Abstract
There is a gap of knowledge about the clinical and pathophysiological implications resulting from the interaction between primary hyperlipidemias and type 2 diabetes (T2D). Most of the existing evidence comes from sub-analyses of cohorts; scant information derives from randomized clinical trials. The expected clinical implications of T2D in patients with primary hyperlipidemias is an escalation of their already high cardiovascular risk. There is a need to accurately identify patients with this dual burden and to adequately prescribe lipid-lowering therapies, with the current advancements in newer therapeutic options. This review provides an update on the interactions of primary hyperlipidemias, such as familial combined hyperlipidemia, familial hypercholesterolemia, multifactorial chylomicronemia, lipoprotein (a), and type 2 diabetes.
Keywords: Primary hyperlipidemias, Diabetes, Type 2 diabetes, Interactions, Diabetes complications, Diabetes incidence, Familial combined hyperlipidemia, Combined hyperlipidemia, Familial hypercholesterolemia, Multifactorial chylomicronemia, Familial dysbetalipoproteinemia, Type III hyperlipidemia, Lp(a)
Key Summary Points
| Several primary hyperlipidemias (i.e., familial combined hyperlipidemias, familial hypertriglyceridemia, and dysbetalipoproteinemia) are associated with a higher risk of having type 2 diabetes (T2D). |
| Familial hypercholesterolemia patients have a lower prevalence of T2D than the general population. |
| The association between multifactorial chylomicronemia and T2D varies between 25 and 76%. |
| There is an inverse relationship between Lipoprotein (a) and T2D, but those with T2D and high levels of lipoprotein (a) have more risk for cardiovascular events. |
| The interaction between primary hyperlipidemias and T2D has not been addressed in clinical guidelines. |
Introduction
Primary hyperlipidemias are a group of monogenic and polygenic diseases characterized by severe disorders of lipid and lipoprotein metabolism. These entities are characterized by severe forms of hypercholesterolemia, hypertriglyceridemia, or both (mixed hyperlipidemias). These groups of disorders are relatively common and pose a significant burden due to increased risk for cardiovascular disease (CVD). It is known that several primary hyperlipidemias have a bidirectional risk for the development of diabetes. However, little attention has been focused on the interaction of different primary hyperlipidemias and its clinical implications. In this review, we focus on the association of primary hyperlipidemias and type 2 diabetes (T2D). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Brief Overview of Changes in Lipid Metabolism Associated with Type 2 Diabetes
The interactions between lipoproteins and diabetes are the subject matter of several recent reviews [1, 2] and are summarized in Fig. 1. Clinically, these alterations are presented as either elevated levels of triglycerides (TG) (> 200 mg/dl) and low high-density lipoprotein cholesterol (HDL-C) levels (< 40 mg/dl) with increased levels of apolipoprotein B (apoB) but slightly increased low-density lipoprotein cholesterol (LDL-C), or a mixed hyperlipidemia (TG > 200 mg/dl and LDL-C > 100 mg/dl). However, the increase in ASCVD is caused by an increased apoB containing lipoproteins. For example, those mixed with hypertriglyceridemia (HTG) and high levels of apoB have a higher risk of developing ASCVD (HR 3.3, 95% CI 2.06–5.30; p = 0.0008) than those with mixed lipid phenotype was 2.17 (1.38–3.40; p < 0.0001) compared with those with the optimal lipid phenotype [3].
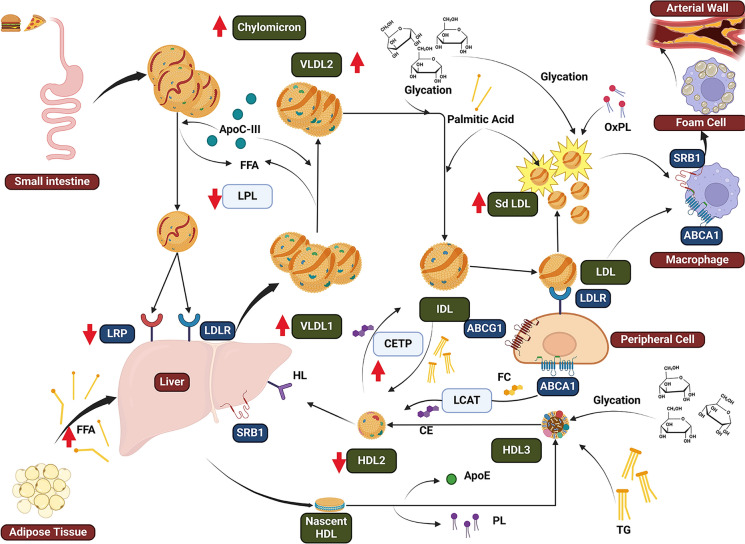
Fig. 1.
Overview of changes in type 2 diabetes dyslipidemia. Chylomicron in T2D there is an increased secretion of apoB-48, which is also stimulated by high circulating FFA. The delayed catabolism of chylomicrons is mainly due to metabolomic enrichment of apoC-III and reduced expression of LRP. VLDL overproduction, mainly VLDL1, produces an increased level of TG. There is both an increased production and delayed catabolism due to the increased FFA flux from adipocytes to liver. Metabolomic changes of VLDL include enrichment of apoC-III an inhibitor of LPL, which associated to glycation of apolipoproteins causes a reduced elimination. LDL has a reduced catabolism in T2D, inducing a longer half-life in plasma and promoting oxidation and production of sdLDL. As a consequence of hyperglycemia, there is glycation of LDL which further reduce the affinity to the receptors. HDL undergoes several changes in T2D as there is an increased activity of CETP and there is an enrichment of TG in HDLs. This promotes HL activity and results in an increased elimination of HDL from circulation. Also in T2D its known that HDL undergoes glycation and therefore has metabolomic changes (loss of phospholipid content and reduced apoE). apoB-100 apolipoprotein B-100, apoB-48 apolipoprotein B-48, apoC-III apolipoprotein C-III, apoE apolipoprotein E, CE cholesteryl ester, CETP cholesteryl ester transfer protein, CM chylomicron, FFAs free fatty acids, glycLDL glycated low-density lipoprotein, HL hepatic lipase, IDL intermediate density lipoprotein, LDLR low-density lipoprotein receptor, LPL lipoprotein lipase, sdLDL small, dense low-density lipoproteins, SR-B1 scavenger receptor B1, TG triglycerides, VLDL very-low-density lipoprotein
Genetic Link Between T2D and Hyperlipidemias
The connection between the predisposition of T2D and hyperlipidemias is shown consistently in Mendelian randomization studies. There is an inverse association between LDL-C and the risk of developing T2D [4–6]. Genome-wide association studies of patients with T2D have found that in 130 single-nucleotide peptide scores for which each standard deviation (SD) of (38 mg/dl) estimated increase in LDL-C, the risk of T2D was reduced by 21% [R 0.79 (0.71–0.88)), as was the case for 130 SNPs scores every 16 mg/dl estimated increase in HDL-c (OR 0.83 (0.76–0.90)] [7].
Genetic variants associated with lipid metabolism are also linked to risk of developing T2D. Some studies have found the genetic predisposition to low HDL and or high TG are associated with an increase in T2D risk. In this same study, they also evaluated the collective contribution of multiple genetic variants and found that for each additional risk allele in the genotype scores of HDL cholesterol or triglycerides it was a ~ 2–3% increment in the T2D risk [8]. Other studies have shown that higher levels of cholesterol, specifically on large and extra-large HDL and LDL-C are associated with lower fasting glucose and T2D, respectively. Other enzymes related to metabolism of HDL have also been associated with a greater risk of diabetes. For example, Dixit et al. demonstrated that the prevalence of two CETP variants (rs708272, rs708272) was higher in T2D [9].
The effect of TG on the risk on T2D has been conflicting. Variants in LPL and angiopoietin-like protein 4 (ANGPTL4) that lowered TG there was lower risk for developing T2D [10]. Two other studies have also shown that genetic variants associated with high TG are linked to a higher risk of T2D [8, 11]. However, it is important to note that other studies have not found any conclusive effects [7].
Association of Primary Hyperlipidemias and Diabetes
Familial Combined Hyperlipidemia
Familial combined hyperlipidemia (FCHL) is the most common primary dyslipidemia in the general population (prevalent in up to 3%) and in those with history of MI (prevalent up to 20–38%) [12]. FCHL is characterized by abnormally elevated levels of apoB and mixed hyperlipidemia, isolated hypercholesterolemia, hypertriglyceridemia, or even a normal lipid profile [13]; spontaneous fluctuations in serum lipid concentrations are common in this condition.
FCHL is a high-risk condition for having ASCVD. The prevalence of CAD in patients with FCHL younger than 60 years has been estimated to be near 15% [14–16], and being five-fold higher in men. Diagnosis of FCHL has changed over the years. Due to the polygenic nature of its non-Mendelian trait, FCHL diagnosis is done clinically. Classically, it is based on the identification of at least one of the usual phenotypes (HTG, HCT, or mixed dyslipidemia), high levels of apoB (> 90th percentile or > 120 mg/dl) alongside first degree relatives with premature coronary artery disease (CAD) with any of the mentioned phenotypes. Many patients with FCHL remain undiagnosed [17]. Still, a high diagnostic uncertainty exists in categorization as normal or abnormal members of the family. This was further explored in a 5-year follow-up study where it showed that based on a single observation up to 40% of patients can be misclassified. This becomes critical, especially by the fact a single patient can switch phenotypes in follow-up, while family members could have a different phenotype [18, 19]. Therefore a fixed percentile cut-off may be difficult to use. The FCHL criteria have overlap with the metabolic syndrome (MetS) [15]. However, the FCHL diagnosis is established when apoB is abnormally high (> 90th percentile of the population) [13]. All diagnostic criteria are mentioned in Table 1.
Table 1.
Primary dyslipidemias diagnosis criteria
| Primary hyperlipidemia | Genetic defect | Diagnosis criteria |
|---|---|---|
| Familial combined hyperlipidemia | Polygenic trait, variants of GCKR, FOXC2, CERS4, TNFRSF1B, and TCF7L2, HNF4A, APOA1/C3/A4/A5 gene cluster, and USF1 |
HTG, HCT or mixed dyslipidemia High levels of apoB (> 90th percentile or > 120 mg/dl) First-degree relatives with premature CAD with any phenotype |
| Familial hypercholesterolemia | Defects in LDLR, PCSK9, or APOB genes | > 8 of Dutch Lipid Clinical Network criteria |
| Multifactorial chylomicronemia | Polygenic nature, large variants effects in LPL, APOC2, APOA5, GPIHBP1, and LMF1 |
TG > 1000 mg/dl Genotyping for known variants |
| Familial dysbetalipoproteinemia | Homozygous for apo ε2/ε2 |
Definite diagnosis can only be achieved with genotype Others include: TG between 150 and 1000 mg/dl and VLDL-C/TG ratio > 0.30 VLDL-C/TG ratio > 0.194 |
| Lipoprotein (a) | Genetically determined by variants of LPA gene |
Measurement of Lp(a) > 50 mg/dl > 125 nmol/l |
GCKR glucokinase regulator, FOXC2 Forkhead box protein C2, CERS4 ceramide synthase 4, TCF7L2 transcription factor 7-like 2, HNF4A hepatocyte nuclear factor 4 alpha, APOA1/C3/A4/A5 apolipoprotein A1, C3, A4, A5, USF1 upstream stimulatory factor 1, LDLR low-density lipoprotein receptor, APOB apolipoprotein B, PCSK9 proprotein convertase subtilisin/kexin type 9, LPL lipoprotein lipase, APOC2 apolipoprotein C2, GPIHBP1 glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1, LMF1 lipase maturation factor 1, HTG hypertriglyceridemia, HCT hypercholesterolemia, apoB apolipoprotein B, CAD coronary artery disease, TG triglycerides, VLDL-C very-low-density lipoprotein cholesterol, Lp(a) lipoprotein (a)
FHCL phenotype is frequently accompanied by other cardiometabolic risk factors such as metabolic syndrome, obesity, IR, MAFLD, and hypertension, and those who share these factors are shown to be independent markers of CVD in FHCL [14, 20, 21]. FCHL and T2D are characterized by an increased number of circulating TG-rich very-low-density lipoprotein (VLDL)1 particles, which explains the combined phenotype of HTG and HC, which also produces reduced levels of HDL-C levels and the plasma accumulation of small dense LDL particles [22–24]. These lipid changes are also observed in T2D, which confers a three-times higher risk of developing macrovascular complications [25]. However, a clinical characteristic of FCHL is an early elevation of apoB in young adults, even in the absence of insulin resistance [26].
The development of T2D in FCHL is up to 6.3 times higher than unaffected relatives [27]. Recent studies suggest that in a 5–15-year follow-up period, approximately 10–26% of individuals may develop T2D [16, 27, 28]. Follow-up cohort studies have found a higher prevalence of CAD in those with FCHL and T2D when compared to those without any MetS component (26.5 vs. 11.1%, p < 0.001). This trend continues even after adjustment for age, sex, and smoking [16]. All studies have consistently shown that DM is a major independent variable for developing CAD, with an adjusted HR of 11.4 (95% CI 5.49–23.66) [16].
Genetically, FCHL share several linked pathophysiological mechanisms with T2D. Several genes identified for FCHL also have an increased risk of developing T2D. These genes are associated with hepatic fat accumulation and increased VLDL secretion (GCKR), thrifty genes (i.e., FOXC2), ceramide synthase (CERS4), inflammation regulators (TNFRSF1B and TCF7L2), and glucose and lipid regulators (HNF4A, APOA1/C3/A4/A5 gene cluster, and USF1) genes [29] are associated with both T2D and FCHL.
Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is an autosomal dominant, rarely recessive, genetic disease characterized by elevated levels of LDL-C and premature CVD. The most frequent mutations are found in the LDL-receptor (LDL-R) gene (85–90%), followed by APOB (5%), or gain-of-function of PCSK9 (1–3%) [30, 31]. Other mutations have also been identified in the APOE gene and the LDL-R adaptor protein type 1 gene (with an autosomal recessive inheritance pattern) [32, 33]. However, up to 40% of patients with a clinical FH phenotype result negative to all previous mutations and may be the cause of severe polygenic forms of hypercholesterolemia, or other non-described mutations [34]. Diagnostic criteria are summarized in Table 1.
Historically, the prevalence of T2D in FH is lower than the general population. In the 2019 European Society of Atherosclerosis-Familial Hypercholesterolemia Studies Collaboration [35] report, the overall prevalence of diabetes was 5%, but varied by region and both were more common with increasing age. For example, the highest prevalence is in the Eastern Mediterranean with 26.5%, followed by South East Asia and the Western Pacific in 11.2%, Americas in 7.8%, Europe excluding the Netherlands in 7.4%, Netherlands in 2.5%, and the Africa population was the lowest at 1.3%. However, the genetic relationship between FH mutations and DM has not been consistent.
In 2015, Besseling and colleagues [36] found that patients with mutations defining FH had less history of DM than those without identified mutations (1.75 vs. 2.93%, respectively); and remained significant despite adjustment for confounders. Interestingly, individuals with LDL receptor mutations had lower DM prevalence (1.63%), and those with mutations on APOB had an intermediate risk for DM (2.42%). Furthermore, those negative to mutation on the LDL receptor had lower DM prevalence (1.41%) than carriers (1.80%). Similar findings were found in other studies; for example, PCSK9 InsLEU mutations had a higher prevalence of DM and a lower prevalence of CHD, but other studies have not replicated the results [37, 38].
The cause of this lower prevalence is not clearly known yet. It is now known that any genetic variant associated with low levels of cholesterol have shown an overall increased risk of diabetes. In a large meta-analysis of genetic association of LDL cholesterol–lowering alleles in or near HMGCR, NPC1L1, PCSK9, LDLR, and ABCG5/G8, it was estimated the odds ratio for T2D and found that variants at NPC1L1 were directly associated with T2D (OR for a genetically predicted 38.6 mg/dl (1-mmol/l) reduction in LDL-C of 2.42 [95% CI 1.70–3.43]; P < 0.001). For PCSK9 variants, the OR per 38.6 mg/dl (1-mmol/l) genetically predicted reduction in LDL-C was 1.19 (95% CI 1.02–1.38; p = 0.03) [39]. In patients with FH, the severity of the LDLR mutation was inversely related to diabetes prevalence [36, 40], and it has been shown that there is no difference in β-cell function markers and impaired glucose metabolism (such as insulin, C-peptide, fasting plasma glucose) regardless of their insulin sensitivity [41–43]. In line with these findings, murine models have shown that the lack of LDLR pancreatic β-cells are protected from accumulation of cholesterol and its related dysfunction [44, 45]. These data have suggested that statins may increase new-onset diabetes mellitus (NODM) through lipotoxicity, but interestingly, in this effect in patients with FH when compared to those with FHCL on statin treatment, the latter outweighed FH in T2D prevalence (13 vs. 2%) in 10 years of follow-up [46]. This is due to the reduced functionality of LDLR in FH patients, which cannot sustain cholesterol accumulation in β-cells even when there is an increased expression of LDLR as a consequence of statin treatment. These findings support the key role of LDLR in the dysfunction of pancreatic β-cells.
Other factors may play a role in the development of DM in FH, such as environmental factors. This has been studied in the Spanish dyslipidemia registry as patients with HeFH without CVD showed a lower BMI and smoking than controls, suggesting that the lower prevalence of T2D could be partially explained by a healthier lifestyle in patients with FH [47]. Another factor that could play a role is lipoprotein (a), which has an inverse association with the risk of developing T2D, but this relationship has not yet been explained or explored in the FH population [48].
Despite this lower prevalence in the last years, it has been shown that individuals with FH and T2D act as an independent risk factor for the development of ASCVD [49]. The results of a recent Swedish nationwide register showed that coexistence of FH and T2D had higher levels of LDL-C and had a higher risk of cardiovascular mortality (HR 2.40 [2.19–2.63]) and of a cardiovascular event (2.73 [2.58–2.89]) [50]. This was similar to what has been published earlier in the SAFEHEART registry, where poorer metabolic control also acted as a risk factor in this population [51].
Several studies have assessed the phenotypic features of diabetes-related dyslipidemia in FH. It has been shown that patients with HeFH and T2D are older, with a higher prevalence of arterial hypertension and higher BMI when compared to those without T2D. As in most of the patients with T2D, the lipid profiles show higher TG, lower HDL-C, and apoA-I levels, as well as higher inflammation biomarkers (hsCRP and neutrophils). As expected CVD was higher in patients with T2D, there is a greater risk for CVD (OR, 2.01; 95% CI 1.18–3.43; p = 0.010), and HbA1c was an independent risk factor for both the presence and severity of CAD [OR 2.321 (1.098–4.904), p = 0.027) in these studies [37, 52, 53].
Due to heterogeneous risk for CVD among individuals with the same mutations of FH, the development of predictive models of CV risk stratification, designed mainly for HeFH, have not added DM in their evaluation [51, 54]. This was then explored in a small sample of 1412 patients (only 73 with DM) from the FH Canada Registry. In this study, patients with DM had higher CVD than those without. However, the inclusion of DM in the model of the Montral-FH-SCORE did not improve its CVD risk prediction [53]. More studies are needed in this particular factor.
Multifactorial Chylomicronemia Syndrome
Primary chylomicronemia is a monogenic disorder characterized by a significant reduction in LPL activity. This enzymatic deficiency impairs the clearance of triglyceride-rich lipoproteins (TRL) from plasma [55]. However, the majority of cases associated with chylomicronemia syndrome (CS) occur due to the polygenic form of HTG named multifactorial chylomicronemia syndrome (MCS) [56, 57]. MCS is a complex disorder characterized by severe HTG, typically defined by triglyceride levels > 1000 mg/dl (> 10 mmol/l). With a global prevalence estimated to range from 1:250 to 1:600, MCS stands out as a clinically significant condition [58]. Chylomicronemia, the hallmark of MCS, arises from a combination of common small-effect variants and rare heterozygous large-effect variants within genes implicated in HTG (LPL, APOC2, APOA5, GPIHBP1, and LMF1, and many others such as PPARG) [59]. The cumulative effect of these genetic predispositions is quantified by a polygenic TG risk score, representing an individual's susceptibility to chylomicronemia [56]. Heterozygosity for pathogenic variants is associated with highly variable TG phenotypes. The heterozygous LPL and APOAV-deficient phenotype is highly variable both within and between patients [60, 61]. Diagnostic criteria are summarized in Table 1.
Those affected by MCS have an increased risk of acute pancreatitis, CVD, non-alcoholic steatohepatitis (NASH), and DM. Secondary factors modulate the phenotypic severity [60, 61]. Recent findings have shown a higher prevalence of NASH in individuals with MCS compared to those with familial chylomicronemia syndrome (FCS) or moderate HTG, as reported in a study [62]. This elevated prevalence of NASH in MCS cases is often associated with IR, MetS, and DM. Moreover, MCS precipitates a pro-atherogenic metabolic environment characterized by postprandial lipemia, making its management more complicated [63].
The association between MCS and DM is well documented, with reported prevalence rates in cohorts with severe HTG varying between 25 and 76%. This is particularly more notable in populations with additional secondary factors such as obesity, alcohol consumption, and the use of TG-raising medications. Among these cohorts, individuals of Hispanic descent appear to be disproportionately affected. Notably, patients with DM with poor glycemic control are at higher risk for developing severe TG [64–67]. Interestingly, the correlation between TG levels in affected individuals and those of their first-degree relatives is not consistent, given its multifactorial nature [66, 68].
Poorly controlled DM contributes to HTG through several mechanisms. Firstly, it enhances the conversion of free fatty acids released from adipose tissue into TG. Additionally, it stimulates de novo lipogenesis in the liver, leading to increased production of TRL, thereby exacerbating the HTG. Finally, DM promotes overexpression of apoC-III, which results in lower LPL activity and prolonged lifetime of TRL [69–71]. The impact of glucose-lowering therapy may often lead to a significant reduction in TG levels: however, this may not achieve TG target levels in all patients [66].
Familial Dysbetalipoproteinemia
Familial dysbetalipoproteinemia (FD), formerly known as type III hyperlipoproteinemia [72], is a genetic lipoprotein metabolism disorder associated with a tenfold increased risk for premature CVD [73]. It is characterized by a combination of lipoprotein phenotype and genotype that consists of mutations of the apoE gene (APOE). apo E contributes to the removal of lipoproteins by serving as a ligand for the LDLR, LDLR-related protein, VLDL-receptor and the heparan sulfate proteoglycans receptor.
The APOE polymorphisms are a combination of two variants, rs429358 and rs7412, which results in three common isoforms: ApoE2 (ε2), apoE3 (ε3), and apoE4 (ε4), leading to six common combinations; ε2/ε2, ε3/ε2, ε4/ε2, ε3/ε3, ε4/ε3, and ε4/ε4 genotypes [74]. These six combinations can vary between individuals and populations, but ε3/ε3 is the common allele in most populations [74]. The ε2 allele is associated with high triglycerides and reduced LDL-C, which is more pronounced when ε2/ε2 is present [75].
The homozygous ε2/ε2 is the most common genetic defect of FD. Approximately 0.7% of the general population is homozygous for the ɛ2 allele in the APOE gene, but interestingly the majority of carriers do not express the lipid phenotype (80%) [75]. The apoE2 isoform, which is only one amino acid different from apoE3 (Arg158Cys), has a lower affinity for the LDLR (< 2% of binding activity compared to apoE3) [76, 77]. Thus, when a precipitating factor that acts by decreasing remnants clearance, increasing VLDL production and/or decreasing LPL activity, this phenotype becomes clinically apparent. However, the development of the FD phenotype is associated with the appearance of secondary factors such as IR, obesity, T2D, diet, alcohol, hypothyroidism, pregnancy, estrogen therapy, menopause, or high polygenic, thus, generally occurring until the third or fourth decade of life [78].
Phenotypically, FD is characterized by mixed hyperlipidemia with moderately severe elevations in plasma triglyceride and cholesterol levels; typically, these values both range from 300 to 400 mg/dl, apoB < 120 mg/dl, and low LDL-C levels. Due to the impaired clearance of VLDL, remnants, and chylomicrons, their plasma residence time is markedly prolonged. Given this prolonged period in circulation, they become cholesterol-enriched as they acquire excess cholesterol ester due to CETP–mediated core lipid exchanges [79]. Therefore, clinically, this is shown as a nearly equally elevated level of TC and TG and an altered non–HDL-C/apoB ratio (> 2.5) and VLDL-C/triglyceride ratio (> 0.3) [80]. Some physical findings could also be found, like xanthochromia striata palmaris, but this only appears to be present in a minority of patients.
Diagnosis can be challenging since the gold standard Fredrickson's criteria for the diagnosis of FD (TG between 150 and 1000 mg/dl and VLDL-C/TG ratio > 0.30) required ultracentrifugation, which is an expensive test and not widely available in the clinical care setting [81]. Therefore, some groups have proposed other simple criteria for the diagnosis. For example, the apoB/total cholesterol ratio [82], the apoB algorithm of Sniderman [79, 83], the non-HDL-C/apoB ratio [84], the remnant cholesterol/TG ratio [85], and the most recent by Sampson et al., which is based on a new formula to calculate VLDL-C, which includes apoB, and then used as a VLDL-C/TG ratio > 0.194 (sensitivity = 73.9%; specificity = 82.6%; and area under the curve (AUC) = 0.8685) to identify FD [86]. However, the only certain way to diagnose the pathology is though the identification of the genotype. Each is summarized in Table 1.
The FD relationship with T2D has not been fully explored. As mentioned above, the development of T2D may be a precipitating factor for the development of the FD phenotype in those at risk, but if there is a genetic link between them has not been explored. Although in the majority of cohort studies the prevalence of T2D in FD is considered to be higher than what is seen in the general population (8–20% in FD) [79, 87–89], and the coexistence of both is associated with a higher risk of the development of CVD. This combination is probably underdiagnosed by the assumption that the mixed dyslipidemia in a T2D patient may be due to diet habits and to T2D by itself, and by the fact that dietary intervention in combination with treatment of associated disorders (such as overweight, diabetes mellitus) may normalize plasma lipid levels [90].
Lipoprotein (a)
Similar to LDL, lipoprotein (a) (Lp(a)) is an apoB-containing lipoprotein with an additional protein, an apolipoprotein (a) (apo(a)), which is covalently bound. The Lp(a) has been identified as a causal factor for coronary heart disease based on epidemiological and genetic findings [91–93]. Apo(a) contains large Kringle-shaped protein structures which vary in number, and therefore affect the size and production rate. Thus, there is an inverse relationship between circulating levels Lp(a) and size [94]. The gene that codes for apo(a) is LPA, where variations determine its size. Of importance is the polymorphisms of LPA Kringle IV type 2 (KIV-2) repeat, which is defined by a 5.6-kb repeat that can occur two to more than 43 times per allele and, therefore, determines the number of apolipoprotein(a) Kringle structures [95, 96].
Although the circulating levels of Lp(a) are much lower than other lipoproteins, it has been shown that per-particle ASCVD risk of Lp(a) is six times higher than LDL (point estimate of 6.6; 95% CI 5.1–8.8). It is expected that up to 1% of the population has extreme levels of Lp(a) above 430 nmol/l (180 mg/dl), which is associated with a more than threefold increased risk of CVD and the same lifetime risk for ASCVD as untreated heterozygous familial hypercholesterolemia [94].
Mendelian randomization studies have shown that low levels of Lp(a) and a high LPA KIV-2 sums of repeats are clearly associated with an increased risk for T2D. This has also been supported by a prospective study of 26,746 women with 13-year follow-up. They found an inverse association of Lp(a) with risk of T2D, with approximately 20–50% lower relative risk in quintiles 2–5 compared with quintile 1 [97]. A recent meta-analysis of four prospective studies found that the risk of T2D was higher in those with the lowest Lp(a) concentration, with the highest risk in those with a Lp(a) less than 7 mg/dl [48]. The basis of this relationship remains unclear, but it has been hypothesized that it may be related to either the isoform size or an inverse relationship with TG [98].
Therapeutic Implications
Familial Combined Hyperlipidemia
Treatment of mixed hyperlipidemia should achieve a significant lowering of LDL-C, non–HDL-C, and apoB. As mentioned above, the coexistence of FCHL and T2D implies the categorization of cases at least as a high risk status (according to the European Atherosclerosis Society). Intermediate-risk patients with diabetes (i.e., young type 2 diabetes cases with a time since diagnosis lower than 10 years, free of chronic complications) should be considered as a high-risk status because of the coexistence of FCHL. Cases with ten or more years of exposure to hyperglycemia or with one or more chronic complications should be considered as a very high-risk status. As guidelines recommend, patients at high risk need to achieve a 50% LDL-C decrement compared to the baseline concentrations and a during therapy LDL-C level < 70 mg/dl. The same recommendation applies for the very high-risk cases, but the on-therapy LDL-C should be below 55 mg/dl [99]. The main first-line therapy to reduce cardiovascular risk should be statins, and if goals are not attained, the addition of a combined therapy with ezetimibe, PCSK9i, bempedoic acid, or inclisiran should be considered. In cases with a non-HDL-C > 220 mg/dl, a statin/ezetimibe combination should be considered as initial therapy. If there is a persistence of TG > 200 mg/dl despite dietary therapy, a fibrate or eicosapentaenoic acid ethyl ester should be added, although its capability to reduce CVD is controversial [100].
A novel therapeutic strategy that might be relevant for FCHL and T2D is apoC-III inhibition because increased levels of apoC-III are associated with insulin resistance and drive CV risk in T2D [101]. The effects of apoC-III inhibition on T2D dyslipidemia have been supported by several trials. The use of antisense oligonucleotides (ASO) like volanesorsen, a second-generation non-triantennary N-acetylgalactosamine (GalNAc) ASO. A recent meta-analysis of the phase 2 and phase 3 clinical trials on volanesorsen in chylomicronemia showed an impressive lowering of VLDL-C (− 73%), TG (− 68%), apoC-III (− 74%), and increasing HDL-C (+ 40%), but also LDL-C (+ 136%) and apoB (+ 20%) [102]. This has to be further studied in FCHL. In the BROADEN study for familial partial lipodystrophy (FPL), the subgroup of T2D benefited from decreased TG (– 88%) and apo CIII levels (– 80%) without any effect in hemoglobin A1c (HbA1c) [103]. In a randomized placebo-controlled trial for patients with T2D with hypertriglyceridemia, 300 mg of volanesorsen once a week decreased plasma apoC-III levels by 88%, TG levels by 69%, and increased HDL-C levels by 42% compared to placebo. Insulin sensitivity measured with the gold standard methodology (the hyperinsulinemic-euglycemic clamp) increased by 57% during volanesorsen treatment and was significantly correlated with a decrease in plasma apoC-III and TG levels [104]. However, in the COMPASS trial, HbA1c increased by 0.3–0.7% among those with T2D and HOMA-IR increased in patients without T2D. However, the follow-up of patients with volanesorsen over 5 years has not shown chronic deleterious effects on glucose homeostasis [105]. This will be further addressed in ongoing open-label trials with volanesorsen [106].
Two novel agents targeting APOC3 (olezarsen and plozasiran) are in clinical development in the hope of retaining the benefits seen in volanesorsen while avoiding thrombocytopenia. Olezarsen, a third-generation triantennary N-acetylgalactosamine (GalNac) conjugates APOC3-ASO, is in ongoing phase 3 clinical studies covering patients with FCS (NCT05130450), severe HTG (NCT05681351), and atherosclerotic cardiovascular disease (NCT05610280). In a phase 2 study, BRIDGE-TIMI 73, a total of 154 patients with moderate HTG (150–499 mg/dl) and high CVD risk or severe HTG (> 500 mg/dl) were treated with 50 and 80 mg of olezarsen; with 68% of patients with a T2D diagnosis. They showed that TG levels were reduced in 49.3 and 53.1% of the cases, respectively. In this trial, 86 and 93% of patients, respectively, achieved TG levels < 150 mg/dl compared to 12% in the placebo group. Treatment with olezarsen lowered apoC-III levels in 64–73% [107]. Currently, the ESSENCE study will evaluate the change from baseline to week 25 in fasting TG on patients with TG between 200 and 500 mg/dl and ASCVD or increased risk for ASCVD and TG > 500 mg/dl (NCT05610280). Of note, in the recent phase 3 BALANCE trial, a total of 43 patients were treated with olezarsen and 23% of the treated patients had diabetes. This trial showed that there was a 43.5% reduction of TG at 6 months of treatment on the 80 mg group, which also showed a 73.7% reduction of apoC-III, no outcomes of diabetes or HbA1c were reported.
A second novel apoC-III antagonist is plozasiran, formerly known as ARO-APOC3, an APOC3 GalNAc-conjugated small interfering RNA (siRNA), currently under phase 3 studies in FCS, severe HTG and HTG at high CVD risk. In phase 1/2 trials, it has been shown that treatment with plozasiran can have apoC-III reductions in up to 94% and up to 64% in TG without safety issues. Of relevance in this context, FHCL pathophysiology can have a reduction in VLDL-C up to 68%, LDL-C up to 25%, and an increase in HDL-C up to 69%. The phase 2 MUIR trial, which evaluated the safety and efficacy of plozasiran in 353 patients with mixed hyperlipidemia, showed that treatment with plozasiran decreased apoC-III in a dose-dependent manner up to 80%. The TG reduction was significant by 52–64% and reduced non-HDL-C in 27%, 19% apoB, 55% remnant cholesterol, and increased HDL-C in 51%. Recently, the MUIR-3 trial was published where they evaluated the TG change with plozasiran in adults with TG > 150–499 mg/dl and LDL-C < 130 mg/dl on statins. They showed that treatment with plozasiran significantly reduced apoC-III, TG, Non-HDL-C, and LDL-C in a dose-dependent manner. Plozasiran 10, 25, and 50 mg s.c. Q12W reduced apoC-III by 58.9, 74.1 and 80.1%, respectively, compared to placebo at week 24. This was accompanied by reductions in TG of 49.8, 56, and 62.4%, respectively, compared to placebo. In addition, non-HDL-C was reduced by 19.3, 20.1, and 26.9%, respectively, and apoB was reduced by 9.5, 12.2 and 18.3%, respectively, compared to placebo [108]. In the same trial, they also reported up to 20% in glycemic control outcomes. The results of the SHASTA-2 trial, a phase 2b study, have been published where 64% of population had T2D [109]. This trial showed that the treatment with plozasiran led to a modest worsening of glycemic control compared to placebo, leading to discontinuation in one patient [109]. In the analysis, it showed that the glycemic events were confined to those with diabetes at baseline. In those without T2D, there were no changes in insulin sensitivity, evaluated through HOMA-IR. The authors hypothesized that this might be due to an increased substrate delivery to the liver to drive gluconeogenesis. Although this needs further investigation, long-term use of volanesorsen for up to 5 years in patients with FCS has not revealed any chronic deleterious effects on glucose homeostasis. This suggests that these effects may be transient rather than long term [105, 106].
Other strategies can come in the new future as angiopoietin-like protein 3 (ANGPTL3) siRNA as ARO-ANG3 (zodasiran), which recently published the results of a double-blind, placebo-controlled phase 2b study to evaluate the efficacy and safety in adults with mixed dyslipidemia [110]. Zodasiran 50, 100, and 200 mg s.c. reduced ANGPTL3 by 54.3, 69.8, and 73.7%, respectively, compared to placebo at week 24 (all p < 0.0001). This was accompanied by reductions in TG of 51.2, 56.6, and 63.1%, respectively, compared to placebo (all p < 0.0001). In addition, remnant cholesterol was reduced by 72.6, 75.9, and 82.0%, respectively, compared to placebo (all p < 0.0001), and apoB was reduced by 18.7, 15.2, and 21.9%, respectively, compared to placebo (p < 0.0001, p < 0.05, p < 0.0001). Similarly, solbinsiran, a GalNAc conjugated siRNA targeting ANGPTL3 is in an ongoing trial. A phase 1 ascending and repeat-dose study of solbinsiran in patients with mixed dyslipidemia (fasting TG ≥ 150 mg/dl and < 500 mg/dl, as well as LDL-C ≥ 70 mg/dl) has been completed (NCT04644809) and results are soon to be published.
A new monoclonal antibody against ANGPTL3/8 complex has shown in preliminary results of phase 1 and 2 [111] that single doses a monoclonal antibody targeting the ANGPTL3/8 complex achieved significant reductions in TG and remnant cholesterol levels among patients with mixed dyslipidemia. The preliminary study, involving 48 participants with plasma TG levels exceeding 135 mg/dl (1.5 mmol/l) and plasma LDL-C levels > 70 mg/dl (1.8 mmol/l) showed remarkable TG reductions of 59, 65, and 70% at 15 days with LY3475766 doses of 100, 300, and 600 mg, respectively. Additionally, LDL-C levels showed reductions of up to 17, 22, and 37%, while decreases in apoB were at 14, 21, and 31% for the respective doses. Furthermore, a dose-dependent reduction in cholesterol remnants and a corresponding increase in HDL-C were observed. The effects of the treatments are summarized in Table 2.
Table 2.
Current therapies for primary hyperlipidemias
| Drug | Mechanism of action | Indications | LDL-C | HDL-C | TG | Lp(a) | ApoB |
|---|---|---|---|---|---|---|---|
| Statins | Inhibits HMG-CoA reductase | Hypercholesterolemia | ↓20–60% | ↑5–15% | ↓0–35% | ↑8–24% | ↓20–30% |
| Fibrates | PPAR alpha agonist | Hypertriglyceridemia | ↓0–15% | ↑5–15% | ↓20–50% | ↑12% | ↓11% |
| Ezetimibe | Inhibits NPC1L1 | Hypercholesterolemia | ↓15–25% | ↑1–3% | ↓10–20% | - | ↓11–17% |
| PCSK9 inhibitors | mAb targeting PCSK9 | Hypercholesterolemia | ↓50–60% | ↑5–15% | ↓5–20% | ↓6.2–46.7% | ↓40–62% |
| Bempedoic acid | ATP- citrate lyase | Hypercholesterolemia | ↓15–25% | ↓5–6% | No change | ↑2.4% | ↓8–13.2% |
| Volanesorsen | ASO inhibiting APOC3 | MCS and mixed hyperlipidemia | ↑136% | ↓40% | ↓58.9–88.5% | – | ↑20% |
| Olezarsen | ASO inhibiting APOC3 | MCS and mixed hyperlipidemia | ↑7.7–9.9% | ↑39.6% | ↓49–53.1% | – | 18.2–18.5% |
| Plozasiran | siRNA APOC3 | Mixed hyperlipidemia | ↓0.9–10% | ↑37–50% | ↓49–62% | ↑19–33.9% | ↓9.5–18.3% |
| Evinacumab | ASO inhibiting ANGPTL3 |
Mixed hyperlipidemia Hypercholesterolemia |
↓29.9–47.2% | ↓19–27.9% | ↓38–53.4% | ↓8.9–10.3% | ↓19.9–38.8% |
| Zodasiran | siRNA-inhibiting ANGPTL3 | Mixed hyperlipidemia | ↓7.3–15.8% | ↓7.8–24.5% | ↓52–63% | ↓3.3–20% | ↓6.8–21.9% |
| Solbisiran | siRNA-inhibiting ANGPTL3 |
Mixed hyperlipidemia (Not published) |
– | – | – | – | – |
| LYS3475766 | mAb-targeting ANGPTL3/8 complex |
Mixed hyperlipidemia (Not published) |
↓17–37% | ↓37% | ↓59–70% | – | – |
LDL-C low-density lipoprotein cholesterol, TG triglycerides, HDL-C high-density lipoprotein cholesterol, Lp(a) lipoprotein (a), apoB apolipoprotein B, HMG-CoA β-hydroxy β-methylglutaryl-CoA, PPAR β-hydroxy β-methylglutaryl-CoA, NPC1L1 Niemann–Pick C1-like 1, mAb monoclonal antibody, PCSK9 proprotein convertase subtilisin/kexin type 9, ATP adenosine triphosphate, ASO antisense oligonucleotide, APOC3 apolipoprotein C-III gene, siRNA silencing ribonucleic acid, ANGPTL3 angiopoietin-like protein 3
Currently, FCHL populations are considered at high risk for CVD, but when combined with DM, which is frequent this population, they should be treated as high or very high risk. Although there are no direct randomized trials with a FCHL population as underrepresented in trials, we can extrapolate the experience of high-risk populations to choose and individualize the right treatment. There is a need to further study new treatments in this population.
Familial Hypercholesterolemia
Statins with ezetimibe continue to be the mainstay of treatment in FH. However, up to 80% of patients fail to achieve LDL-C with this lipid-lowering therapy [112]. Therefore, an addition of newer medications like PCSK9i are required. For example, both alirocumab and evolocumab have been tested for HeFH. In the Rutheford-2 trial, evolocumab lowered LDL-C by 60%, non-HDL-C in 56% and apoB by 49%, Lp(a) by 31 and TG by 22%. In the Odyssey FH I and FH II studies, alirocumab lowered LDL-C by 55%, non-HDLC by 50%, apoB by 43%, and Lp(a) by 19% [113].
Inclisiran is a long-acting siRNA that interferes with the translation of PCSK9. Its effect in LDL-C in HeFH was explored in the ORION-9 study [114]. These patients were administered with 284 mg (n = 242) of inclisiran or placebo (n = 240) for 510 days. The LDL-C levels of these patients were reduced by 47.9%, non-HDL-C by 44%, Lp(a) by 17.2%, and TG by 12% compared to placebo. Interestingly, the reduction of LDL-C was similar in all genotypes.
Recently, evinacumab, a monoclonal antibody against ANGPTL3, has been approved in the EU, UK, and US for the treatment of homozygous FH (HoFH). In the ELIPSE HoFH trial [115], an intravenous infusion of evinacumab 15 mg/kg every 4 weeks (n = 42) showed a 47% reduction in LDL-C and 41% in apoB levels compared to placebo (n = 25). Interestingly, in those with null-null LDL receptor variants, the treatment with evinacumab resulted in a 43% decrease in LDL-C. Also, treated patients showed a 55% decrease in TG and 30% increase in HDL-C. In 2020, this was also shown for patients with HeFH and refractory hypercholesterolemia. In a double-blind, placebo-controlled trial, 272 patients with HeFH with refractory hypercholesterolemia (LDL-C > 70 mg/dl with ASCVD or > 100 mg/dl without ASCVD) were treated with evinacumab 15 mg/kg [116]. In this trial, it was shown that LDL-C levels were decreased in 50% compared to placebo. In contrast to HoFH in the ELIPSE, in this study HeFH also presented a decrease in Lp(a) by 16%. Currently, there are no cardiovascular outcomes studies. The effects of the treatments are summarized in Table 2.
In summary, the clinical implications of DM in FH are of relevance for a higher risk of presenting CVD. Although some studies have been controversial, the consensus of treatment suggests that those who coexist with FH and DM or CHD should be considered as very high-risk subjects and be treated more intensively, and, as guidelines recommend, patients at very high risk need to achieve LDL-C goals of 50% reduction and LDL-C < 55 mg/dl (< 1.8 mmol/l), which is not achieved in 50% of this population [50, 99]. An unmet need is the development of a tool to determine the degree of risk that T2D confers in this population. However, FH registries are heterogeneous in nature; the difficulties in realizing a genetic diagnosis leads to a too wide range of registries based solely on clinical criteria (such as Dutch Lipid Clinical Network or Simone Broom). The need to standardize registries based on molecular diagnosis is needed for a better understanding of FH heterogeneity.
Multifactorial Chylomicronemia
Diet remains the cornerstone of treatment of all forms of primary hypertriglyceridemia, but the response to treatment is influenced by the extent of genetic variants associated with TG metabolism. Those with minimal genetic contributions often exhibit a favorable response to treatment and with the control of secondary causes. However, individuals with a high genetic burden may experience a poorer response, with need for intensive therapeutic interventions. Targeting specific pathways implicated in TG metabolism opens up exciting possibilities for more tailored and effective treatments.
Volanesorsen, in a double-blind randomized placebo-controlled trial, showed significantly reduced plasma apoC-III (– 88%, p = 0.02), triglycerides (– 69%, p = 0.02) and increased HDL-cholesterol levels (+ 42%, p = 0.03) in patients with DM compared to placebo [104]. However, in the COMPASS Study, participants with T2D had an HbA1c increase of 0.3–0.7%, but we still do not know the mechanism or the clinical relevance of this. The most frequent adverse effect was thrombocytopenia and site injection reactions. Volanesorsen was also approved for FCS [102, 117], and MCS [106], with reductions of 77 and 71%, respectively.
Similarly, recently published results from the BALANCE study where at 6 months of treatment, TG levels were significantly reduced with olezarsen 80 mg (− 43.5%; 95% CI − 69.1 to − 17.9; p < 0.001) [118], but not with olezarsen 50 mg (− 22.4%; 95% CI − 47.2 to 2.5; p = 0.08). At 12 months, placebo-adjusted reductions in TG and apoC-III were 59 and 81%, respectively. By 53 weeks of treatment there was only one episode of acute pancreatitis in each olezarsen group compared to the 11 in the placebo group. Treatment with olezarsen had no drug-related adverse effects [118]. Currently, an open-label extension of the BALANCE study is undergoing, and also phase 3 clinical trials for severe HTG (CORE and CORE2 study), which aim to evaluate the safety and efficacy of the drug in severe HTG will provide more data (NCT05079919, NCT05552326).
Plozasiran [119] is currently phase 1/2a (including patients with FCS and MCS) and has shown a decrease of TG levels in 87% in those with FCS compared to 84% in those without FCS. The phase 2b SHASTA-2 study evaluated patients with severe HTG, showed a TG in 90%, apoC-III in 96%, non-HDL-c in 35%, apoB in 19%, and also showed an increase HDL-C in 75% to 99% but also of LDL-C 13–22% [120]. Among plozasiran-treated patients, 91% achieved a TG level of < 500 mg/dl at week 24. However, currently active is the PALISADE trial (NCT05089084), which will evaluate the changes of TG at 10 months of treatment in patients with FCS and TG > 880 mg/dl. The patients who complete the randomized period will continue in a 2-year open-label extension period where all participants will receive plozasiran.
ANGPTL3 inhibitors and ANGPTL3,8 complex inhibitors require a minimal LPL enzymatic activity, therefore they are not useful in patients with FCS. Their utility is in polygenic and MCS and are reviewed above [121]. However, it is important to note that evinacumab and vupanorsen have recently been suspended for severe HTG, as it was associated with higher adverse events (acute pancreatitis and increased hepatic fat, respectively).
Finally, pegozafermin is a long-acting glycopegylated analog human fibroblast growth factor 21 (FGF21) [122, 123] in development for the treatment of severe hypertriglyceridemia and nonalcoholic steatohepatitis [122]. The outcomes of the ENTRIGUE Study [124] (NCT0441186) a phase 2, double-blind, randomized, five-arm trial evaluating pegozafermin at four varying doses compared to placebo over an 8-week period in patients diagnosed with severe hypertriglyceridemia, defined as triglycerides ≥ 500 mg/dl and ≤ 2000 mg/dl. Treatment with pegozafermin reached its primary endpoint by achieving reductions in TG that varied from 36.4 to 63.4% in a dose-dependent manner. No serious adverse events related to the administration of the drug were reported. However, phase 3 trials are still undergoing. The effect of treatments are summarized in Table 2.
In summary, severe HTG is influenced by a complex interplay of genetic predisposition and secondary factors like diabetes, with additional nuances related to race/ethnicity and sociocultural disparities. These multifaceted risk factors pose challenges for clinical management, necessitating tailored therapeutic approaches. While current strategies target the prevention of both CVD and pancreatitis, the novel drug targets and treatment approaches hold promise for addressing these challenges. By targeting the underlying genetic and secondary contributors to HTG, these emerging therapies aim to reduce the risk of both CVD and pancreatitis, offering improved outcomes and prevention strategies tailored to individual needs.
Familial Dysbetalipoproteinemia
Treatment of FD starts with lifestyle modification with a reduced intake of fats and/carbohydrates. If dyslipidemia continues after controlling precipitating factors and lifestyle changes, which is the case in 60% of patients [80], non-HDL-C should be the primary lipid treatment target, this is particularly important in FD as LDL-C levels are usually low. The effect of different pharmacological treatments on CVD outcomes in FD is not known as there is a lack of RCT, where most of the clinical trials in FD use intermediate or lipid levels as surrogate for endpoints. Statin treatment in these patients show that they reduce LDL-C as in non FD patients but with a lesser impact in TG. As guidelines recommend, in most cases of FD, when TG levels are over 200 mg/dl a fibrate should be added. In fact, in FD, the addition of FD has shown to improve the lipid profile. Some studies have shown that adding bezafibrate significantly improved non-HDL-C, TG, HDL, and apoB compared to standard lipid-lowering level.
Achievement of non-HDL-C target levels in FD only occurs in 40% of patients (PMID: 25,768,710). Therefore, the addition of other therapies could be of use in this population. This was evaluated in the EVOLVE-FD trial where the use of the PCSK9i evolocumab in patients with FD, showed a large reduction of non-HDL-C, IDL-C, and apoB of 51% (95% CI 43–57%), 44% (95% CI 30–55%), and 48% (95% CI 42–53%), respectively [125]. A follow-up study showed that evolocumab reduced particle number of all apoB-containing particles, but PCSK9i reduced more cholesterol content than TG (VLDL-C 48%, 95% CI 29–63%, and VLDL-TG 20%, 95% CI 6.3–41%). Other treatments targeted to inhibit apoC-III or ANGPTL3 could be beneficial in patients with FD, although these treatments have not been explored in this context.
In summary, the link between FD and DM is not quite clear. This is due to the difficulty of diagnosis of FD and the underrepresentation of primary dyslipidemias in DM trials. It is true that generally with DM standard care, the phenotype of FD might disappear, but it should be suspected more frequently as therapeutic goals and follow-up may change. The addition over statins in these patients is, as mentioned above, dependent on the achievement of goals, which is low. Newer therapeutics such as PCSK9i have proven to be effective in this population.
Lipoprotein (a)
Although the relationship between Lp(a) and diabetes is inverse, the cardiovascular risk of Lp(a) in diabetes is directly proportional. In the BiomarCaRE study, elevated levels of Lp(a) were associated with increased risk of CAD in T2D [126]. In another study, one standard deviation of change in Lp(a) (26.5 mg/dl in pre DM and 26.0 mg/dl in DM) was associated with 32.7% and 38.6% increased risk of CVEs in pre-DM and DM, respectively [127]. This was further replicated in the ARIC study with ASCVD events in Caucasian participants with prediabetes (hazard ratio [HR] = 1.35; 95% confidence interval [CI] 1.07–1.69); p = 0.03) and diabetes (HR = 1.42; 95% CI 1.10–1.84; p < 0.01) [128].
However, there is a lot of work to be done in assessing these interactions. A recent case-cohort study showed that Lp(a) is associated with CVD, neuropathy, and nephropathy in patients with diabetes, suggesting its value as a biomarker of outcomes in diabetes [129]. Although new drugs have shown a potent lowering of Lp(a), their benefits and impact on diabetes and its complications have yet to be proven.
Conclusions
The interactions between primary hyperlipidemias and T2D can be complex, with limited available data on the associated risks and links, which have therapeutic implications. Most data come from sub-analyses of cohorts; frequently patients with primary dyslipidemia are underrepresented in all T2D clinical trials. The general clinical implications of T2D in primary hyperlipidemias is the higher risk of CVD than those presented separately. Consequently, there is a need to accurately identify patients with this dual burden and to adequately prescribe lipid-lowering therapies with the current advancements in newer therapeutic options.
Author Contributions
Conceptualization: Rafael Zubirán and Carlos A. Aguilar-Salinas; Methodology: Rafael Zubirán performed the literature search; Writing—original draft preparation: Rafael Zubirán and Ivette Cruz-Bautista; Writing: Rafael Zubirán and Ivette Cruz-Bautista, Review and editing: Rafael Zubirán and Carlos A. Aguilar-Salinas.
Funding
No funding or sponsorship was received for the publication of this article.
Declarations
Conflict of Interest
Rafael Zubirán is full-time US government employee. Ivette Cruz-Bautista and have declared that no potential conflicts of interest exist. Carlos A. Aguilar-Salinas is an editorial board member of Diabetes Therapy. Carlos A. Aguilar-Salinas was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Rafael Zubirán funding was provided by intramural DIR research funds from National Heart, Lung and Blood Institute. Ivette Cruz-Bautista and Carlos A. Aguilar received no funding.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Luciani L, Pedrelli M, Parini P. Modification of lipoprotein metabolism and function driving atherogenesis in diabetes. Atherosclerosis. 2024;394:117545. 10.1016/j.atherosclerosis.2024.117545 [DOI] [PubMed] [Google Scholar]
- 2.Martagon AJ, Zubiran R, Gonzalez-Arellanes R, Praget-Bracamontes S, Rivera-Alcantara JA, Aguilar-Salinas CA. HDL abnormalities in type 2 diabetes: Clinical implications. Atherosclerosis. 2023;394:117213. 10.1016/j.atherosclerosis.2023.117213 [DOI] [PubMed] [Google Scholar]
- 3.Pencina KM, Pencina MJ, Dufresne L, Holmes M, Thanassoulis G, Sniderman AD. An adverse lipoprotein phenotype-hypertriglyceridaemic hyperapolipoprotein B-and the long-term risk of type 2 diabetes: a prospective, longitudinal, observational cohort study. Lancet Healthy Longev. 2022;3(5):e339–46. 10.1016/S2666-7568(22)00079-4 [DOI] [PubMed] [Google Scholar]
- 4.Marazziti D, Placidi GF, Cassano GB, Akiskal HS. Lack of specificity of reduced platelet imipramine binding in different psychiatric conditions. Psychiatry Res. 1989;30(1):21–9. 10.1016/0165-1781(89)90168-6 [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow DI, Sattar N. Blood Lipids and Type 2 Diabetes Risk: Can Genetics Help Untangle the Web? Diabetes. 2015;64(7):2344–5. 10.2337/db15-0458 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Lleo AM, Sanchez-Hernandez RM, Boronat M, Wagner AM. Diabetes and familial hypercholesterolemia: interplay between lipid and glucose metabolism. Nutrients. 2022;14(7):1–23. 10.3390/nu14071503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016;1(6):692–9. 10.1001/jamacardio.2016.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Q, Liang L, Doria A, Hu FB, Qi L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61(3):745–52. 10.2337/db11-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixit M, Bhattacharya S, Mittal B. Association of CETP TaqI and APOE polymorphisms with type II diabetes mellitus in North Indians: a case control study. BMC Endocr Disord. 2005;5:7. 10.1186/1472-6823-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49(12):1758–66. 10.1038/ng.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong THT, Mo JMY, Zhou M, Zhao JV, Schooling CM, He B, et al. A two-sample Mendelian randomization study explores metabolic profiling of different glycemic traits. Commun Biol. 2024;7(1):293. 10.1038/s42003-024-05977-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwers MC, van Greevenbroek MM, Stehouwer CD, de Graaf J, Stalenhoef AF. The genetics of familial combined hyperlipidaemia. Nat Rev Endocrinol. 2012;8(6):352–62. 10.1038/nrendo.2012.15 [DOI] [PubMed] [Google Scholar]
- 13.Zubiran R, Vargas-Vazquez A, Olvera FDR, Cruz-Bautista I, Martagon-Rosado A, Sampson M, et al. Performance of the enhanced Sampson-NIH equation for VLDL-C and LDL-C in a population with familial combined hyperlipidemia. Atherosclerosis. 2023;386: 117364. 10.1016/j.atherosclerosis.2023.117364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoumas I, Masoura C, Aznaouridis K, Metaxa V, Tsokanis A, Papadimitriou L, et al. Impact of cardiometabolic risk factors on major cardiovascular events in patients with familial combined hyperlipidemia. Circ J. 2013;77(1):163–8. 10.1253/circj.CJ-12-0320 [DOI] [PubMed] [Google Scholar]
- 15.Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation. 2003;108(5):519–23. 10.1161/01.CIR.0000081777.17879.85 [DOI] [PubMed] [Google Scholar]
- 16.Skoumas J, Papadimitriou L, Pitsavos C, Masoura C, Giotsas N, Chrysohoou C, et al. Metabolic syndrome prevalence and characteristics in Greek adults with familial combined hyperlipidemia. Metabolism. 2007;56(1):135–41. 10.1016/j.metabol.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Naukkarinen J, Ehnholm C, Peltonen L. Genetics of familial combined hyperlipidemia. Curr Opin Lipidol. 2006;17(3):285–90. 10.1097/01.mol.0000226121.27931.3f [DOI] [PubMed] [Google Scholar]
- 18.Ylitalo K, Syvanne M, Salonen R, Nuotio I, Taskinen MR, Salonen JT. Carotid artery intima-media thickness in Finnish families with familial combined hyperlipidemia. Atherosclerosis. 2002;162(1):171–8. 10.1016/S0021-9150(01)00691-8 [DOI] [PubMed] [Google Scholar]
- 19.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17(12):3542–56. 10.1161/01.ATV.17.12.3542 [DOI] [PubMed] [Google Scholar]
- 20.Gaddi A, Cicero AF, Odoo FO, Poli AA, Paoletti R, Atherosclerosis, Metabolic Diseases Study G. Practical guidelines for familial combined hyperlipidemia diagnosis: an up-date. Vasc Health Risk Manag. 2007;3(6):877–86. [PMC free article] [PubMed] [Google Scholar]
- 21.Keulen ET, Voors-Pette C, de Bruin TW. Familial dyslipidemic hypertension syndrome: familial combined hyperlipidemia, and the role of abdominal fat mass. Am J Hypertens. 2001;14(4 Pt 1):357–63. 10.1016/S0895-7061(00)01280-2 [DOI] [PubMed] [Google Scholar]
- 22.Bredie SJ, Kiemeney LA, de Haan AF, Demacker PN, Stalenhoef AF. Inherited susceptibility determines the distribution of dense low-density lipoprotein subfraction profiles in familial combined hyperlipidemia. Am J Hum Genet. 1996;58(4):812–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Vakkilainen J, Jauhiainen M, Ylitalo K, Nuotio IO, Viikari JS, Ehnholm C, Taskinen MR. LDL particle size in familial combined hyperlipidemia: effects of serum lipids, lipoprotein-modifying enzymes, and lipid transfer proteins. J Lipid Res. 2002;43(4):598–603. 10.1016/S0022-2275(20)31489-9 [DOI] [PubMed] [Google Scholar]
- 24.Guerin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes : impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol. 2001;21(2):282–8. 10.1161/01.ATV.21.2.282 [DOI] [PubMed] [Google Scholar]
- 25.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260(13):1917–21. 10.1001/jama.1988.03410130125037 [DOI] [PubMed] [Google Scholar]
- 26.ter Avest E, Sniderman AD, Bredie SJ, Wiegman A, Stalenhoef AF, de Graaf J. Effect of aging and obesity on the expression of dyslipidaemia in children from families with familial combined hyperlipidaemia. Clin Sci (Lond). 2007;112(2):131–9. 10.1042/CS20060234 [DOI] [PubMed] [Google Scholar]
- 27.Brouwers M, de Graaf J, Simons N, Meex S, Ten Doeschate S, van Heertum S, et al. Incidence of type 2 diabetes in familial combined hyperlipidemia. BMJ Open Diabetes Res Care. 2020;8(1):1–8. 10.1136/bmjdrc-2019-001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skoumas I, Ioakeimidis N, Vlachopoulos C, Chrysohoou C, Michalakeas C, Georgakopoulos C, et al. Statin therapy and risk of diabetes mellitus in aging patients with heterozygous familial hypercholesterolemia or familial combined hyperlipidemia: a 10-year follow-up. Angiology. 2018;69(3):242–8. 10.1177/0003319717718331 [DOI] [PubMed] [Google Scholar]
- 29.Taghizadeh E, Farahani N, Mardani R, Taheri F, Taghizadeh H, Gheibihayat SM. Genetics of familial combined hyperlipidemia (FCHL) disorder: an update. Biochem Genet. 2022;60(2):453–81. 10.1007/s10528-021-10130-2 [DOI] [PubMed] [Google Scholar]
- 30.Bruikman CS, Hovingh GK, Kastelein JJP. Molecular basis of familial hypercholesterolemia. Curr Opin Cardiol. 2017;32(3):262–6. 10.1097/HCO.0000000000000385 [DOI] [PubMed] [Google Scholar]
- 31.Mehta R, Zubiran R, Martagon AJ, Vazquez-Cardenas A, Segura-Kato Y, Tusie-Luna MT, Aguilar-Salinas CA. The panorama of familial hypercholesterolemia in Latin America: a systematic review. J Lipid Res. 2016;57(12):2115–29. 10.1194/jlr.R072231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cenarro A, Etxebarria A, de Castro-Oros I, Stef M, Bea AM, Palacios L, et al. The p.Leu167del Mutation in APOE gene causes autosomal dominant hypercholesterolemia by down-regulation of LDL receptor expression in hepatocytes. J Clin Endocrinol Metab. 2016;101(5):2113–21. 10.1210/jc.2015-3874 [DOI] [PubMed] [Google Scholar]
- 33.Soutar AK, Naoumova RP, Traub LM. Genetics, clinical phenotype, and molecular cell biology of autosomal recessive hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2003;23(11):1963–70. 10.1161/01.ATV.0000094410.66558.9A [DOI] [PubMed] [Google Scholar]
- 34.De Castro-Oros I, Pocovi M, Civeira F. The genetic basis of familial hypercholesterolemia: inheritance, linkage, and mutations. Appl Clin Genet. 2010;3:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collaboration EASFHS. Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet. 2021;398(10312):1713–25. 10.1016/S0140-6736(21)01122-3 [DOI] [PubMed] [Google Scholar]
- 36.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313(10):1029–36. 10.1001/jama.2015.1206 [DOI] [PubMed] [Google Scholar]
- 37.Climent E, Perez-Calahorra S, Benaiges D, Pinto X, Suarez-Tembra M, Plana N, et al. Clinical and genetic differences between heterozygous familial hypercholesterolemia patients with and without type 2 diabetes. Rev Esp Cardiol (Engl Ed). 2020;73(9):718–24. 10.1016/j.recesp.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Ryan KA, Jaworek TJ, Southam L, Reid JG, Overton JD, et al. Familial hypercholesterolemia and type 2 diabetes in the old order Amish. Diabetes. 2017;66(7):2054–8. 10.2337/db17-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA. 2016;316(13):1383–91. 10.1001/jama.2016.14568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vohl MC, Gaudet D, Moorjani S, Tremblay G, Perron P, Gagne C, et al. Comparison of the effect of two low-density lipoprotein receptor class mutations on coronary heart disease among French-Canadian patients heterozygous for familial hypercholesterolaemia. Eur J Clin Invest. 1997;27(5):366–73. 10.1046/j.1365-2362.1997.1250669.x [DOI] [PubMed] [Google Scholar]
- 41.Paolisso G, Ferrannini E, D’Amore A, Volpe C, Varricchio M, D’Onofrio F. Effects of physiological plasma insulin levels on glucose turnover parameters in familial hypercholesterolemia. Atherosclerosis. 1993;101(1):111–5. 10.1016/0021-9150(93)90106-5 [DOI] [PubMed] [Google Scholar]
- 42.Galvan AQ, Santoro D, Natali A, Sampietro T, Boni C, Masoni A, et al. Insulin sensitivity in familial hypercholesterolemia. Metabolism. 1993;42(10):1359–64. 10.1016/0026-0495(93)90138-E [DOI] [PubMed] [Google Scholar]
- 43.Karhapaa P, Voutilainen E, Kovanen PT, Laakso M. Insulin resistance in familial and nonfamilial hypercholesterolemia. Arterioscler Thromb. 1993;13(1):41–7. 10.1161/01.ATV.13.1.41 [DOI] [PubMed] [Google Scholar]
- 44.Kruit JK, Kremer PH, Dai L, Tang R, Ruddle P, de Haan W, et al. Cholesterol efflux via ATP-binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol-induced impairment of beta cell function in mice. Diabetologia. 2010;53(6):1110–9. 10.1007/s00125-010-1691-2 [DOI] [PubMed] [Google Scholar]
- 45.Mbikay M, Sirois F, Mayne J, Wang GS, Chen A, Dewpura T, et al. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 2010;584(4):701–6. 10.1016/j.febslet.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 46.Skoumas I, Masoura C, Pitsavos C, Tousoulis D, Papadimitriou L, Aznaouridis K, et al. Evidence that non-lipid cardiovascular risk factors are associated with high prevalence of coronary artery disease in patients with heterozygous familial hypercholesterolemia or familial combined hyperlipidemia. Int J Cardiol. 2007;121(2):178–83. 10.1016/j.ijcard.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 47.Perez-Calahorra S, Civeira F, Guallar-Castillon P, Pinto X, Banegas JR, Pedro-Botet J, et al. Behavioural cardiovascular risk factors and prevalence of diabetes in subjects with familial hypercholesterolaemia. Eur J Prev Cardiol. 2020;27(15):1649–60. 10.1177/2047487319896138 [DOI] [PubMed] [Google Scholar]
- 48.Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, et al. Lipoprotein(a) and incident type-2 diabetes: results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc Diabetol. 2017;16(1):38. 10.1186/s12933-017-0520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Climent E, Perez-Calahorra S, Marco-Benedi V, Plana N, Sanchez R, Ros E, et al. Effect of LDL cholesterol, statins and presence of mutations on the prevalence of type 2 diabetes in heterozygous familial hypercholesterolemia. Sci Rep. 2017;7(1):5596. 10.1038/s41598-017-06101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinck J, Hagstrom E, Natman J, Franzen S, Eeg-Olofsson K, Nathanson D, Eliasson B. Cardiovascular outcomes in patients with both diabetes and phenotypic familial hypercholesterolemia: a nationwide register-based cohort study. Diabetes Care. 2022;45(12):3040–9. 10.2337/dc22-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez de Isla L, Alonso R, Mata N, Fernandez-Perez C, Muniz O, Diaz-Diaz JL, et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation. 2017;135(22):2133–44. 10.1161/CIRCULATIONAHA.116.024541 [DOI] [PubMed] [Google Scholar]
- 52.Sun D, Cao YX, You XD, Zhou BY, Li S, Guo YL, et al. Clinical and genetic characteristics of familial hypercholesterolemia patients with type 2 diabetes. J Endocrinol Invest. 2019;42(5):591–8. 10.1007/s40618-018-0959-0 [DOI] [PubMed] [Google Scholar]
- 53.Paquette M, Bernard S, Ruel I, Blank DW, Genest J, Baass A. Diabetes is associated with an increased risk of cardiovascular disease in patients with familial hypercholesterolemia. J Clin Lipidol. 2019;13(1):123–8. 10.1016/j.jacl.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 54.Costas MJ, Cameselle JC. The inhibition of fructose 1,6-bisphosphatase by fructose 2,6-bisphosphate is enhanced by EDTA and diminished by zinc(II). Biochem Int. 1988;16(4):747–53. [PubMed] [Google Scholar]
- 55.Baass A, Paquette M, Bernard S, Hegele RA. Familial chylomicronemia syndrome: an under-recognized cause of severe hypertriglyceridaemia. J Intern Med. 2020;287(4):340–8. 10.1111/joim.13016 [DOI] [PubMed] [Google Scholar]
- 56.Dron JS, Wang J, Cao H, McIntyre AD, Iacocca MA, Menard JR, et al. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol. 2019;13(1):80–8. 10.1016/j.jacl.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 57.Chait A. Multifactorial chylomicronemia syndrome. Curr Opin Endocrinol Diabetes Obes. 2024;31(2):78–83. 10.1097/MED.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 58.Paquette M, Bernard S. The evolving story of multifactorial chylomicronemia syndrome. Front Cardiovasc Med. 2022;9: 886266. 10.3389/fcvm.2022.886266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glodowski M, Christen S, Saxon DR, Hegele RA, Eckel RH. Novel PPARG mutation in multiple family members with chylomicronemia. J Clin Lipidol. 2021;15(3):431–4. 10.1016/j.jacl.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 60.Perera SD, Wang J, McIntyre AD, Dron JS, Hegele RA. The longitudinal triglyceride phenotype in heterozygotes with LPL pathogenic variants. J Clin Lipidol. 2023;17(1):87–93. 10.1016/j.jacl.2022.11.007 [DOI] [PubMed] [Google Scholar]
- 61.Perera SD, Wang J, McIntyre AD, Hegele RA. Variability of longitudinal triglyceride phenotype in patients heterozygous for pathogenic APOA5 variants. J Clin Lipidol. 2023;17(5):659–65. 10.1016/j.jacl.2023.08.003 [DOI] [PubMed] [Google Scholar]
- 62.De Villers-Lacasse A, Paquette M, Baass A, Bernard S. Non-alcoholic fatty liver disease in patients with chylomicronemia syndromes. J Clin Lipidol. 2023;17(4):475–82. 10.1016/j.jacl.2023.05.096 [DOI] [PubMed] [Google Scholar]
- 63.Paragh G, Nemeth A, Harangi M, Banach M, Fulop P. Causes, clinical findings and therapeutic options in chylomicronemia syndrome, a special form of hypertriglyceridemia. Lipids Health Dis. 2022;21(1):21. 10.1186/s12944-022-01631-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldberg RB, Chait A. A comprehensive update on the chylomicronemia syndrome. Front Endocrinol (Lausanne). 2020;11: 593931. 10.3389/fendo.2020.593931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrera Echegoyen FX, Szeto A, Mendez AJ, Garg R, Goldberg RB. The nature and characteristics of hypertriglyceridemia in a large cohort with type 2 diabetes. J Diabetes Complications. 2023;37(2): 108387. 10.1016/j.jdiacomp.2022.108387 [DOI] [PubMed] [Google Scholar]
- 66.Yotsapon T, Surat K, Veekij V, Kewalin W, Soontaree N, Sirinate K, Thep H. Recurrent hypertriglyceridemia-induced pancreatitis due to multifactorial chylomicronemia syndrome in a patient with ketosis-prone diabetes mellitus. Clin Med Insights Case Rep. 2022;15:11795476221119444. 10.1177/11795476221119445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangeshkar S, Nazarenko N, Varrias D, Spanos M, Borkowski P, Alhuarrat MAD, et al. A case of type V hyperlipoproteinemia resistant to insulin treatment. Cureus. 2023;15(7): e41424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SY, Sheth CA. Eruptive xanthoma associated with severe hypertriglyceridemia and poorly controlled type 1 diabetes mellitus. J Community Hosp Intern Med Perspect. 2019;9(4):344–6. 10.1080/20009666.2019.1650591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adiels M, Taskinen MR, Bjornson E, Andersson L, Matikainen N, Soderlund S, et al. Role of apolipoprotein C-III overproduction in diabetic dyslipidaemia. Diabetes Obes Metab. 2019;21(8):1861–70. 10.1111/dom.13744 [DOI] [PubMed] [Google Scholar]
- 70.Giammanco A, Spina R, Cefalu AB, Averna M. APOC-III: a gatekeeper in controlling triglyceride metabolism. Curr Atheroscler Rep. 2023;25(3):67–76. 10.1007/s11883-023-01080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Rocha NA, McCullough PA. Contribution of ApoCIII to diabetic dyslipidemia and treatment with volanesorsen. Rev Cardiovasc Med. 2018;19(1):13–9. 10.31083/j.rcm.2018.01.890 [DOI] [PubMed] [Google Scholar]
- 72.Fredrickson DS. An international classification of hyperlipidemias and hyperlipoproteinemias. Ann Intern Med. 1971;75(3):471–2. 10.7326/0003-4819-75-3-471 [DOI] [PubMed] [Google Scholar]
- 73.Hopkins PN, Wu LL, Hunt SC, Brinton EA. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J Am Coll Cardiol. 2005;45(7):1003–12. 10.1016/j.jacc.2004.11.062 [DOI] [PubMed] [Google Scholar]
- 74.Abondio P, Sazzini M, Garagnani P, Boattini A, Monti D, Franceschi C, et al. The genetic variability of APOE in different human populations and its implications for longevity. Genes (Basel). 2019;10(3):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–11. 10.1001/jama.298.11.1300 [DOI] [PubMed] [Google Scholar]
- 76.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50 suppl(Suppl):S183–8. 10.1194/jlr.R800069-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252(5014):1817–22. 10.1126/science.2063194 [DOI] [PubMed] [Google Scholar]
- 78.Koopal C, van der Graaf Y, Asselbergs FW, Westerink J, Visseren FL, group Ss. Influence of APOE-2 genotype on the relation between adiposity and plasma lipid levels in patients with vascular disease. Int J Obes (Lond). 2015;39(2):265–9. 10.1038/ijo.2014.105 [DOI] [PubMed] [Google Scholar]
- 79.Sniderman A, Tremblay A, Bergeron J, Gagne C, Couture P. Diagnosis of type III hyperlipoproteinemia from plasma total cholesterol, triglyceride, and apolipoprotein B. J Clin Lipidol. 2007;1(4):256–63. 10.1016/j.jacl.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 80.Koopal C, Retterstol K, Sjouke B, Hovingh GK, Ros E, de Graaf J, et al. Vascular risk factors, vascular disease, lipids and lipid targets in patients with familial dysbetalipoproteinemia: a European cross-sectional study. Atherosclerosis. 2015;240(1):90–7. 10.1016/j.atherosclerosis.2015.02.046 [DOI] [PubMed] [Google Scholar]
- 81.Fredrickson DS, Morganroth J, Levy RI. Type III hyperlipoproteinemia: an analysis of two contemporary definitions. Ann Intern Med. 1975;82(2):150–7. 10.7326/0003-4819-82-2-150 [DOI] [PubMed] [Google Scholar]
- 82.Blom DJ, O’Neill FH, Marais AD. Screening for dysbetalipoproteinemia by plasma cholesterol and apolipoprotein B concentrations. Clin Chem. 2005;51(5):904–7. 10.1373/clinchem.2004.047001 [DOI] [PubMed] [Google Scholar]
- 83.Sniderman AD, de Graaf J, Thanassoulis G, Tremblay AJ, Martin SS, Couture P. The spectrum of type III hyperlipoproteinemia. J Clin Lipidol. 2018;12(6):1383–9. 10.1016/j.jacl.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 84.Boot CS, Middling E, Allen J, Neely RDG. Evaluation of the non-HDL cholesterol to apolipoprotein B ratio as a screening test for dysbetalipoproteinemia. Clin Chem. 2019;65(2):313–20. 10.1373/clinchem.2018.292425 [DOI] [PubMed] [Google Scholar]
- 85.Nakajima K, Daimon M, Kamiyama K, Takanashi K, Suzuki Y, Watanabe M, et al. Serum remnant lipoprotein cholesterol/triglyceride ratio as an index for screening familial type III hyperlipidaemia. Ann Clin Biochem. 2007;44(Pt 4):353–9. 10.1258/000456307780945787 [DOI] [PubMed] [Google Scholar]
- 86.Sampson M, Wolska A, Meeusen JW, Donato LJ, Jaffe AS, Remaley AT. Identification of dysbetalipoproteinemia by an enhanced Sampson-NIH equation for very low-density lipoprotein-cholesterol. Front Genet. 2022;13: 935257. 10.3389/fgene.2022.935257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satny M, Todorovova V, Altschmiedova T, Hubacek JA, Dlouha L, Lanska V, et al. Genetic risk score in patients with the APOE2/E2 genotype as a predictor of familial dysbetalipoproteinemia. J Clin Lipidol. 2024;18(2):e230–7. 10.1016/j.jacl.2023.11.010 [DOI] [PubMed] [Google Scholar]
- 88.Heidemann BE, Marais AD, Mulder MT, Visseren FLJ, Roeters van Lennep JE, Stroes ESG, et al. Composition and distribution of lipoproteins after evolocumab in familial dysbetalipoproteinemia: A randomized controlled trial. J Clin Lipidol. 2023;17(5):666–76. 10.1016/j.jacl.2023.07.004 [DOI] [PubMed] [Google Scholar]
- 89.Heidemann BE, Koopal C, Roeters van Lennep JE, Stroes ES, Riksen NP, Mulder MT, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol measurement in Familial Dysbetalipoproteinemia. Clin Chim Acta. 2023;539:114–21. 10.1016/j.cca.2022.11.035 [DOI] [PubMed] [Google Scholar]
- 90.Smelt AH, de Beer F. Apolipoprotein E and familial dysbetalipoproteinemia: clinical, biochemical, and genetic aspects. Semin Vasc Med. 2004;4(3):249–57. 10.1055/s-2004-861492 [DOI] [PubMed] [Google Scholar]
- 91.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–46. 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kronenberg F, Mora S, Stroes ESG. Consensus and guidelines on lipoprotein(a)—seeing the forest through the trees. Curr Opin Lipidol. 2022;33(6):342–52. 10.1097/MOL.0000000000000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–60. 10.1161/ATV.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatia HS, Becker RC, Leibundgut G, Patel M, Lacaze P, Tonkin A, et al. Lipoprotein(a), platelet function and cardiovascular disease. Nat Rev Cardiol. 2024;21(5):299–311. 10.1038/s41569-023-00947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53. 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kamstrup PR. Lipoprotein(a) and ischemic heart disease–a causal association? A review Atherosclerosis. 2010;211(1):15–23. 10.1016/j.atherosclerosis.2009.12.036 [DOI] [PubMed] [Google Scholar]
- 97.Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56(8):1252–60. 10.1373/clinchem.2010.146779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74(24):2982–94. 10.1016/j.jacc.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 99.Authors/Task Force M, Guidelines ESCCfP, Societies ESCNC. ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;2019(290):140–205. [DOI] [PubMed] [Google Scholar]
- 100.Drexel H, Tamargo J, Kaski JC, Lewis BS, Saely CH, Fraunberger P, et al. Triglycerides revisited: is hypertriglyceridaemia a necessary therapeutic target in cardiovascular disease? Eur Heart J Cardiovasc Pharmacother. 2023;9(6):570–82. 10.1093/ehjcvp/pvad044 [DOI] [PubMed] [Google Scholar]
- 101.Kanter JE, Shao B, Kramer F, Barnhart S, Shimizu-Albergine M, Vaisar T, et al. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J Clin Invest. 2019;129(10):4165–79. 10.1172/JCI127308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381(6):531–42. 10.1056/NEJMoa1715944 [DOI] [PubMed] [Google Scholar]
- 103.Oral EA, Garg A, Tami J, Huang EA, O’Dea LSL, Schmidt H, et al. Assessment of efficacy and safety of volanesorsen for treatment of metabolic complications in patients with familial partial lipodystrophy: results of the BROADEN study: Volanesorsen in FPLD ;The BROADEN Study. J Clin Lipidol. 2022;16(6):833–49. 10.1016/j.jacl.2022.08.008 [DOI] [PubMed] [Google Scholar]
- 104.Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, et al. Antisense-mediated lowering of plasma apolipoprotein c-iii by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39(8):1408–15. 10.2337/dc16-0126 [DOI] [PubMed] [Google Scholar]
- 105.Jones A, Peers K, Wierzbicki AS, Ramachandran R, Mansfield M, Dawson C, et al. Long-term effects of volanesorsen on triglycerides and pancreatitis in patients with familial chylomicronaemia syndrome (FCS) in the UK Early Access to Medicines Scheme (EAMS). Atherosclerosis. 2023;375:67–74. 10.1016/j.atherosclerosis.2023.05.008 [DOI] [PubMed] [Google Scholar]
- 106.Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(5):264–75. 10.1016/S2213-8587(21)00046-2 [DOI] [PubMed] [Google Scholar]
- 107.Bergmark BA, Marston NA, Prohaska TA, Alexander VJ, Zimerman A, Moura FA, et al. Olezarsen for hypertriglyceridemia in patients at high cardiovascular risk. N Engl J Med. 2024;390(19):1770–80. 10.1056/NEJMoa2402309 [DOI] [PubMed] [Google Scholar]
- 108.Ballantyne CM, Vasas S, Azizad M, Clifton P, Rosenson RS, Chang T, et al. Plozasiran, an RNA interference agent targeting APOC3, for mixed hyperlipidemia. N Engl J Med. 2024. [DOI] [PubMed]
- 109.Gaudet D, Pall D, Watts GF, Nicholls SJ, Rosenson RS, Modesto K, et al. Plozasiran (ARO-APOC3) for severe hypertriglyceridemia: the SHASTA-2 Randomized Clinical Trial. JAMA Cardiol. 2024. [DOI] [PMC free article] [PubMed]
- 110.Rosenson RS, Gaudet D, Hegele RA, Ballantyne CM, Nicholls SJ, Lucas KJ, et al. Zodasiran, an RNAi therapeutic targeting ANGPTL3, for mixed hyperlipidemia. N Engl J Med. 2024. [DOI] [PubMed]
- 111.Ruotolo DGMGXSGMJKLOBTBG. A first-in-human single ascending dose study of a monoclonal antibody against the ANGPTL3/8 complex in subjects with mixed hyperlipidemia. Atherosclerosis. 2022;355:E12. 10.1016/j.atherosclerosis.2022.06.034 [DOI] [Google Scholar]
- 112.Ray KK, Haq I, Bilitou A, Manu MC, Burden A, Aguiar C, et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023;29: 100624. 10.1016/j.lanepe.2023.100624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. 10.1056/NEJMoa1913805 [DOI] [PubMed] [Google Scholar]
- 115.Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20. 10.1056/NEJMoa2004215 [DOI] [PubMed] [Google Scholar]
- 116.Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19. 10.1056/NEJMoa2031049 [DOI] [PubMed] [Google Scholar]
- 117.Witztum JL, Gaudet D, Arca M, Jones A, Soran H, Gouni-Berthold I, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome: long-term efficacy and safety data from patients in an open-label extension trial. J Clin Lipidol. 2023;17(3):342–55. 10.1016/j.jacl.2023.03.007 [DOI] [PubMed] [Google Scholar]
- 118.Stroes ESG, Alexander VJ, Karwatowska-Prokopczuk E, Hegele RA, Arca M, Ballantyne CM, et al. Olezarsen, acute pancreatitis, and familial chylomicronemia syndrome. N Engl J Med. 2024;390(19):1781–92. 10.1056/NEJMoa2400201 [DOI] [PubMed] [Google Scholar]
- 119.Chebli J, Larouche M, Gaudet D. APOC3 siRNA and ASO therapy for dyslipidemia. Curr Opin Endocrinol Diabetes Obes. 2024;31(2):70–7. 10.1097/MED.0000000000000857 [DOI] [PubMed] [Google Scholar]
- 120.Gaudet D, Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, et al. RNA interference therapy targeting apolipoprotein C-III in hypertriglyceridemia. NEJM Evid. 2023;2(12):EVIDoa2200325. 10.1056/EVIDoa2200325 [DOI] [PubMed] [Google Scholar]
- 121.Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, et al. Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial. Nat Med. 2023;29(3):729–37. 10.1038/s41591-023-02222-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bailey NN, Peterson SJ, Parikh MA, Jackson KA, Frishman WH. Pegozafermin is a potential master therapeutic regulator in metabolic disorders: a review. Cardiol Rev. 2023. [DOI] [PubMed]
- 123.Rosenstock M, Tseng L, Pierce A, Offman E, Chen CY, Charlton RW, et al. The novel GlycoPEGylated FGF21 analog pegozafermin activates human FGF receptors and improves metabolic and liver outcomes in diabetic monkeys and healthy human volunteers. J Pharmacol Exp Ther. 2023;387(2):204–13. 10.1124/jpet.123.001618 [DOI] [PubMed] [Google Scholar]
- 124.Bhatt DL, Bays HE, Miller M, Cain JE 3rd, Wasilewska K, Andrawis NS, et al. The FGF21 analog pegozafermin in severe hypertriglyceridemia: a randomized phase 2 trial. Nat Med. 2023;29(7):1782–92. 10.1038/s41591-023-02427-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heidemann BE, Koopal C, Roeters van Lennep JE, Stroes ESG, Riksen NP, Mulder MT, et al. Effect of evolocumab on fasting and post fat load lipids and lipoproteins in familial dysbetalipoproteinemia. J Clin Lipidol. 2023;17(1):112–23. 10.1016/j.jacl.2022.10.006 [DOI] [PubMed] [Google Scholar]
- 126.Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jorgensen T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490–8. 10.1093/eurheartj/ehx166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–8. 10.2337/dc19-0274 [DOI] [PubMed] [Google Scholar]
- 128.Saeed A, Sun W, Agarwala A, Virani SS, Nambi V, Coresh J, et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: the Atherosclerosis Risk in Communities study. Atherosclerosis. 2019;282:52–6. 10.1016/j.atherosclerosis.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moosaie F, Firouzabadi FD, Abouhamzeh K, Esteghamati S, Meysamie A, Rabizadeh S, et al. Lp(a) and Apo-lipoproteins as predictors for micro- and macrovascular complications of diabetes: A case-cohort study. Nutr Metab Cardiovasc Dis. 2020;30(10):1723–31. 10.1016/j.numecd.2020.05.011 [DOI] [PubMed] [Google Scholar]