Abstract
From clinical trials and observational data, oral semaglutide has proven to be the most effective second-line oral therapy for the management of patients with type 2 diabetes. This review aims to describe the perspective of an Italian expert panel that addressed the potential challenges arising during the use of oral semaglutide in the free-living conditions of routine clinical care. A group of Italian experts discussed and generated insights into the use of oral semaglutide in clinical practice. Key topics included the effectiveness of oral semaglutide in clinical practice, the positioning of the agent to optimize the treatment benefits, the possibility to adopt flexibility in the administration schedule, critical issues encountered, the role of patient communication and information in the importance of dose escalation and management of adverse events. Available data on efficacy and effectiveness of oral semaglutide from randomized clinical trials and real-world studies were reported, along with factors that determine tolerability and persistence on treatment. The debate over a fixed versus a flexible dosing schedule was critically addressed, providing anecdotical clues from a small case series and a real-world database. Additionally, a set of recommendations for clinicians to consider when prescribing oral semaglutide and during the process of patient monitoring were provided.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-024-01625-3.
Keywords: Clinical practice, Glucagon-like peptide 1 receptor agonists, Oral semaglutide, Real-world evidence, Type 2 diabetes
Key Summary Points
| Oral semaglutide has been demonstrated to be the most effective second-line oral therapy in patients with type 2 diabetes, having a crucial role in the treatment and management of this population. |
| This review presents insights from an Italian expert panel regarding the challenges associated with using oral semaglutide in the real-world setting of routine clinical care in terms of optimal positioning within treatment regimens, flexibility in dosing schedules, encountered challenges, patient communication, dose escalation, and adverse event management. |
| The debate over fixed versus flexible dosing schedules is addressed, supplemented by anecdotal evidence from case series and real-world databases. |
| Practical recommendations have been provided by the Italian expert panel to support healthcare professionals in gaining greater confidence regarding the appropriate use of oral semaglutide in clinical practice. |
Introduction
In Italy, the use of innovative drugs for diabetes management has been steadily increasing, with a significant proportion of patients intensifying treatment regimen by adding oral medications more often than injectable ones. However, despite the advances in therapeutic options, a considerable number of patients continue to fail to reach their therapeutic goals. This paradox suggests that although there are an ever-widening range of innovative medicines, addressing the multifaceted nature of diabetes remains a complex challenge. The unhalted occurrence of complications highlights the need for continued exploration and improvement of diabetes management. This includes strategies that reinforce the importance of tailoring treatment plans to individual patient needs, exploring novel therapeutic approaches, and enhancing patient education and engagement.
Among the latest innovative oral therapeutic option, results from clinical trials indicate that oral semaglutide is the most effective second-line oral therapy [1, 2].
Oral semaglutide is the first innovative orally delivered glucagon-like peptide 1 receptor agonist (GLP-1 RA) developed for oral administration for the treatment of type 2 diabetes (T2D) [3]. Co-formulation of semaglutide with the absorption enhancer sodium N-(8-[2-hydroxybenzoyl]amino)caprylate (SNAC) increases its bioavailability, promoting absorption across the gastric epithelium. SNAC works by increasing the local pH in the stomach and thereby protecting the drug from proteolytic degradation by enzymes and stomach acid and facilitating its absorption in the intestinal tract via the transcellular route [4]. Thanks to its mechanism of action, by removing the injectable barrier, this formulation represents the first oral peptidic hormone-based therapy for the management of T2D. Trials and real-world data have established oral semaglutide as an extremely effective option for glycated hemoglobin (HbA1c) reduction across various durations of disease, backgrounds of therapy, and comorbidities [5, 6].
Semaglutide aids the management of hyperglycemia by stimulating insulin secretion in beta cells while diminishing glucagon secretion from alpha cells. Moreover, the medication slows the emptying of the stomach, fostering a feeling of fullness and aiding in weight loss [7, 8]. Semaglutide diminishes both body weight and fat mass by decreasing overall energy intake, with a general reduction in appetite. Moreover, it influences dietary choices by decreasing the inclination towards high-fat foods. Furthermore, semaglutide exhibits positive effects on lipid levels, contributes to lower systolic blood pressure, and mitigates diabetes-associated chronic inflammation [5, 9].
The current recommendations advise using oral semaglutide to treat people with T2D with inadequate metabolic control, along with diet and exercise. It can be used as an add-on therapy to other glucose-lowering agents or as monotherapy if metformin is not tolerated or contraindicated [10, 11].
In this manuscript, we describe the perspective of an Italian expert panel that addressed the potential challenges arising during the use of oral semaglutide in the free-living conditions of routine clinical care. We briefly review the available data on efficacy and effectiveness of oral semaglutide from randomized controlled trials (RCTs) and real-world studies, along with factors that determine tolerability and persistence on treatment. We critically address the debate over a fixed versus a flexible dosing schedule, providing anecdotical clues from a small case series and a real-world database. Case reports were shared by some of the authors and discussed during the expert panel and reported in Supplementary Material. We finally provide a set of recommendations for clinicians to consider when prescribing oral semaglutide and during the process of patient monitoring.
Ethical review by an ethics committee was not applicable as this article is based on previously conducted studies and direct observations from real-world clinical practice. Case reports derive from authors’ daily practice on clinical use of oral semaglutide. Written informed consent was obtained from each patient for the publication of the data reported in the case reports.
Overview of Evidence from Clinical Trials on Efficacy of Oral Semaglutide
The efficacy of oral semaglutide was widely assessed in the PIONEER (Peptide InnOvatioN for Early diabEtes tReatment) program of 10 RCTs, which included more than 9500 patients and examined the use of oral semaglutide in different phases of T2D, on various background therapies. Oral semaglutide showed significant improvements in glycemic control and weight reduction versus various comparators.
As for the comparison of oral semaglutide with other oral agents, in the PIONEER 2 open-label trial, oral semaglutide (14 mg once daily) was evaluated against the sodium-glucose co-transporter 2 inhibitor (SGLT2i) empagliflozin (25 mg once daily) for 52 weeks in 822 patients with T2D uncontrolled on metformin with a mean HbA1c level of 8.1%. Semaglutide was superior to empagliflozin in reducing HbA1c (− 1.3% vs. − 0.8%; p < 0.001) and body weight (− 4.7 kg vs. − 3.8 kg; p = 0.01) after 52 weeks of treatment. Moreover, a higher number of patients reached the composite endpoint (HbA1c < 7% without severe or symptomatic hypoglycemia and no weight gain) with oral semaglutide compared to empagliflozin. The likelihood of achieving this endpoint was significantly greater with oral semaglutide (OR 2.03, 95% CI 1.50–2.74) [12].
PIONEER 3 was a randomized double-blind trial that compared the efficacy of oral semaglutide vs. sitagliptin in patients with T2D not controlled on metformin with or without sulfonylurea. Among 1864 randomized patients, over 26 weeks, mean HbA1c changes from baseline were estimated as − 0.6%, − 1.0%, and − 1.3% for semaglutide at doses of 3, 7, and 14 mg/day, respectively, and − 0.8% for sitagliptin. Both 7 and 14 mg/day doses achieved statistically significant greater reduction in HbA1c when compared to sitagliptin (− 0.3% [95% CI − 0.4% to − 0.1%] and − 0.5% [95% CI − 0.6% to − 0.4%], respectively). Also, mean body weight changes from baseline were estimated as − 1.2, − 2.2, and − 3.1 kg for semaglutide 3, 7, and 14 mg/day, respectively, and − 0.6 kg for sitagliptin. A statistically significant greater reduction in body weight compared to sitagliptin was obtained (− 1.6 kg [95% CI − 2.0 to − 1.1 kg] and − 2.5 kg [95% CI − 3.0 to − 2.0 kg], for semaglutide 7 and 14 mg/day respectively). Furthermore, in both the 7 mg/day and 14 mg/day semaglutide groups, a significantly higher percentage of patients attained the composite outcome of achieving HbA1c levels below 7.0%, without experiencing hypoglycemia or weight gain, compared to the sitagliptin group (estimated treatment difference [ETD] 14%, 95% CI 8–14 for semaglutide 7 mg/day; ETD 26%, 95% CI 20–32 for semaglutide 14 mg/day) [13].
In the PIONEER 7 study, a 52-week, open-label, randomized trial, 504 patients with T2D uncontrolled with one or two oral glucose-lowering drugs were allocated to receive either oral semaglutide with flexible dose adjustments (3, 7, or 14 mg once daily) or sitagliptin 100 mg once daily. Oral semaglutide with flexible dose adjustment provided superior glycemic control and weight loss compared with sitagliptin 100 mg. Overall, 58% of patients treated with semaglutide reached the HbA1c target of < 7% vs. 25% of participants with sitagliptin. The odds of achieving HbA1c < 7% were higher with oral semaglutide than with sitagliptin (OR 4.40, 95% CI 2.89–6.70) [14]. The efficacy in HbA1c and weight reduction persisted over time as demonstrated in the extension phase of the PIONEER 7, representing the longest treatment period with oral semaglutide to date, spanning 2 years. A continuous treatment with oral semaglutide with flexible dose adjustments allowed a sustained improvement in glycemic control (− 1.5% at week 52 and − 1.3% at week 104) and resulted in further weight loss (− 2.8 kg at week 52 and − 3.7 kg at week 104). The switch from sitagliptin to oral semaglutide yielded HbA1c reductions with no need to add other glucose-lowering agents. This switch improved the likelihood of achieving an HbA1c target of < 7.0% (52.6% vs. 28.6% in the semaglutide and sitagliptin group, respectively; p = 0.0011) and may provide additional benefits on body weight. Finally, the “satisfaction with treatment” item of the DTSQ (Diabetes Treatment Satisfaction Questionnaire) was significantly improved with oral semaglutide versus sitagliptin (ETD 0.34, 95% CI 0.02–0.65) [15].
More than two-thirds of treated patients in the PIONEER program achieved the glycemic target of HbA1c < 7% [1, 5, 12–14, 16–18]. Subgroup analyses of PIONEER trials also highlighted that patients with higher baseline HbA1c achieved greater HbA1c reductions, irrespective of baseline body mass index (BMI) or background medication [19, 20].
Since the prevention of atherosclerotic cardiovascular disease (ASCVD) requires addressing its risk factors, early and effective intervention on modifiable risk factors can prevent or slow the progression of ASCVD. Oral semaglutide has demonstrated capacity to tackle cardiovascular risk biomarkers, encompassing improvements in blood pressure, lipid profiles, abdominal adiposity, ectopic fat deposition, and inflammation [12, 21].
The results of the PIONEER trial program have shown that oral semaglutide is the most effective medication for lowering blood glucose levels and body weight among the currently available oral agents for T2D [2].
These characteristics position oral semaglutide as an excellent option for initiating treatment in individuals with T2D after the failure of metformin or, as a monotherapy, when metformin cannot be used because of contraindications or intolerance. This underscores its potential for early intervention in such cases.
Standard Recommended Dosing Schedule
Prescribing information instructs taking oral semaglutide in the fasting state, followed by a post-dose fasting period of at least 30 min. This standardized recommendation is based on the pharmacokinetic properties of the drug and is designed to optimize its absorption through the gastric mucosa. Pre-dose fasting is required to protect the drugs from enzymatic degradation, while waiting less than 30 min to eat or drink after semaglutide dosing may decrease absorption due to dilution. Taking other oral medications within 30 min after semaglutide dosing may affect exposure to both semaglutide and the concomitantly administered medication. This may be particularly relevant for levothyroxine, which needs to be taken 30 min before breakfast itself. On the other side, the semaglutide exposure is not expected to be significantly modified by the pre-existence of upper gastrointestinal (GI) tract disease or symptoms [22]. Though GI side effects may be more common among patients with as compared to those without pre-existing upper GI tract disease, concomitant treatment with oral semaglutide and proton pump inhibitor (omeprazole) does not significantly modify semaglutide exposure [23].
A pharmacokinetic study performed among healthy subjects explored various dosing schedules [24] and found that the duration of pre-dose fasting is more important than the duration of post-dose fasting to ensure appropriate semaglutide exposure. Despite these data support dosing of oral semaglutide in accordance with prescribing information in the fasting state, it remains unclear to what extent these data obtained in healthy individuals translate into clinical benefits for people with T2D.
Real-World Evidence on Use of Oral Semaglutide in Patients with T2D
Data on the use of oral semaglutide in real-world settings are limited and new evidence is emerging.
One of the most important pieces of evidence came from the IGNITE study. This was a retrospective, observational cohort study based on electronic health records. It included 782 patients prescribed oral semaglutide, with a mean age of 57.8 years and a mean HbA1c baseline level of 8.4%; 54.5% of patients were woman and 66.0% received their prescription from a primary care practitioner. The population in which semaglutide was initiated was predominantly overweight (mean BMI 36.2 ± 7.6 kg/m2), affected by other comorbidities, and treated with different treatment backgrounds. Results indicated that oral semaglutide is effective in improving glycemic; in particular, a mean reduction of 0.9% in HbA1c was observed in 6 months. However, a significant proportion of patients (37%) were administered only the 3-mg starting dose, underlining a therapeutic inertia and a large room for improvement when using therapeutic dosages [25].
In a single-center retrospective observational study conducted in 88 Japanese patients with T2D treated with oral semaglutide, the use of this agent was effective in reducing HbA1c regardless of age, sex, BMI, renal function, or diabetes duration. Individuals receiving oral semaglutide experienced a reduction in HbA1c by 1.2% and a weight loss of 1.4 kg, typically with an average dose slightly above 7 mg. The proportion of patients reaching HbA1c < 7% underwent a significant increase, rising from 14% at baseline to 48%. Other cardiometabolic parameters were also reduced, such as alanine aminotransferase (from 24 IU/L to 23 IU/L; p = 0.008), total cholesterol (from 177 mg/dl to 172 mg/dl; p = 0.009), triglycerides (from 146 mg/dl to 144 mg/dl; p = 0.028), and non-high-density lipoprotein (HDL) cholesterol (from 120 mg/dl to 115 mg/dl; p = 0.002) [26].
In a real-world Italian retrospective study, the effectiveness and tolerability of oral semaglutide were assessed in 129 patients with T2D in add-on to background agents (ADD-ON group) or after switching from other glucose-lowering agents (SWITCH group), demonstrating both to be effective and safe. Patients were on average 72 years old and had baseline HbA1c levels of 7.2%. Oral semaglutide led to a significant reduction in both HbA1c levels and body weight among patients with T2D, reaching the target HbA1c recommended level of < 7%. Overall, despite the high mean age and the low baseline HbA1c levels in the ADD-ON and in the SWITCH groups, oral semaglutide resulted in a median decrease in HbA1c levels of − 0.4% and − 0.3%, respectively. When looking in specific subgroups in the SWITCH group, according to the glucose-lowering agent used before oral semaglutide, there was a significant HbA1c reduction in subgroups switching from dipeptidyl peptidase 4 (DPP4) inhibitors (p < 0.001) or SGLT2i (p = 0.01). As for BMI, there was a significantly greater reduction observed in the ADD-ON group (− 1.2 kg/m2) compared to the SWITCH group (− 0.7 kg/m2) [27].
The effectiveness of oral semaglutide was also observed in another retrospective study, using claims data. A total of 744 adult patients with at least one pharmacy claim for oral semaglutide and with a diagnosis code for T2D were selected. The study demonstrated an average HbA1c reduction of 0.8%; furthermore, patients with a higher starting HbA1c levels (HbA1c ≥ 9%) experienced greater HbA1c reductions than those with HbA1c < 9% (− 2.0% vs. − 0.4%; p < 0.001) within 6 months following initiation with oral semaglutide. Furthermore, patients that were persistent users of oral semaglutide (> 90 days) reached lower HbA1c levels. In this study, however, one-third of the involved population remained with the starting dose of 3 mg despite the recommendation reported in the summary of product characteristics and only 16% reached the maintenance dose of 14 mg [28].
Additionally, improvements in cardiovascular risk factors were observed. In particular, a noteworthy reduction of 6.2 mmHg in systolic blood pressure was observed after a 6-month period. There was also a significant decrease in low-density lipoprotein (LDL)-cholesterol levels after 6 months, and non-HDL-cholesterol levels showed a tendency to decrease over the same period. Furthermore, the urinary albumin to creatinine ratio (UACR) demonstrated a tendency to decrease after both 3 and 6 months. The decline in cardiovascular biomarkers plays a role in mitigating cardiovascular and renal events [29].
In a recent cross-sectional survey conducted among Italian endocrinologists and diabetologists, known as the PIONEERING EXPERIENCE study, there was strong support for the early implementation of oral semaglutide as a therapeutic approach to overcome therapeutic inertia and improve management patients with T2D. Patients identified as candidates for initiating therapy with oral semaglutide were aged 56–71 years, had a relatively short disease duration (42% of patients had < 5 years of diabetes duration) despite a high-to-very high cardiovascular risk (61% high cardiovascular [CV] risk; 34% very high risk), and had HbA1c levels ranging from 7.2% to 8.4%. When physicians were requested to indicate the reason behind initiating oral semaglutide for each patient, the most prevalent reasons were enhancing metabolic control (80.3%), reducing cardiovascular risk (82.9%), weight management (63.9%), lowering microvascular risk (29.3%), and mitigating the risk of hypoglycemia (14.5%). The adoption of oral semaglutide resulted in a significant reduction in the use of sulfonylureas, pioglitazone, DPP4 inhibitors, and insulin, with no substantial change in the utilization of SGLT2 inhibitors. In addition, assessment of the projected glycemic effectiveness associated with oral semaglutide based on trial data indicated that 62% of patients would achieve an HbA1c level below 7%, and 43% would achieve their optimal individualized HbA1c target. The achievement of HbA1c < 7% is much more often achieved with oral semaglutide (67%) than with empagliflozin (25%) or sitagliptin (31%) [30].
Recently, the GLIMPLES Italian multicenter retrospective study investigated the effectiveness on HbA1c levels and body weight of oral semaglutide in 166 patients with T2D who initiated the medication and were followed for 18 months. This study represents the longest real-world investigation of oral semaglutide to date. Over the observation period, a reduction of − 0.9% in HbA1c was observed, and 42.1% of patients with a baseline HbA1c above 7.0% achieved a value below 7.0%. Additionally, there was a significant decrease in body weight, with an estimated change of − 3.4 kg. At 6 months post-treatment initiation, systolic blood pressure decreased significantly by 6.2 mmHg (95% CI − 10.7 to − 1.8 mmHg) and total cholesterol significantly decreased by 14.4 mg/dl (95% CI − 21.0 to − 7.7 mg/dl) [6]. New data from matched cohorts of the GLIMPLES study illustrate that, in the specific population of early individuals with T2D who initiated oral semaglutide, the use or both formulations of semaglutide (injectable or oral) can exert similar effects on the improvement in HbA1c and body weight, despite greater persistence on injectable than on oral semaglutide [31].
Finally, results of the PIONEER REAL studies are becoming available for several countries [32–34]. This was a series of prospective observational studies of 34–44 weeks duration, performed with the aim of understanding clinical outcomes with oral semaglutide in adults with T2D. In the various studies, HbA1c declined by 0.9–1.2% and body weight declined by 4–6 kg, and around half of participants achieved an HbA1c levels < 7.0%.
Safety Profile of Oral Semaglutide
As a result of the stimulation of GLP-1 receptors in the GI tract, semaglutide may directly influence GI motility, secretion, and sensitivity, contributing to GI symptoms such as nausea, vomiting, diarrhea or constipation, and abdominal discomfort. It also affects delaying gastric emptying, slowing the absorption of nutrients, but also causing feelings of fullness, bloating, and nausea [35, 36].
GI side effects typically range from mild to moderate in severity, often occur temporarily, commonly beginning during the period of initiation and dose escalation and typically resolving after reaching the maintenance dose. The safety profile of oral semaglutide across the PIONEER clinical trials was consistent with the known profile of the GLP-1 RA class. Interruption of therapy as a result of GI tolerability issues was reported in 2–12% of patients in oral semaglutide groups [1, 9, 12–14, 16–18].
The most frequently reported adverse events are GI manifestations such as nausea (15%), diarrhea (10%), and vomiting (7%) [2, 12–14]. Data on side effects from observational studies are more heterogeneous. The diverse nature of real-world settings, patient populations, and treatment protocols in this type of study can contribute to a more varied and less standardized collection of side effect data. This variability may stem from differences in patient demographics, comorbidities, treatment adherence, and other factors, making the findings less uniform or consistent compared to the more controlled conditions of clinical trials.
In the Italian study by Candido et al. GI adverse events, nausea, and diarrhea were reported by 6.2% of patients, while hypoglycemic episodes occurred in 3% of patients. Discontinuation of oral semaglutide occurred in 10% of patients over 6 months; among these patients, almost 80% were due to an adverse event [27].
Persistence and Adherence to Therapy as Barriers to Optimal Care
The occurrence of GI side effects poses a potential challenge in garnering widespread patient acceptance of the medication and consequently it may result in suboptimal therapeutic adherence. This issue can limit the effectiveness of prescribed treatments, resulting in unmet treatment goals. In individuals with T2D, poor adherence and persistence represent barriers to achieving optimal care [37]. Furthermore, the onset of GI complications could limit or interfere with the recommended dose escalation up to 14 mg, which represents the dosage for which the maximum effectiveness of the product is appreciated.
In the diabetes pharmacotherapy area, a systematic review assessed that only 56.2% of patients with T2D continued their treatments at 1 year post-initiation [38]. Among the primary reasons reported for low adherence and persistence, the severity of adverse events was most common [37].
Overcoming these barriers becomes mandatory for diabetologists and healthcare providers. Efforts to address and manage GI side effects in the use of oral semaglutide, whether through patient education, proactive symptom management, or considering alternative strategies, are essential in promoting better patient tolerance and adherence to the prescribed medication regimen. By actively addressing and mitigating these challenges, healthcare professionals involved in diabetes care play a crucial role in ensuring that patients receive the full therapeutic benefits of the prescribed medication, contributing to more effective diabetes management and improved overall patient outcomes.
Need for Comprehensive Practical Guidance and Expert Insights
From clinical trials and observational data, oral semaglutide has proven to be an effective therapeutic approach to the management of patients with T2D. However, physicians face various practical questions that cannot be answered by guidelines or recently published clinical trials or observational studies results.
This article aims to provide practical guidance on the use of oral semaglutide to optimize therapeutic effects, while minimizing possible GI effects. A group of experts discuss and generate insights into these topics to try to fill the gaps that have been found in the use of the agent in conditions of normal clinical practice. It is a commentary based on the expert opinion and clinical experience of the authors. It does not involve new studies with human participants performed by any of the authors.
Topics included the effectiveness of oral semaglutide in clinical practice, the positioning of oral semaglutide to optimize the treatment benefits, critical issues encountered, the role of patient communication and information in the importance of dose escalation and management of adverse events.
Clinical Insights Derived from Expert Experiences with Use of Oral Semaglutide in Clinical Practice
Effectiveness
The introduction of oral semaglutide in routine clinical practice has brought to light a multitude of important considerations. In this section, we describe the perceived benefits of oral semaglutide in routine care according to the panel’s perspective, and the possible challenges in maximizing the achievement of therapeutic goals.
Oral semaglutide has demonstrated notable effectiveness in enhancing metabolic control within routine clinical practice, confirming results obtained in clinical trials. Blood glucose level control, both pre- and post-prandial, has shown marked improvement in patients on oral GLP-1 RA treatment, with substantial glycemic and extraglycemic benefits. It is important to highlight that its maximum effectiveness can, however, be appreciated upon reaching the 14-mg dosage, making it one of the most effective oral glucose-lowering agents available.
Clinical use of oral semaglutide indicated its ability to decrease systolic and diastolic blood pressure and improve lipid profiles by reducing triglycerides and LDL-cholesterol levels. These broader metabolic benefits contribute to its role in mitigating cardiovascular risk factors and addressing the multifaceted nature of T2D.
Its effectiveness in improving metabolic parameters underscores its significance as a valuable therapeutic option in the management of T2D.
A reduction in food intake and diminished feelings of hunger have been observed, contributing to a better-controlled dietary pattern. This dual effect not only assists in reducing caloric intake but also plays a crucial role in promoting a more structured and controlled dietary regimen, thereby promoting healthier eating habits.
Notably, experts reported that the use of oral semaglutide is associated with an enhanced sense of well-being and heightened performance, potentially contributing to better adherence to therapy regimens. This positive impact on patients’ quality of life should not be overlooked. Recognizing and acknowledging the broader benefits, including improved well-being and increased capacity for daily activities, is crucial in comprehensively understanding the positive outcomes associated with the use of oral semaglutide in everyday diabetes management.
Tolerability
In routine clinical practice, there exists the need to promptly offer viable alternatives to manage and mitigate potential side effects associated with glucose-lowering agents.
Although GI side effects associated with the use of oral semaglutide are limited, they may prompt some patients to discontinue the medication, with consequent rapid resolution of GI effects. It should, however, be noted, that the rate of treatment discontinuation in the experience of participating experts is in line with that of the GLP-1 RA class.
Authors highlighted the need in their clinical practice to have viable approaches to mitigate side GI effects to suggest the patient. Some of these approaches, despite not currently being listed in the summary of product characteristics, can effectively mitigate the occurrence and severity of adverse effects, notably enhancing patients’ adherence to treatment and persistence.
Management of GI Events to Benefit the Efficacy of Oral Semaglutide
Clinical Experience with Oral Semaglutide
Clinical experience with the use of oral semaglutide highlighted a reduction in efficacy attributed to inadvertent errors patients make in drug dosing and administration. This led clinicians to search for more flexible approaches in managing therapy with this novel glucose-lowering agent, aiming to maintain effectiveness, without compromising quality of life and ensuring adherence and compliance to the treatment regimen. This flexibility in administration aims to enhance treatment adherence by accommodating individual lifestyles and preferences. Case reports in the Supplementary Material exemplify the possibility of more flexible approaches in the timing of administration.
Evidence from GLIMPLES Study
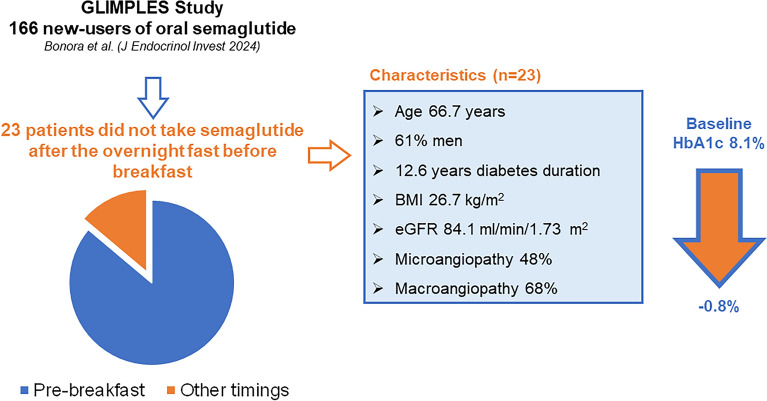
An analysis of data from 166 patients treated with oral semaglutide and described in the GLIMPLES study [6] provides some support to the feasibility of an alternative dosing schedule. There were 143 participants who took the drug before breakfast and 23 participants (13.9%) who took the drug at other timings (before lunch and before dinner) since their treatment initiation. They were always advised to fast 4–5 h before dosing and at least 30 min after dosing. We calculated HbA1c nadir during up to 18 months of treatment in the two groups. In these participants, HbA1c significantly declined by 0.8% from a baseline value of 8.1% (Fig. 1). These findings suggest that the effectiveness of oral semaglutide in reducing HbA1c levels was preserved in individuals who took the drug at times other than the morning fast, provided that a pre- and post-dose fasting was respected.
Fig. 1.
Oral semaglutide effectiveness taken at different timings: results from the GLIMPLES Study. BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin
Expert Consideration and Recommendations
Suggested Strategies for Optimal Oral Semaglutide Administration
Ensuring that patients who start oral semaglutide therapy receive comprehensive education from their healthcare provider is crucial. This education should encompass various aspects such as instructions on timing and method of administration, expectations regarding efficacy and adverse events, preventive measures to minimize the occurrence of side effects and on how to address them promptly. Thus, thorough patient education not only enhances treatment adherence but also promotes proactive management of potential challenges, ultimately promoting better overall outcomes.
The different aspects, suggested by the experts, to consider in subjects starting therapy with oral semaglutide were the following:
- Patient communication
- Adopt specific and proactive counseling as a key component in the management of patients with T2D initiating oral semaglutide treatment.
- Recommend and explain the dose escalation strategy, with a starting dose of 3 mg once daily for at least 1 month, which can be then increased to 7 mg. After at least 1 month the dose can be increased to a maintenance dose of 14 mg once daily if needed to further improve glycemic control and body weight. Staying longer than 1 month on the 7-mg dose may help diluting GI side effects, but should not delay the achievement of individual targets.
- Inform patients about the potential occurrence of GI side effects in the initial weeks of oral semaglutide treatment, particularly during dose escalation periods. Patients should be reassured that in most cases, GI symptoms are mild to moderate and typically resolve naturally over time, encouraging them to persist on treatment.
- Method and timing of administration
- Communicate the specific instructions related to mode of administration and their importance to patients initiating oral semaglutide treatment, to guarantee an effective drug bioavailability. Underline the necessity for a pre-dose fasting condition (enough to have an empty stomach) and observing an additional fasting period of at least 30 min post-dose, along with avoiding pharmacological interactions to allow correct semaglutide absorption.
- Recommend taking the medication in a time slot based on patient daily routine, when the stomach is empty.
- Address the patient’s lifestyle and needs, to evaluate whether taking oral semaglutide after the overnight fast is the most suitable option or whether an alternative dosing time could be considered. In the latter case, provide the patients with clear written dosing instructions, regarding pre- and post-dose fasting.
- Dose escalation schedule
- Extend the escalation periods beyond the recommended duration, if tolerability concerns arise at lower doses, i.e., maintain longer than 1 month on 7 mg before increasing the dose to 14 mg.
- Reduce the dose temporarily if adverse events persist and resume dose escalation once the symptoms diminish or resolve.
- Presence of diseases or conditions affecting adherence and prescription of therapy.
- Emphasize adherence to specific administration timing recommendations, such as taking oral semaglutide before any other oral medications and waiting for at least 30 min before other oral medications.
Adherence to recommended eating behaviors for patients with T2D (details reported in Table 1).
Table 1.
Eating behaviors recommendations for patient with T2D
| Recommend eating in smaller portions: individuals are advised to learn to recognize the feeling of fullness, gradually reducing portion sizes, using smaller sized plates and determining the optimal amount |
| Recommend reducing fat intake taking into account the following nutrients intake in accordance with the clinical practice of the diabetes clinic: carbohydrates 45–60%, proteins 10–29%, fats 25–30% calories intake 25–40 kcal/kg. Suggest opting for fewer snacks, employing alternative cooking methods like grilling, boiling, or steaming, and controlling oil usage are effective measures. Recommend limiting the amount of cheese by choosing a portion the size of a fist; limit mayonnaise and other sauces not exceeding a quantity equal to the tip of the index finger; establish intake of meat, fish, and other protein sources, choosing a portion size limited to the palm of a hand and the thickness of a finger |
| Recommend eating slowly: it allows to register fullness and prevent overeating |
| Recommend starting the meal with a quantity of vegetables contained in both palms of hands |
| Recommend using knife and fork to eat smaller bites |
| Recommend avoiding doing other things while eating (no multitasking) |
| Recommend taking short breaks mid-meal |
| When eating at restaurants or out of the home suggest preferring a single dish or smaller portions (half portion or starters) |
| Consider alternative administration timings to before breakfast, such as before lunch or dinner |
Conclusions
The growing recognition of GLP-1 RAs’ advantages in international recommendations for T2D treatment emphasizes their early initiation in the disease’s management. The efficacy and effectiveness of oral semaglutide in reducing HbA1c levels and body weight, coupled with its favorable clinical profile, positions it as an optimal choice for people with T2D.
The oral formulation provides an attractive therapeutic option for patients who may be more likely to use GLP-1 RA at an early stage of disease.
The importance of providing comprehensive education to individuals with T2D is established. This approach aims to empower the patient with information regarding the different aspects of available treatment options, including flexibility, complexity, route, and frequency of administration. By doing so, physicians can tailor the treatment regimen to patients’ lifestyle and preferences. A fundamental component that guarantees optimal results involves, in particular, correct and complete information on potential side effects and the optimization of administration times. Educating patients on these aspects significantly contributes to improving adherence to therapeutic protocols, thus maximizing the benefits deriving from oral semaglutide.
Some degree of flexibility in the administration schedule of oral semaglutide would represent an opportunity that both physicians and patients could benefit from. We acknowledge there is quite limited evidence from trials or observational studies to strongly support alternative dosing times for oral semaglutide administration. This is an important evidence gap in the literature and we encourage new research on this topic. Although following an overnight fast should remain the standard timing of oral semaglutide administration, the flexibility option may allow patients to integrate diabetes treatment into their daily routine, potentially improving treatment adherence and overall glycemic control. At the same time, flexibility could help in minimizing GI adverse events. Ultimately, the emphasis on flexibility in oral semaglutide administration schedules would create a dynamic and collaborative therapeutic environment that is conducive to successful diabetes management.
This personalized approach acknowledges that a unique strategy may not be optimal for all patients and that tailoring treatment to individual circumstances and needs contributes to better overall outcomes. The use of this approach emphasizes the importance of patient engagement, education, and ongoing communication in a patient-centric model of care to ensure the successful integration of oral semaglutide into the daily lives of individuals managing T2D.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Medical writing service was provided by Giorgia De Berardis (Coresearch). Éthos S.r.l. offered their invaluable organizational and coordination support in providing technical assistance throughout all stages of the development of this paper. These services were funded by Novo Nordisk.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author Contributions
All authors (Riccardo Candido, Chiara Di Loreto, Paolo Desenzani, Paola Pantanetti, Cristina Romano, Silvio Settembrini, Sebastiano Bruno Solerte, Gian Paolo Fadini) made substantial contributions to the conception of the work, drafted and critically revised the manuscript, and approved the final version to be published. Chiara Di Loreto, Paolo Desenzani, and Paola Pantanetti contributed by providing the case reports reported in the manuscript.
Funding
This manuscript was supported by an unrestricted grant from Novo Nordisk S.p.A. Medical writing assistance was provided by Giorgia De Berardis from Éthos S.r.l. and Coresearch S.r.l. Novo Nordisk S.p.A. did not influence the content of the publication and was not involved in its writing. Novo Nordisk S.p.A. reviewed this document for factual accuracy only. The views expressed in this publication are those of the authors. The journal’s Rapid Service Fee was funded by Éthos S.r.l. through the Novo Nordisk unrestricted grant.
Declarations
Conflict of Interest
Riccardo Candido declared to receive: an honoraria for advisory board membership from Novo Nordisk; consulting fees from Abbott, Novo Nordisk, Sanofi, Aventis, MSD Italia, Eli Lilly, Menarini Diagnostics, Bayer; honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Roche Diabetes Care, Sanofi-Aventis. Chiara Di Loreto declared to receive: an honoraria for advisory board membership from Novo Nordisk and Lilly, consulting fees from Novo Nordisk; honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Abbott, Novo Nordisk, Roche Diabetes Care. Gian Paolo Fadini is an Editorial Board member of Diabetes Therapy. Gian Paolo Fadini was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Gian Paolo Fadini declared to receive: an honoraria for advisory board membership from Novo Nordisk; consulting fees from AstraZeneca and Lilly; honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Abbott, Novo Nordisk, AstraZeneca, Servier, Boehringer, Lilly; support for attending meetings and/or travel from Novo Nordisk. Cristina Romano, Paolo Desenzani, Paola Pantanetti, Silvio Settembrini, Sebastiano Bruno Solerte declare no competing interests.
Ethical Approval
Ethical review by an ethics committee was not applicable as this article is based on previously conducted studies and direct observations from real-world clinical practice. Case reports derive from authors’ daily practice on clinical use of oral semaglutide. Written informed consent was obtained from each patient for the publication of the data reported in the case reports.
References
- 1.Aroda VR, Rosenstock J, Terauchi Y, et al. Randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–32. 10.2337/dc19-0749. 10.2337/dc19-0749 [DOI] [PubMed] [Google Scholar]
- 2.Rodbard HW, Dougherty T, Taddei-Allen P. Efficacy of oral semaglutide: overview of the PIONEER clinical trial program and implications for managed care. Am J Manag Care. 2020;26(16 Suppl):S335–43. 10.37765/ajmc.2020.88554. 10.37765/ajmc.2020.88554 [DOI] [PubMed] [Google Scholar]
- 3.Andersen A, Knop FK, Vilsbøll T. A pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs. 2021;81(9):1003–30. 10.1007/s40265-021-01499-w. 10.1007/s40265-021-01499-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. 10.1126/scitranslmed.aar7047. 10.1126/scitranslmed.aar7047 [DOI] [PubMed] [Google Scholar]
- 5.Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22(8):1263–77. 10.1111/dom.14054. 10.1111/dom.14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonora BM, Russo G, Leonetti F, et al. Effectiveness of oral semaglutide on glucose control and body weight up to 18 months: a multicenter retrospective real-world study. J Endocrinol Invest. 2024. 10.1007/s40618-024-02309-2. 10.1007/s40618-024-02309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavernia F, Blonde L. Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes compared with other oral antihyperglycemic agents and placebo. Postgrad Med. 2020;132(sup2):15–25. 10.1080/00325481.2020.1798638. 10.1080/00325481.2020.1798638 [DOI] [PubMed] [Google Scholar]
- 8.Wright EE Jr, Aroda VR. Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes considered for injectable GLP-1 receptor agonist therapy or currently on insulin therapy. Postgrad Med. 2020;132(sup2):26–36. 10.1080/00325481.2020.1798127. 10.1080/00325481.2020.1798127 [DOI] [PubMed] [Google Scholar]
- 9.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. 10.1056/NEJMoa1901118. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 10.ElSayed NA, Aleppo G, Aroda VR, et al. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140–57. 10.2337/dc23-S009. 10.2337/dc23-S009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannucci E, Candido R, Monache LD, et al. Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol. 2022;59(5):579–622. 10.1007/s00592-022-01857-4. 10.1007/s00592-022-01857-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–81. 10.2337/dc19-0883. 10.2337/dc19-0883 [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–80. 10.1001/jama.2019.2942. 10.1001/jama.2019.2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–39. 10.1016/S2213-8587(19)30194-9. (Erratum in: Lancet Diabetes Endocrinol. 2019;7(9):e21). 10.1016/S2213-8587(19)30194-9 [DOI] [PubMed] [Google Scholar]
- 15.Buse JB, Bode BW, Mertens A, et al. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020;8(2):e001649. 10.1136/bmjdrc-2020-001649. 10.1136/bmjdrc-2020-001649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. 10.1016/S0140-6736(19)31271-1. (Erratum in: Lancet. 2019; 394(10192):e1). 10.1016/S0140-6736(19)31271-1 [DOI] [PubMed] [Google Scholar]
- 17.Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–71. 10.2337/dc19-0898. 10.2337/dc19-0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–27. 10.1016/S2213-8587(19)30192-5. (Erratum in: Lancet Diabetes Endocrinol. 2019;7(9):e21). 10.1016/S2213-8587(19)30192-5 [DOI] [PubMed] [Google Scholar]
- 19.Pratley RE, Crowley MJ, Gislum M, et al. Oral semaglutide reduces HbA1c and body weight in patients with type 2 diabetes regardless of background glucose-lowering medication: PIONEER subgroup analyses. Diabetes Ther. 2021;12(4):1099–116. 10.1007/s13300-020-00994-9. 10.1007/s13300-020-00994-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabe D, Deenadayalan S, Horio H, et al. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: a subgroup analysis by baseline variables in the PIONEER 9 and PIONEER 10 trials. J Diabetes Investig. 2022;13(6):975–85. 10.1111/jdi.13764. 10.1111/jdi.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosenzon O, Capehorn MS, De Remigis A, Rasmussen S, Weimers P, Rosenstock J. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. 2022;21(1):172. 10.1186/s12933-022-01585-7. 10.1186/s12933-022-01585-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier JJ, Granhall C, Hoevelmann U, et al. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes. Diabetes Obes Metab. 2022;24(4):684–92. 10.1111/dom.14632. 10.1111/dom.14632 [DOI] [PubMed] [Google Scholar]
- 23.Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14(8):869–77. 10.1080/17425255.2018.1488965. 10.1080/17425255.2018.1488965 [DOI] [PubMed] [Google Scholar]
- 24.van Hout M, Forte P, Jensen TB, Boschini C, Bækdal TA. Effect of various dosing schedules on the pharmacokinetics of oral semaglutide: a randomised trial in healthy subjects. Clin Pharmacokinet. 2023;62(4):635–44. 10.1007/s40262-023-01223-9. 10.1007/s40262-023-01223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aroda VR, Faurby M, Lophaven S, Noone J, Wolden ML, Lingvay I. Insights into the early use of oral semaglutide in routine clinical practice: the IGNITE study. Diabetes Obes Metab. 2021;23(9):2177–82. 10.1111/dom.14453. 10.1111/dom.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada H, Yoshida M, Funazaki S, et al. Retrospective analysis of the effectiveness of oral semaglutide in type 2 diabetes mellitus and its effect on cardiometabolic parameters in Japanese clinical settings. J Cardiovasc Dev Dis. 2023;10(4):176. 10.3390/jcdd10040176. 10.3390/jcdd10040176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Candido R, Gaiotti S, Giudici F, et al. Real-world retrospective study into the effects of oral semaglutide (as a switchover or add-on therapy) in type 2 diabetes. J Clin Med. 2023;12(18):6052. 10.3390/jcm12186052. 10.3390/jcm12186052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frazer M, Swift C, Gronroos NN, et al. Real-world hemoglobin A1c changes, prescribing provider types, and medication dose among patients with type 2 diabetes mellitus initiating treatment with oral semaglutide. Adv Ther. 2023;40(11):5102–14. 10.1007/s12325-023-02677-w. 10.1007/s12325-023-02677-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanai H, Hakoshima M, Adachi H, Katsuyama H. A significant effect of oral semaglutide on cardiovascular risk factors in patients with type 2 diabetes. Cardiol Res. 2022;13(5):303–8. 10.14740/cr1441. 10.14740/cr1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morieri ML, Candido R, Frontoni S, et al. Clinical features, cardiovascular risk profile, and therapeutic trajectories of patients with type 2 diabetes candidate for oral semaglutide therapy in the Italian specialist care. Diabetes Ther. 2023;14(12):2159–72. 10.1007/s13300-023-01490-6. 10.1007/s13300-023-01490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadini GP, Bonora BM, Ghiani M, et al. Oral or injectable semaglutide for the management of type 2 diabetes in routine care: a multicentre observational study comparing matched cohorts. Diabetes Obes Metab. 2024. 10.1111/dom.15554. 10.1111/dom.15554 [DOI] [PubMed] [Google Scholar]
- 32.van Houtum W, Schrömbges P, Amadid H, et al. Real-world use of oral semaglutide in adults with type 2 diabetes in the PIONEER REAL Netherlands multicentre, prospective, observational study. Diabetes Ther. 2024. 10.1007/s13300-024-01588-5. 10.1007/s13300-024-01588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain AB, Reichert SM, Amadid H, et al. Use of once-daily oral semaglutide and associated clinical outcomes among adults with type 2 diabetes in routine clinical practice in Canada: A multicentre, prospective real-world study (PIONEER REAL Canada). Diabetes Obes Metab. 2024;26(5):1799–807. 10.1111/dom.15493. 10.1111/dom.15493 [DOI] [PubMed] [Google Scholar]
- 34.Kick A, M’Rabet-Bensalah K, Acquistapace F, et al. Real-world use of oral semaglutide in adults with type 2 diabetes: the PIONEER REAL Switzerland multicentre, prospective, observational study. Diabetes Ther. 2024;15(3):623–37. 10.1007/s13300-023-01525-y. 10.1007/s13300-023-01525-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niman S, Hardy J, Goldfaden RF, et al. A review on the efficacy and safety of oral semaglutide. Drugs R D. 2021;21(2):133–48. 10.1007/s40268-021-00341-8.34. 10.1007/s40268-021-00341-8.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danielson E, Melin-Johansson C, Modanloo M. Adherence to treatment in patients with chronic diseases: from alertness to persistence. Int J Community Based Nurs Midwifery. 2019;7(4):248–57. 10.30476/IJCBNM.2019.81303.0. 10.30476/IJCBNM.2019.81303.0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DSU, Lee H. Adherence and persistence rates of major antidiabetic medications: a review. Diabetol Metab Syndr. 2022;14(1):12. 10.1186/s13098-022-00785-1. 10.1186/s13098-022-00785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–96. 10.1185/03007995.2015.1053048. 10.1185/03007995.2015.1053048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.