28.8) | 33 (27.7) | 21 (30.9) | 25 (24.5) | 204 (29.6) |
| Obese (30<) | 79 (7.8) | 95 (11.6) | 15 (12.6) | 5 (7.3) | 13 (12.7) | 48 (7.0) |
| NA | 59 (5.8) | 13 (1.6) | 3 (2.5) | 3 (4.4) | 3 (2.9) | 35 (5.1) |
| Smoke (n (%)) | ||||||
| Current | 108 (10.7) | 88 (10.8) | 14 (11.8) | 11 (16.2) | 10 (9.8) | 72 (10.4) |
| Past | 248 (28.1) | 206 (25.2) | 28 (23.5) | 17 (25.0) | 23 (22.5) | 178 (25.8) |
| Sex (n (%)) | ||||||
| Male | 639 (63.3) | 522 (64.0) | 73 (61.3) | 41 (60.3) | 60 (58.8) | 428 (62.0) |
| Female | 370(36.7) | 294 (36.0) | 46 (38.7) | 27 (39.7) | 42 (41.2) | 262 (38.0) |
| HT (n (%)) | ||||||
| Yes | 334 (33.1) | 282 (34.6) | 34 (28.6) | 24 (35.3) | 26 (25.5) | 242 (35.1) |
| No | 668 (66.2) | 528 (64.7) | 84 (70.6) | 44 (64.7) | 75 (73.5) | 444 (64.3) |
| NA | 7 (0.7) | 6 (0.7) | 1 (0.8) | 0 (0) | 1 (1.0) | 4 (0.6) |
| DM (n (%)) | ||||||
| Yes | 172 (17.0) | 136 (16.7) | 21 (17.6) | 18 (26.5) | 14 (13.7) | 109 (15.8) |
| No | 827 (82.0) | 672 (82.4) | 96 (80.8) | 49 (72.0) | 87 (85.3) | 573 (83.0) |
| NA | 10 (1.0) | 8 (0.9) | 2 (1.6) | 1 (1.5) | 1 (1.0) | 8 (1.2) |
| CVD (n (%)) | ||||||
| Yes | 64 (6.3) | 56 (6.9) | 3 (2.5) | 3 (4.4) | 2 (1.9) | 41 (6.0) |
| No | 937 (92.9) | 754 (92.4) | 114 | 64 (94.1) | 99 (97.1) | 628 (91.0) |
| NA | 8 (0.8) | 6 (0.7) | 2 (1.6) | 1 (1.5) | 1 (1.0) | 21 (3.0) |
| Malignancy (n (%)) | ||||||
| Yes | 69 (6.8) | 53 (6.5) | 6 (5.0) | 2 (2.9) | 6 (5.9) | 39 (5.7) |
| No | 931 (92.3) | 757 (92.8) | 110 (92.4) | 65 (95.6) | 93 (91.2) | 646 (93.6) |
| NA | 9 (0.9) | 6 (0.7) | 3 (2.5) | 1 (1.5) | 3 (2.9) | 5 (0.7) |
| Autoimmune disease (n (%)) | ||||||
| Yes | 19 (1.9) | 15 (1.8) | 3 (2.5) | 3 (4.4) | 2 (1.9) | 10 (1.4) |
| No | 980 (97.1) | 794 (97.3) | 114 (95.8) | 64 (94.1) | 99 (97.1) | 673 (97.5) |
| NA | 10 (1.0) | 7 (0.9) | 2 (1.7) | 1 (1.5) | 1 (1.0) | 7 (1.0) |
| COPD (n (%)) | ||||||
| Yes | 32 (3.2) | 24 (3.0) | 7 (5.9) | 4 (5.9) | 6 (5.9) | 22 (3.2) |
| No | 967 (95.8) | 784 (96.1) | 110 (92.4) | 63 (92.6) | 95 (93.1) | 662 (95.9) |
| NA | 10 (1.0) | 8 (0.9) | 2 (1.7) | 1 (1.5) | 1 (1.0) | 6 (0.9) |
| Asthma (n (%)) | ||||||
| Yes | 54 (5.4) | 42 (5.2) | 9 (7.6) | 6 (8.8) | 9 (8.8) | 37 (5.4) |
| No | 940 (93.2) | 763 (93.5) | 107 (89.9) | 60 (88.2) | 92 (90.2) | 646 (93.6) |
| NA | 15 (1.5) | 11 (1.3) | 3 (2.5) | 2 (3.0) | 1 (1.0) | 7 (1.0) |
| HUA (n (%)) | ||||||
| Yes | 103 (10.2) | 90 (11.0) | 9 (7.6) | 7 (10.3) | 6 (5.9) | 75 (10.9) |
| No | 897 (88.9) | 719 (88.1) | 108 (90.8) | 60 (88.2) | 95 (93.1) | 608 (88.1) |
| NA | 9 (0.9) | 7 (0.9) | 2 (1.6) | 1 (1.5) | 1 (1.0) | 7 (1.0) |
| CLD (n (%)) | ||||||
| Yes | 35 (3.5) | 29 (3.6) | 4 (3.4) | 3 (4.4) | 2 (1.9) | 27 (3.9) |
| No | 958 (94.9) | 773 (94.7) | 113 (95.0) | 65 (95.6) | 98 (96.2) | 654 (94.8) |
| NA | 16 (1.6) | 14 (1.7) | 2 (1.6) | 0 (0) | 2 (1.9) | 9 (1.3) |
| CKD (n (%)) | ||||||
| Yes | 44 (4.4) | 39 (4.8) | 3 (2.5) | 1 (1.5) | 3 (2.9) | 34 (4.9) |
| No | 948 (94.0) | 763 (93.5) | 113 (95.0) | 66 (97.0) | 96 (94.2) | 624 (90.4) |
| NA | 17 (1.7) | 14 (1.7) | 3 (2.5) | 1 (1.5) | 3 (2.9) | 32 (4.6) |
| ICU hospitalization (n (%)) | ||||||
| Yes | 98 (9.7) | 80 (9.8) | 13 (10.9) | 8 (11.8) | 11 (10.8) | 68 (9.9) |
| No | 894 (88.6) | 722 (88.5) | 103 (86.6) | 60 (88.2) | 88 (86.3) | 609 (88.3) |
| NA | 17 (1.7) | 14 (1.7) | 3 (2.5) | 0 (0) | 3 (2.9) | 13 (1.9) |
| Blood test (Mean (SD)) | ||||||
| WBC (/μL) | 5359.76 (2162.99) | 5323.27 (2186.28) | 5407.83 (2033.30) | 5633.48 (2023.19) | 5380.68 (2059.16) | 5405.74 (2170.85) |
| Neu (%) | 67.32 (12.62) | 67.46 (12.77) | 68.38 (12.10) | 69.51 (12.92) | 67.97 (12.79) | 68.19 (12.55) |
| Lym (%) | 23.42 (10.39) | 23.3 (10.43) | 22.76 (10.03) | 22.41 (11.32) | 23.11 (10.45) | 22.81 (10.20) |
| Hb (g/dL) | 14.06 (1.82) | 14.12 (1.75) | 14.17 (1.76) | 14.07 (1.82) | 14.08 (1.86) | 14.10 (1.70) |
| PLT (×10^4/μL) | 37.74 (56.69) | 38.07 (58.75) | 35.29 (46.38) | 39.38 (53.56) | 32.86 (40.68) | 38.29 (59.56) |
| Cr (mg/dL) | 0.87 (0.52) | 0.88 (0.57) | 0.84 (0.28) | 0.84 (0.32) | 0.84 (0.30) | 0.88 (0.47) |
| LDH (U/L) | 252.89 (96.76) | 252.88 (93.30) | 273.00 (124.68) | 290.88 (123.31) | 269.33 (126.66) | 256.98 (97.56) |
| KL‐6 (U/mL) | 276.88 (185.56) | 277.00 (177.84) | 295.94 (255.32) | 322.12 (303.36) | 270.92 (180.56) | 280.14 (192.96) |
| Fibrinoge (mg/dL) | 485.75 (152.34) | 486.27 (150.87) | 495.82 (166.57) | 517.63 (179.78) | 491.45 (153.49) | 492.29 (153.98) |
| D‐dimer (μg/mL) | 1.44 (3.43) | 1.37 (3.29) | 1.97 (4.82) | 2.25 (4.86) | 1.77 (4.33) | 1.50 (3.62) |
| CRP (mg/dL) | 4.24 (5.42) | 4.33 (5.45) | 4.75 (5.96) | 5.72 (6.90) | 4.47 (5.47) | 4.55 (5.66) |
BMI, body mass index; CKD, chronic kidney disorder; CLD, chronic liver disorder; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; HT, hypertension; HUA, hyperuricemia.
Frequency and trends of brain fog
Next, we determined the number of individuals with brain fog, memory impairment, or poor brain concentration (Fig. 1A). The frequency of individuals reporting brain fog symptoms decreased from 26.3% during hospitalization to 12.2% at 3 months postdiagnosis. However, 6 months postdiagnosis, it remained constant at 13.0%, and at 13.1% at 12 months postdiagnosis, indicating that the proportion of individuals with brain fog remained stable at 3 months postdiagnosis. When focusing on each symptom, memory impairment slightly increased from 3 to 12 months after diagnosis (6.9% at 3 months, 7.5% at 6 months, and 8.1% at 12 months). Poor concentration consistently decreased up to 12 months postdiagnosis, although the rate of decrease between 6 and 12 months was modest (10.4% at 3 months, 10.9% at 6 months, and 9.1% at 12 months). Some patients exhibited brain fog symptoms (memory impairment or poor concentration) that emerged during the follow‐up period.
Figure 1.
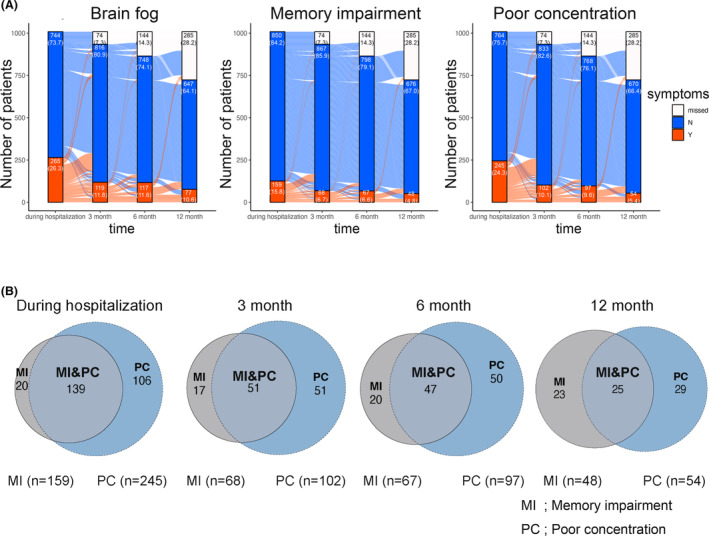
Trends of patients who had brain fog, memory impairment, and poor concentration as sequelae at 3, 6, and 12 months. The trends in patients with brain fog, memory impairment, and poor concentration are shown (A). The red area represents patients with symptoms, the blue area represents those without symptoms, and the white area represents cases in which information is missing. The blue curve illustrates the progression in individuals who remained asymptomatic during their hospital stay, whereas the red curve represents individuals who developed symptoms during hospitalization. Furthermore, we showed the distribution of patients with overlapping symptoms during hospitalization at 3, 6, and 12 months (B). The gray area represents patients with memory impairment, and the blue area represents those with poor concentration. The numbers within each circle indicate the number of patients. MI, memory impairment; PT, poor concentration.
A graph was used to visualize symptom differences between patients with or without brain fog. Patients with brain fog generally had more symptoms compared with those without brain fog (Fig. S2).
The relationship between the symptoms of memory impairment or poor concentration during hospitalization and at 3, 6, and 12 months after diagnosis was visualized using Venn diagrams (Fig. 1B). During hospitalization, most individuals who reported memory impairment also presented poor concentration. However, as time passed, during hospitalization and at 3, 6, and 12 months after diagnosis, the number of individuals with poor concentration decreased (139, 51, 47, and 25). Furthermore, the number of cases with only memory impairment remained unchanged (20, 17, 20, and 23). On the contrary, concerning poor concentration, almost half of the cases had memory impairment throughout the observation period.
Brain fog has been reported to have a strong association with fatigue 38 . We analyzed the association between fatigue and brain fog in our study cohort and identified >40% of patients with brain fog who reported experiencing fatigue (Fig. S2).
Combination of brain fog with symptoms during hospitalization
To determine the clinical features of patients experiencing neurological symptoms as part of long COVID, we used heat maps to visualize the association between symptoms during hospitalization and brain fog, memory impairment, and poor concentration as long COVID manifestations after 3 months. For brain fog as a long COVID manifestation, strong associations were observed between combinations of symptoms during hospitalization, for example, numbness and loss of smell (63.16%), numbness and loss of taste (62.96%), numbness and hair loss (62.50%), or numbness and sleep disturbances (60.87%) during hospitalization and brain fog at any point at 3, 6, and 12 months postdischarge (Fig. 2). Similarly, combinations of sleep disturbances and hair loss (65.45%), or sleep disturbances and stomach aches (62.16%) during hospitalization were strongly associated with brain fog as long COVID manifestations. For memory impairment (Fig. S2) and poor concentration (Fig. S3), the most frequent symptom combinations were numbness and loss of smell (55.56%) or taste (55.56%). These findings suggest that these neurological symptoms (brain fog, memory impairment, and poor concentration) are associated with sensory disturbances such as numbness, smell, and taste disorders during hospitalization.
Figure 2.
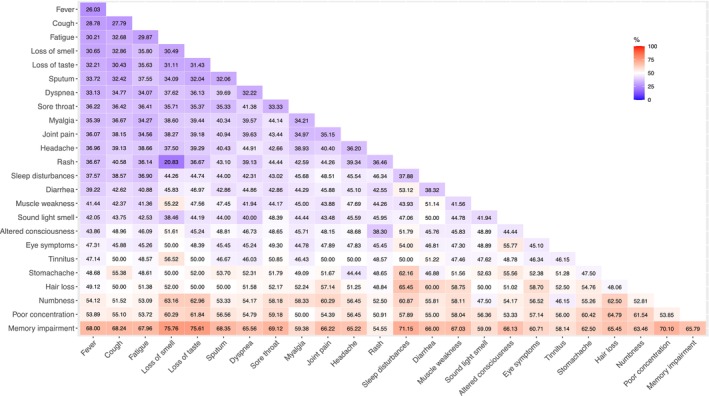
Pattern of symptoms during hospitalization in patients with brain fog at either 3, 6, or 12 months. The percentages in this figure indicate how frequently patients, who had a combination of two symptoms observed during hospitalization, experienced brain fog at 3, 6, and 12 months postdiagnosis. Symptoms refer to a combination of 24 symptoms assessed during hospitalization.
Risk factor analysis of cognitive symptoms as Long COVID
Following, we performed a GLMM analysis to identify the risk factors for brain fog, memory impairment, and poor concentration. Forest plots show the odds ratio of each risk factor for brain fog (Fig. 3, on the left), memory impairment (Fig. 3, on the middle), and poor concentration (Fig. 3, on the right). The odds ratios for brain fog, at 3–12 months consistently exceeded 1 across most categories, including age, 1.001 (95% CI: 0.990 to 1.010); sex, 1.27 (0.86–1.88); oxygen requirement, 1.14 (0.68 to 1.93); autoimmune disorders, 0.76 (0.22 to 2.65); COPD, 1.59 (0.69–3.66); asthma, 1.73 (0.85 to 3.53); CRP, 1.042 (1.00 to 1.09); and D‐dimer, 1.022 (0.97 to 1.07). A similar trend, with odds ratios exceeding 1 in most categories, was also observed for memory impairment and poor concentration. Notably, memory impairment was significantly associated with age 1.024 (95% CI: 1.010 to 1.040, p = 0.009).
Figure 3.
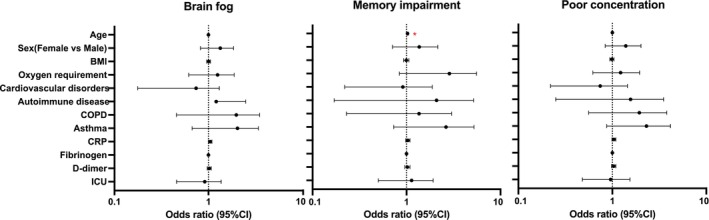
Risk factors for having brain fog symptoms between 3, 6, and 12 months. Odds ratios (ORs) were analyzed for patients with brain fog, memory impairment, or poor concentration using risk factors identified in previous reports. BMI, body mass index; COPD, chronic obstructive pulmonary disease; CI, confidence interval; ICU, intensive care unit.
Social impact of brain fog sequelae: Association with absolute and relative presenteeism
The average absolute presenteeism score was lower in participants with brain fog (42.7 points) than in those without (63.7 points) at 3 months postdiagnosis (Fig. 4, on the left). In patients experiencing brain fog, a similar trend of low absolute presenteeism was observed at both 6 and 12 months. Additionally, differences in scores based on the presence or absence of brain fog were minimal for relative presenteeism (Fig. 4, on the right).
Figure 4.
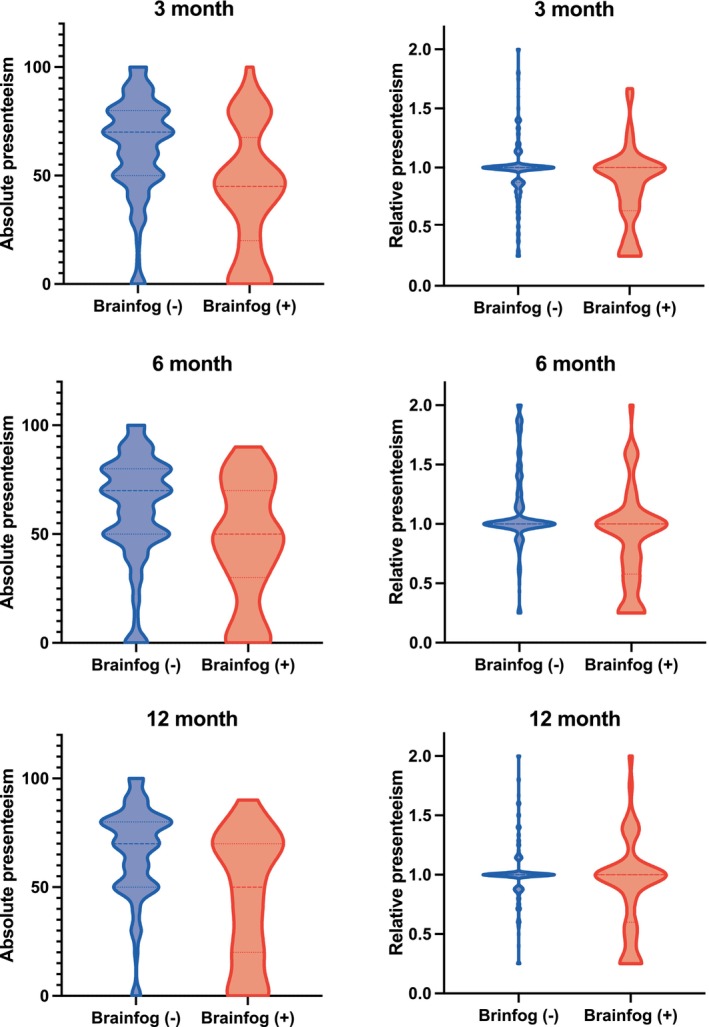
Brain fog causes a decrease in presenteeism values at 3, 6, and 12 months. Comparison of absolute and relative presenteeism in patients with persistent symptoms (red) and those with recovered symptoms (dark blue) at 3, 6, and 12 months after diagnosis. The horizontal dashed line represents the median, while the dotted lines indicate the upper and lower 25th percentiles. Patients with brain fog tend to have lower presenteeism scores than those without.
Individuals with brain fog exhibited significantly lower presenteeism than those without brain fog, regardless of the time point. To analyze longitudinal changes, we conducted an MMRM analysis based on the presence of brain fog at 3 months. In terms of absolute presenteeism scores, BMI (−0.10, 95% CI −0.70 to 0.49), CRP level (−0.25, 95% CI −0.91 to 0.40), sex (−0.85, 95% CI −6.77 to 5.07), ICU admission (−8.30, 95% CI −17.14 to 0.54), education level (−0.89, 95% CI −5.92 to 4.15), and work type (−5.60, 95% CI −10.84 to 0.36) were factors associated with a score reduction (Table 2). In terms of changes over time, reductions of 8.53, 4.34, and 11.01 points were estimated at 3, 6, and 12 months, respectively, compared with during hospitalization. In contrast, relative presenteeism scores showed modest estimates in factors and slight temporal changes (Table S2). Similarly, the estimated scores for memory impairment and poor concentration showed decreases at almost all time points in absolute presenteeism scores (Tables S3 and S4). Furthermore, the relative presenteeism scores for memory impairment and poor concentration did not significantly change (Tables S5 and S6). Our results in relation to presenteeism showed similar trends even when the study population was reduced to 690 patients (Tables [Link], [Link], [Link], [Link], [Link], [Link]).
Table 2.
The relationship between the presence of brain fog symptoms and absolute presenteeism.
| Absolute presenteeism 3, 6, and 12 months (MMRM) | ||||
|---|---|---|---|---|
| Index | Estimated value | Lower | Upper | p |
| Intercept | 67.55 | 47.86 | 87.23 | <.0001 |
| Age | 0.02 | −0.17 | 0.21 | 0.846 |
| Asthma | 1.84 | −8.57 | 12.24 | 0.728 |
| Autoimmune_disease | 9.22 | −6.58 | 25.01 | 0.252 |
| BMI | −0.10 | −0.70 | 0.49 | 0.733 |
| COPD | 5.38 | −6.71 | 17.46 | 0.382 |
| CRP | −0.25 | −0.91 | 0.40 | 0.443 |
| CVD | 1.88 | −8.96 | 12.73 | 0.733 |
| Oxygen requirement | 3.57 | −3.08 | 10.21 | 0.292 |
| Sex(female) | −0.85 | −6.77 | 5.07 | 0.777 |
| D‐dimer | 0.21 | −0.50 | 0.93 | 0.555 |
| Fibrinogen | 0.00 | −0.02 | 0.02 | 0.964 |
| ICU hospitalization | −8.30 | −17.14 | 0.54 | 0.066 |
| Education | −0.89 | −5.92 | 4.15 | 0.729 |
| Work | −5.60 | −10.84 | −0.36 | 0.036 |
| Visit 6 months | −0.09 | −2.42 | 2.24 | 0.938 |
| Visit 12 months | 2.03 | −1.24 | 5.30 | 0.223 |
| Brain fog 3 months* | −8.53 | −15.95 | −1.11 | 0.024 |
| Brain fog 6 months* | −4.34 | −12.02 | 3.34 | 0.267 |
| Brain fog 12 months* | −11.01 | −19.54 | −2.47 | 0.012 |
Brain fog_XX meaning is Brain fog at XX months after diagnosis.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Brain fog_XX months was calculated as the sum of the fixed effect for brain fog during hospitalization and the interaction term, which was between brain fog during hospitalization and the visit at XX months.
Discussion
Although clinicians frequently encounter patients with brain fog, comprehensive studies on this phenomenon are lacking. In this study, we analyzed brain fog considering the presence or absence of memory impairment or poor concentration using data collected from 1,009 individuals across 26 institutions in Japan. The questionnaire approach confirming 24 symptoms allowed for visualizing symptom patterns during hospitalization in cases in which brain fog persisted as a long COVID manifestation. A GLMM analysis incorporating patient background factors examined the risk factors for brain fog. Furthermore, we assessed the impact of brain fog on occupational productivity using WHO‐HPQ scores. This is Japan's first large‐scale cohort study on brain fog associated with COVID‐19.
This study revealed that brain fog symptom prevalence decreases from during hospitalization to 3 months after diagnosis yet remains stable until 12 months. Consistent with previous studies 39 , 40 , from 3 to 12 months postdiagnosis, only limited improvement was observed in symptoms related to brain fog, memory impairment, and poor concentration. Moreover, to clarify the clinical features of patients with brain fog during hospitalization, we visualized the symptoms that tended to be combined with brain fog using a heat map. Through this approach, we suggest that neurological symptoms, such as loss of smell, taste, and numbness, tend to be associated with brain fog during hospitalization. The association between neurological symptoms during hospitalization and brain fog aligns with the findings of basic research suggesting that the SARS‐CoV‐2 virus affects the nervous system 41 . We believe that understanding the clinical features of patients with brain fog during hospitalization could be helpful in clinical settings and in the basic research field.
We evaluated previously reported risk factors for brain fog to elucidate their association in our data set, including the infection severity during hospitalization (oxygen requirements and high CRP levels), autoimmune disorders, asthma, and COPD (Table S1). 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 27 , 33 , 34 , 35 , 36 , 37 Despite being reported as a risk factor for brain fog in previous research, items such as dyslipidemia were not collected in this study and could not be assessed. Our findings suggest potential risk factors for the onset of brain fog similar to those previously reported, and an association was observed between the presence of brain fog and combinations of symptoms during hospitalization. Evaluating these factors may indicate their potential for predicting brain fog symptoms.
Previous studies have reported a significant association between the onset of dementia post‐COVID‐19 infection and the presence of multiple risk factors for dementia 42 , 43 . Reported risk factors for dementia include hypertension, diabetes mellitus, a history of psychiatric disorders, alcohol consumption, the use of antipsychotic medications, and the used of stimulants. Some of these risk factors, including hypertension and diabetes mellitus, were consistently observed in this study, whereas factors such as a history of learning disabilities, head trauma, and the use of stimulants were not investigated. MRI images and cerebrospinal fluid examinations suggest that disruption of the blood–brain barrier may be associated with the onset of dementia; however, we did not obtain sufficient data to analyze this association.
Additionally, our study identified a link between persistent brain fog symptoms and decreased absolute presenteeism scores. Conversely, no significant correlation between brain fog symptoms and scores in relative presenteeism was identified. Absolute presenteeism measures the work performance of an individual, while relative presenteeism evaluates the ways in which their performance compares to that of others in the same workplace or at similar jobs. Absolute presenteeism seems to be more closely linked to health indicators compared to relative presenteeism, based on a previous study 44 . Consistent with that report, we found that brain fog has a stronger impact on absolute presenteeism compared with relative presenteeism. Furthermore, this is the first report to examine the relationship between labor productivity and brain fog in Japan and aligns with similar existing reports from overseas 45 .
Our study has some limitations, including the absence of data from individuals not infected with COVID‐19 and the lack of pre‐infection status information for infected individuals. The decreasing number of responses over time, owing to responses not having been obtained, is another limitation of this study. We analyzed the patients who provided all of the required responses; however, caution is warranted in interpreting the findings. Additionally, challenges arise owing to the clinic's lack of objective diagnostic tests or indicators for brain fog. We categorized brain fog based on memory impairment or poor concentration; however, if a more objective and quantitatively assessable method for evaluating brain fog becomes available, conducting a reanalysis based on that indicator would be desirable. Extended long‐term follow‐up is additionally crucial to identify the necessary medical (e.g., psychological or neurological assessments) and social interventions (e.g., reintegration into society and workplace support) for individuals with brain fog symptoms. Future research should prioritize overcoming these limitations to enhance the understanding of the potential for symptom improvement and specify the required medical and social interventions for patients enduring prolonged symptoms, including incorporating objective measures.
In conclusion, this study depicted the clinical characteristics of patients with brain fog, especially for the symptoms during hospitalization, as the patients with in‐hospital neurological symptoms tended to experience brain fog as a long COVID manifestation. This study also indicated that brain fog was associated with the severity of COVID‐19 in most patients, including oxygen requirement, CRP levels at the time of hospitalization, autoimmune disorders, asthma, and COPD. Furthermore, the study revealed that the persistence of brain fog is linked to a decrease in the workplace productivity indicator presenteeism. Validation of the findings of this study through larger‐scale research could provide valuable data that may be useful for efficiently allocating social resources to address this issue.
Funding Information
This research was funded by the Health Labor Science Special Research Project (20CA2054) and supported by AMED (JP20nk0101612, JP20fk0108415, JP20fk0108452, JP 21fk0108553, JP 21fk0108431, JP 22fk0108510, JP 21fk0108563, JP 21fk0108573, JP 22fk0108573, JP 22fk0108513, JP 22wm0325031), JST CREST (JPMJCR20H2), PRESTO (JPMJPR21R7), MHLW Research on Emerging and Re‐emerging Infectious Diseases and Immunization (Program Grant Number JPMH21HA2011 and JPMH23HA2011), and Taiju Life Social Welfare Foundation.
Conflict of Interest
The corresponding authors have no conflicts of interest related to this manuscript to disclose.
Author Contributions
All authors contributed to the data analysis, drafting of the article, and approval of the publication. All authors are accountable for this research.
Supporting information
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Table S6.
Table S7.
Table S8.
Table S9.
Table S10.
Table S11.
Table S12.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Acknowledgements
Data management and statistical analyses were supported by 3H Medi Solution, Inc. (President: Masashi Ando). We want to thank Editage (www.editage.jp) for English language editing.
These authors contributed equally to this work.
Funding Statement
This work was funded by Japan Agency for Medical Research and Development grants JP 21fk0108431, JP 21fk0108553, JP 21fk0108563, JP 21fk0108573, JP 22fk0108510, JP 22fk0108513, JP 22fk0108573, JP 22wm0325031, JP20fk0108415, JP20fk0108452, and JP20nk0101612; Ministry of Health, Labour and Welfare grants 20CA2054, JPMH21HA2011, and JPMH23HA2011; Core Research for Evolutional Science and Technology grant JPMJCR20H2; Precursory Research for Embryonic Science and Technology grant JPMJPR21R7.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Li X, Cui W, Zhang F. Who was the first doctor to report the COVID‐19 outbreak in Wuhan, China? J Nucl Med. 2020;61(6):782‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19. London. 2020. [PubMed]
- 3. Carfi A, Bernabei R, Landi F, Gemelli Against C‐P‐ACSG. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low‐risk individuals with post‐COVID‐19 syndrome: a prospective, community‐based study. BMJ Open. 2021;11(3):e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowe B, Xie Y, Al‐Aly Z. Postacute sequelae of COVID‐19 at 2 years. Nat Med. 2023;29(9):2347‐2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Groff D, Sun A, Ssentongo AE, et al. Short‐term and long‐term rates of postacute sequelae of SARS‐CoV‐2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentilotti E, Gorska A, Tami A, et al. Clinical phenotypes and quality of life to define post‐COVID‐19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62:102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis HE, McCorkell L, Vogel JM, Topol EJ. Author correction: Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(6):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McWhirter L, Smyth H, Hoeritzauer I, Couturier A, Stone J, Carson AJ. What is brain fog? J Neurol Neurosurg Psychiatry. 2023;94(4):321‐325. [DOI] [PubMed] [Google Scholar]
- 10. Ishikura T, Nakano T, Kitano T, Tokuda T, Sumi‐Akamaru H, Naka T. Serum ferritin level during hospitalization is associated with brain fog after COVID‐19. Sci Rep. 2023;13(1):13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam GY, Damant RW, Ferrara G, et al. Characterizing long‐COVID brain fog: a retrospective cohort study. J Neurol. 2023;270(10):4640‐4646. [DOI] [PubMed] [Google Scholar]
- 12. Taquet M, Skorniewska Z, Hampshire A, et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID‐19 hospitalization. Nat Med. 2023;29(10):2498‐2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lanz‐Luces JR, Aceituno H, Quiroz‐Bravo F, et al. Long‐lasting brain fog is related with severity clusters of symptoms in COVID‐19 patients. Rev Med Chile. 2022;150(11):1484‐1492. [DOI] [PubMed] [Google Scholar]
- 14. Beretta S, Cristillo V, Camera G, et al. Incidence and Long‐term functional outcome of neurologic disorders in hospitalized patients with COVID‐19 infected with pre‐omicron variants. Neurology. 2023;101(9):e892‐e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Largent J, Xie Y, Knuth KB, et al. Cognitive and other neuropsychiatric symptoms in COVID‐19: analysis of person‐generated longitudinal health data from a community‐based registry. BMJ Open. 2023;13(6):e069118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imoto W, Yamada K, Kawai R, et al. A cross‐sectional, multicenter survey of the prevalence and risk factors for Long COVID. Sci Rep. 2022;12(1):22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frontera JA, Yang D, Lewis A, et al. A prospective study of long‐term outcomes among hospitalized COVID‐19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in post‐COVID‐19 syndrome: a systematic review and meta‐analysis. Brain Behav Immun. 2022;101:93‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adingupu DD, Soroush A, Hansen A, Twomey R, Dunn JF. Brain hypoxia, neurocognitive impairment, and quality of life in people post‐COVID‐19. J Neurol. 2023;270(7):3303‐3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng N, Zhao YM, Yan W, et al. A systematic review and meta‐analysis of long term physical and mental sequelae of COVID‐19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28(1):423‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chudzik M, Babicki M, Kapusta J, et al. Long‐COVID clinical features and risk factors: a retrospective analysis of patients from the STOP‐COVID registry of the PoLoCOV study. Viruses. 2022;14(8):1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asadi‐Pooya AA, Akbari A, Emami A, et al. Long COVID syndrome‐associated brain fog. J Med Virol. 2022;94(3):979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansson A, Mohamed MS, Moulin TC, Schioth HB. Neurological manifestations of COVID‐19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stefano GB. Historical insight into infections and disorders associated with neurological and psychiatric sequelae similar to Long COVID. Med Sci Monit. 2021;27:e931447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ocon AJ. Caught in the thickness of brain fog: exploring the cognitive symptoms of chronic fatigue syndrome. Front Physiol. 2013;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Callan C, Ladds E, Husain L, Pattinson K, Greenhalgh T. I can't cope with multiple inputs': a qualitative study of the lived experience of ‘brain fog’ after COVID‐19. BMJ Open. 2022;12(2):e056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonilla H, Quach TC, Tiwari A, et al. Myalgic encephalomyelitis/chronic fatigue syndrome is common in post‐acute sequelae of SARS‐CoV‐2 infection (PASC): results from a post‐COVID‐19 multidisciplinary clinic. Front Neurol. 2023;14:1090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakagawara K, Namkoong H, Terai H, et al. Comprehensive and long‐term surveys of COVID‐19 sequelae in Japan, an ambidirectional multicentre cohort study: study protocol. BMJ Open Respir Res. 2021;8(1):e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terai H, Ishii M, Takemura R, et al. Comprehensive analysis of long COVID in a Japanese nationwide prospective cohort study. Respir Investig. 2023;61(6):802‐814. [DOI] [PubMed] [Google Scholar]
- 30. Aronsson G, Gustafsson K, Dallner M. Sick but yet at work. An empirical study of sickness presenteeism. J Epidemiol Community Health. 2000;54(7):502‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki T, Miyaki K, Sasaki Y, et al. Optimal cutoff values of WHO‐HPQ presenteeism scores by ROC analysis for preventing mental sickness absence in Japanese prospective cohort. PLoS One. 2014;9(10):e111191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kessler RC, Barber C, Beck A, et al. The World Health Organization health and work performance questionnaire (HPQ). J Occup Environ Med. 2003;45(2):156‐174. [DOI] [PubMed] [Google Scholar]
- 33. Caspersen IH, Magnus P, Trogstad L. Excess risk and clusters of symptoms after COVID‐19 in a large Norwegian cohort. Eur J Epidemiol. 2022;37(5):539‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asadi‐Pooya AA, Shahisavandi M, Nemati H, et al. Long‐lasting COVID‐associated brain fog: a follow‐up study. Eur Neurol. 2023;86(3):166‐170. [DOI] [PubMed] [Google Scholar]
- 35. Miskowiak KW, Johnsen S, Sattler SM, et al. Cognitive impairments four months after COVID‐19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nordvig AS, Rajan M, Lau JD, et al. Brain fog in long COVID limits function and health status, independently of hospital severity and preexisting conditions. Front Neurol. 2023;14:1150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou H, Lu S, Chen J, et al. The landscape of cognitive function in recovered COVID‐19 patients. J Psychiatr Res. 2020;129:98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delgado‐Alonso C, Diez‐Cirarda M, Pagan J, et al. Unraveling brain fog in post‐COVID syndrome: relationship between subjective cognitive complaints and cognitive function, fatigue, and neuropsychiatric symptoms. Eur J Neurol. 2023 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernandez‐de‐Las‐Penas C, Cancela‐Cilleruelo I, Rodriguez‐Jimenez J, et al. Trajectory of post‐COVID brain fog, memory loss, and concentration loss in previously hospitalized COVID‐19 survivors: the LONG‐COVID‐EXP multicenter study. Front Hum Neurosci. 2023;17:1259660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim Y, Bae S, Chang HH, Kim SW. Long COVID prevalence and impact on quality of life 2 years after acute COVID‐19. Sci Rep. 2023;13(1):11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bauer L, Laksono BM, de Vrij FMS, Kushner SA, Harschnitz O, van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS‐CoV‐2. Trends Neurosci. 2022;45(5):358‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Apple AC, Oddi A, Peluso MJ, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID‐19. Ann Clin Transl Neurol. 2022;9(2):221‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greene C, Connolly R, Brennan D, et al. Blood‐brain barrier disruption and sustained systemic inflammation in individuals with long COVID‐associated cognitive impairment. Nat Neurosci. 2024;27(3):421‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scuffham PA, Vecchio N, Whiteford HA. Exploring the validity of HPQ‐based presenteeism measures to estimate productivity losses in the health and education sectors. Med Decis Mak. 2014;34(1):127‐137. [DOI] [PubMed] [Google Scholar]
- 45. Chatys‐Bogacka Z, Mazurkiewicz I, Slowik J, et al. Brain fog and quality of life at work in non‐hospitalized patients after COVID‐19. Int J Environ Res Public Health. 2022;19(19):12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
Table S6.
Table S7.
Table S8.
Table S9.
Table S10.
Table S11.
Table S12.
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


