Abstract
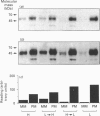
The effect of culture conditions simulating hypo- and hyper-glycaemia on glucose transport and on the subcellular localization of the glucose transporter GLUT-1 was studied in L8 myocytes. Incubation of the cells with 20 mM-glucose for 25 h decreased the rate of 2-deoxy-D-[3H]glucose (dGlc) uptake to 0.106 +/- 0.016 nmol/min per 10(6) cells compared with 0.212 +/- 0.025 in cells maintained at 2 mM-glucose (final glucose concentrations at the end of the incubation period were 16-17 mM and 0.7-1.0 mM respectively). An additional 5 h incubation of these cells with medium containing the opposite glucose concentration (i.e. change from 17 mM to 1 mM and from 1 mM to 17 mM) increased the transport rate to 0.172 +/- 0.033 nmol/min per 10(6) cells in cultures initially conditioned at high glucose, and decreased the transport to 0.125 +/- 0.029 in those conditioned at low glucose. Plasma-membrane- and microsomal-membrane-enriched fractions were prepared from these cells for [3H]cytochalasin B (CB) binding and Western-blot analysis with antibodies against GLUT-1 and GLUT-4. A decrease in glucose concentration increased the number of D-glucose-displaceable CB-binding sites and GLUT-1 protein in the plasma-membrane fraction to the same extent as the increase in dGlc transport. Under downregulatory conditions, the lower dGlc-transport capacity could be accounted for by a decreased number of transporters in the plasma membrane of the cells. No apparent modification of the intrinsic activity of the glucose transporters was observed in up- or down-regulated cells. Under downregulatory conditions, the CB-binding data indicated a large increase in the number of transporters in the intracellular membranes of the myocytes. Western blots of the same membranes also indicated an increase in GLUT-1 content. However, the interaction of the intracellular GLUT-1 protein with the polyclonal antibodies was much weaker than that of the plasma-membrane-associated GLUT-1. The GLUT-4 concentration was too low to permit quantification in membrane fractions. Our findings suggest that autoregulation of glucose transport in L8 myocytes is accompanied by parallel changes in the number of GLUT-1 transporters in the plasma membrane, and that the rate of transporter degradation may be augmented in the upregulated myocytes. These glucose-induced changes are fully reversible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Klip A., Young D. A., Cartee G. D., Holloszy J. O. Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology. 1989 Jan;124(1):449–454. doi: 10.1210/endo-124-1-449. [DOI] [PubMed] [Google Scholar]
- Greco-Perotto R., Assimacopoulos-Jeannet F., Jeanrenaud B. Insulin modifies the properties of glucose transporters in rat brown adipose tissue. Biochem J. 1987 Oct 1;247(1):63–68. doi: 10.1042/bj2470063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco-Perotto R., Zaninetti D., Assimacopoulos-Jeannet F., Bobbioni E., Jeanrenaud B. Stimulatory effect of cold adaptation on glucose utilization by brown adipose tissue. Relationship with changes in the glucose transporter system. J Biol Chem. 1987 Jun 5;262(16):7732–7736. [PubMed] [Google Scholar]
- Haney P. M., Slot J. W., Piper R. C., James D. E., Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991 Aug;114(4):689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman M. F., Goodyear L. J., Wardzala L. J., Horton E. D., Horton E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990 Jan 15;265(2):987–991. [PubMed] [Google Scholar]
- Hirshman M. F., Wallberg-Henriksson H., Wardzala L. J., Horton E. D., Horton E. S. Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 1988 Oct 10;238(2):235–239. doi: 10.1016/0014-5793(88)80486-1. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Kuhn C. E., Sauerheber R. D. Insulin stimulation of glucose transport in isolated rat adipocytes. Functional evidence for insulin activation of intrinsic transporter activity within the plasma membrane. Biochem J. 1985 Nov 15;232(1):245–254. doi: 10.1042/bj2320245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost H. G., Weber T. M., Cushman S. W., Simpson I. A. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986 Aug 5;261(22):10033–10036. [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab Rev. 1985;1(3):203–227. doi: 10.1002/dmr.5610010301. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Moscona R., Rafaeloff R., Illouz Y. G., Armoni M. Discrepancy between glucose transport and transporters in human femoral adipocytes. Am J Physiol. 1989 Jan;256(1 Pt 1):E179–E185. doi: 10.1152/ajpendo.1989.256.1.E179. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sasson S., Cerasi E. Substrate regulation of the glucose transport system in rat skeletal muscle. Characterization and kinetic analysis in isolated soleus muscle and skeletal muscle cells in culture. J Biol Chem. 1986 Dec 25;261(36):16827–16833. [PubMed] [Google Scholar]
- Sasson S., Edelson D., Cerasi E. In vitro autoregulation of glucose utilization in rat soleus muscle. Diabetes. 1987 Sep;36(9):1041–1046. doi: 10.2337/diab.36.9.1041. [DOI] [PubMed] [Google Scholar]
- Sasson S., Kunievsky B., Nathan C., Cerasi E. Failure of fenfluramine to affect basal and insulin-stimulated hexose transport in rat skeletal muscle. Biochem Pharmacol. 1989 Aug 15;38(16):2655–2661. doi: 10.1016/0006-2952(89)90551-0. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Charron M. J., Shah N., Lodish H. F., Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht E., Barnard R. J., Grimditch G. K. Mechanism of insulin action on glucose transport in rat skeletal muscle. Am J Physiol. 1988 May;254(5 Pt 1):E633–E638. doi: 10.1152/ajpendo.1988.254.5.E633. [DOI] [PubMed] [Google Scholar]
- Vilaró S., Palacín M., Pilch P. F., Testar X., Zorzano A. Expression of an insulin-regulatable glucose carrier in muscle and fat endothelial cells. Nature. 1989 Dec 14;342(6251):798–800. doi: 10.1038/342798a0. [DOI] [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Sarabia V., Koivisto U. M., Bilan P. J., Pessin J. E., Klip A. Glucose transport activity in L6 muscle cells is regulated by the coordinate control of subcellular glucose transporter distribution, biosynthesis, and mRNA transcription. J Biol Chem. 1990 Jan 25;265(3):1516–1523. [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Identification of the D-glucose-inhibitable cytochalasin B binding site as the glucose transporter in rat diaphragm plasma and microsomal membranes. Biochim Biophys Acta. 1983 Apr 21;730(1):49–56. doi: 10.1016/0005-2736(83)90315-2. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Watanabe T., Smith M. M., Robinson F. W., Kono T. Insulin action on glucose transport in cardiac muscle. J Biol Chem. 1984 Nov 10;259(21):13117–13122. [PubMed] [Google Scholar]
- Wertheimer E., Sasson S., Cerasi E., Ben-Neriah Y. The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2525–2529. doi: 10.1073/pnas.88.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer E., Sasson S., Cerasi E. Regulation of hexose transport in L8 myocytes by glucose: possible sites of interaction. J Cell Physiol. 1990 May;143(2):330–336. doi: 10.1002/jcp.1041430217. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]
- Zaninetti D., Greco-Perotto R., Assimacopoulos-Jeannet F., Jeanrenaud B. Effects of insulin on glucose transport and glucose transporters in rat heart. Biochem J. 1988 Feb 15;250(1):277–283. doi: 10.1042/bj2500277. [DOI] [PMC free article] [PubMed] [Google Scholar]