Abstract
The present study aims to investigate the current trends in replacing conventional preservatives with multifunctional ingredients with antimicrobial properties for preservation of cosmetics for infants or sensitive population, to decrease their potential for contact dermatitis. We first reviewed the labels of cosmetics purchased from the Chinese market for conventional preservatives and multifunctional ingredients with antimicrobial properties, of which the actual contents were further quantified by chromatographic methods. We identified 7 traditional preservatives (phenoxyethanol, benzoic acid (salts), methylparaben, benzyl alcohol, sorbic acid (salts), propylparaben, and methylisothiazolinone), and 11 alternative ingredients with antimicrobial activities (ethylhexylglycerin, butylene glycol, caprylyl glycol, propylene glycol, 1,2-hexanediol, p-anisic acid, hydroxyacetophenone, pentylene glycol, decylene glycol, caprylhydroxamic acid, and aminomethyl propanol) in descending order of prevalence. The contents of all identified preservatives and ingredients were either below regulatory limits or in the range that is generally regarded to be safe. Further challenge with microorganisms indicated irrespective of the composition of preservation systems, product preservation could be compromised under test conditions. We conclude that multifunctional ingredients with antimicrobial properties in cosmetics have the potential to completely replace or significantly reduce the use of traditional preservatives while retaining comparative preservative efficacy. Future attentions may need to be shifted to the safety of those multifunctional ingredients with antimicrobial properties.
Keywords: Cosmetics, Preservatives, Antimicrobial properties, Contact dermatitis, Allergen
Subject terms: Public health, Risk factors
Introduction
Preservatives are essential ingredients that have long been used for protecting cosmetic products from microbial contamination. The Annex V to the European Cosmetics Regulation (EC) No 1223/2009 lists 57 preservatives that are allowed to be used in cosmetics marketed in the European Union (EU)1. This regulation has also outlined limitations for each preservative regarding applicable product types, maximum allowed concentrations, and wording of conditions of use and warnings on product labels. Those legislated preservatives are expected to be safe when used within certain conditions set forth by the regulation. However, conventional preservatives are typically electrophiles with low molecular weight, which potentiates them to interact with and cause sensitization in skin2. In fact, preservatives have constituted one of the most common causes of allergic contact dermatitis (ACD) attributed to the use of cosmetic products3–5. Thus, most preservatives currently in use in cosmetic formulations have been suggested in the American Contact Dermatitis Society (ACDS) Core Allergen Series for an extended patch testing for screening of contact allergens6–8. Therefore, to reduce the incidence of ACD, the use of single high concentration conventional preservative has been gradually replaced with combinations of various low concentration preservatives without compromising the preservative efficacy. In addition to optimize the use of conventional preservatives, cosmetic industry has also been repurposing artificially synthesized multifunctional ingredients with antimicrobial properties, which are not currently legislated as preservatives, for preservation use. For example, glycols have been increasingly used as antimicrobial agents in addition to its role as humectants based on their emulsifier-like structure, which can disrupt the cellular integrity of microorganisms9. Moreover, several botanical-derived ingredients, that are mainly added to cosmetic preparations as skin-conditioning agents, have shown robust antimicrobial action. Thus, they have been substantially employed alone or in combination with conventional preservatives to protect cosmetic products9,10. The selection of natural compounds has the potential to not only decrease or eliminate use of conventional preservatives but also improve the dermo-cosmetic properties with lower skin irritation and/or contact sensitization potential, though some naturally derived ingredients have been reported to cause allergic contact dermatitis11. Moreover, from a sustainability point of view, cosmetic industry is also in urgent search for natural, organic, and eco-friendly ingredients12. Nowadays, those kinds of cosmetics are increasingly accepted by consumers when labeled or marketed with “green”, “natural”, “paraben-free”, or “preservative-free” claims, because it raises an expectation from consumers that cosmetics marketed and labeled this way are safe to use without unintended adverse effects.
Sensitive skin is a condition of skin hyper-sensitivity characterized by subjective sensory perceptions, including stinging, itching, burning, and a tight sensation, which can be triggered or aggravated by application of cosmetic products13,14. Previous studies have demonstrated that cosmetics induced symptoms occur more commonly in women with sensitive skin than in women with non-sensitive skin15. This increased sensitivity to cosmetics may be correlated with a disrupted barrier function, which allows higher transcutaneous penetration of chemicals like preservatives due to a thinner stratum corneum and a smaller corneocyte area in the sensitive population16. Similarly, infant skin also consists of reduced thickness of stratum corneum and smaller corneocytes compared to those of adult skin17, which also facilitates transdermal absorption of environmental allergens. However, investigative studies have suggested that nearly 90% of cosmetics for babies and children contains at least one contact allergen, which may pose major concern for ACD in babies and children18–20. Contact allergens identified in these cosmetics include fragrances, antioxidants, antiseptics, emollients, emulsifiers, and others, among which preservatives are the most prevalent contact allergens in cosmetics for babies and children18–20. Furthermore, special consideration has been suggested on preservation of cosmetics for the nappy area of infant skin, which is susceptible to contact dermatitis and impaired barrier functions due to the higher water content and pH, and potential contact with feces and urine21. Thus, careful selection of safer preservatives is widely recognized to be more important for cosmetic products intended for infants and sensitive population than cosmetics for general population. However, there is still a knowledge gap between the claimed no use or reduced use of preservatives by manufactures and the actual content of preservatives and/or multifunctional ingredients with antimicrobial properties in cosmetics intended for infants and sensitive population.
In the present study, we seek to investigate the prevalence and to measure the contents of preservatives and multifunctional ingredients with antimicrobial properties in cosmetics intended for infants and sensitive population. We also intend to evaluate the preservative efficacy of selected systems via the preservative challenge test. The purpose is to gain knowledge on the trend in selection of preservatives and multifunctional ingredients with antimicrobial properties, thus to provide references for establishment of safer preservative systems for cosmetics not only intended for infants and sensitive population but also for general population.
Results
General information
General information of the collected sample products in the current study was summarized in Table 1. The sample products could be generally classified into skin care products and general hygiene products, respectively, based on their purposes. Out of the total 83 products collected in our study, 70 (84.3%) were products for infants and 13 (15.7%) were products claimed to be suitable for sensitive skin. The 70 infant products consisted of 28 moisturizers (40.0%), 6 nappy creams (8.6%), 16 sunscreen creams (22.9%), 6 anti-itch gel (8.6%), 4 shampoos (5.7%), 5 body washes (7.1%), and 5 2-in-1 hair and body washes (7.1%). Regarding products for sensitive skin, 12 out of 13 (92.3%) products were face creams and the other one (7.7%) was sunscreen cream.
Table 1.
Sample information in the current study.
| Category | Product Type | N | n (%) | |
|---|---|---|---|---|
| Infant | Sensitive skin | |||
| Skin care† | Moisturizer | 40 | 28 (40.0) | 12 (92.3) |
| Nappy Cream | 6 | 6 (8.6) | / | |
| Sunscreen | 17 | 16 (22.9) | 1 (7.7) | |
| Anti-itch gel | 6 | 6 (8.6) | / | |
| General hygiene‡ | Shampoo | 4 | 4 (5.7) | / |
| Body Wash | 5 | 5 (7.1) | ||
| 2-in-1 | 5 | 5 (7.1) | ||
| Total | 83 | 70 (84.3) | 13 (15.7) | |
†Leave-on products; ‡Rinse-off products.
Prevalence and contents of preservatives and multifunctional ingredients with antimicrobial properties in infant products
We have previously published this part of results in a letter to the Editor of Dermatitis22. To briefly summarize here, phenoxyethanol and benzoic acid (salts) were the top 2 most common conventional preservatives. Methylparaben, benzyl alcohol, sorbic acid (salts), propylparaben, and methylisothiazolinone (MIT) were identified in 14.3%, 12.9%, 8.6%, 7.1%, and 2.9% of infant products, respectively. In case of multifunctional ingredients with antimicrobial properties, ethylhexylglycerin was the most frequently identified, followed by p-anisic acid, hydroxyacetophenone, caprylhydroxamic acid, and aminomethyl propanol. As for glycols, butylene glycol (27.1%) was the most extensively used, followed by caprylyl glycol (21.4%), propylene glycol (17.1%) and 1,2-hexanediol (15.7%). Pentylene glycol (2.9%) and decylene glycol (2.9%) were the least frequently identified on product labels.
The measured contents of phenoxyethanol, benzoic acid (salts), methylparaben, benzyl alcohol, sorbic acid (salts), propylparaben, and MIT ranged between 0.01–0.99%, 0.037–0.65%, 0.014–0.27%, 0.33–0.65%, 0.06–0.52%, 0.045–0.096%, and 0.0066–0.011% (n = 2), respectively. The corresponding mean and median contents were 0.44% and 0.46% for phenoxyethanol, 0.34% and 0.30% for benzoic acid (salts), 0.16% and 0.18% for methylparaben, 0.51% and 0.56% for benzyl alcohol, 0.22% and 0.13% for sorbic acid (salts), and 0.07% and 0.07% for propylparaben, respectively. The content of ethylhexylglycerin was in the range of 0.0079–0.55%, with a mean and median content of 0.18% and 0.096%, respectively. p-Anisic acid, hydroxyacetophenone, and caprylhydroxamic acid ranged between 0.042–0.16%, 0.028–0.51%, and 0.2% (n = 1), respectively, whose mean and median contents were 0.095% and 0.093% for p-anisic acid, and 0.39% and 049% for hydroxyacetophenone, respectively. As for glycols, the contents were 0.039–8.50% for butylene glycol, 0.026–0.56% for caprylyl glycol, 0.097–1.20% for 1,2-hexanediol, 0.0092–6.8% for propylene glycol, 0.23% (n = 1) for pentylene glycol, and 0.30% (n = 1) for decylene glycol, respectively. And their corresponding mean and median contents were 4.44% and 4.65% for butylene glycol, 0.25% and 0.22% for caprylyl glycol, 0.55% and 0.51% for 1,2-hexanediol, and 1.87% and 0.36% for propylene glycol, respectively.
Prevalence and contents of preservatives multifunctional ingredients with antimicrobial properties in products for sensitive skin
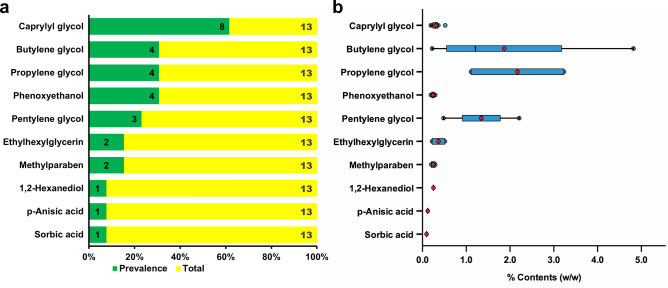
Converse to those identified in the infant products, the number of traditional preservatives in products for sensitive skin was generally decreased while the prevalence of glycols was increased. Phenoxyethanol, methylparaben, and sorbic acid were the only traditional preservatives, with 30.8% (4/13), 15.4% (2/13), and 7.7% (1/13) of products containing phenoxyethanol, methylparaben, and sorbic acid, respectively. On the other hand, caprylyl glycol (61.5%, 8/13), followed by propylene glycol (30.8%, 4/13) and butylene glycol (30.8%, 4/13), was the most common glycol identified in products for sensitive skin. Pentylene glycol, ethylhexylglycerin, and 1,2-hexanediol were found in 23.1% (3/13), 15.4% (2/13), and 7.7% (1/13) of the products, respectively (Fig. 1a).
Figure 1.
The prevalence (a) and contents (b) of traditional preservatives and ingredients with antimicrobial functions in cosmetics for sensitive skin. (a) Information on preservatives and ingredients with antimicrobial functions was collected from product labels. Green column and the number inside the column indicate the prevalence of the correspondent preservative or ingredient in infant products. Yellow column and the number inside the column indicate the total number of products. (b) Generally, traditional preservatives were detected by High-Performance Liquid Chromatography, while polyols were detected by Gas Chromatography. Data were expressed as percentage of product weight (%, w/w). The horizontal line inside each box represents the mean value of contents. The red dot represents the median content of each chemical.
All the contents of traditional preservatives labelled on the products for sensitive skin were above LOQ. The contents of phenoxyethanol, sorbic acid, and methylparaben ranged between 0.19–0.29% (n = 4), 0.09% (n = 1), and 0.2–0.29% (n = 2), respectively. Both the mean and median contents of phenoxyethanol were 0.24%. p-Anisic acid was labelled and detected in only one product with a content of 0.12%. In the case of polyols, 2 out of the 4 products labelled with propylene glycol were below LOQ. The contents of propylene glycol, butylene glycol, pentylene glycol, 1,2-hexanediol, caprylyl glycol, and ethylhexylglycerin were 1.11–3.23% (n = 2), 0.22–4.82% (n = 4), 0.48–2.21% (n = 3), 0.25% (n = 1), 0.19–0.52% (n = 8), and 0.22–0.51% (n = 2), respectively. The mean contents were 1.87%, 1.34%, and 0.31% for butylene glycol, pentylene glycol, and caprylyl glycol, respectively, of which the median contents were 1.21%, 1.34%, and 0.30%, respectively (Fig. 1b).
Performance of selected preservative systems in the preservative challenge test
A total of 14 products (11 for infants and 3 for sensitive skin) were tested in our study, including 4 O/W emulsions, 4 W/O emulsions, 1 water, and 5 washes. The pH ranged between 4.31 to 6.19 (median 5.195). As shown in Table 2, of the 14 preservative systems, 8 consisted of traditional preservatives only, while the other 6 included at least 1 multifunctional ingredient with antimicrobial properties. Of the 8 systems consisted of traditional preservatives only, System No. 2 (0.084% sodium benzoate and 0.079% potassium sorbate) failed to meet both the bacteria and fungi criteria with log reductions at day 7 to be 1.04 and 0.91, respectively. Regarding the 6 systems containing multifunctional ingredients with antimicrobial properties, systems No. 1(1.23% 1,2-hexanediol) 3 (0.51% phenoxyethanol, 0.11% caprylyl glycol, and 0.05% ethylhexylglycerin), and 13 (1.34% pentylene glycol, 0.28% caprylyl glycol, and 0.19% phenoxyethanol) failed to meet the fungi criteria with log reduction at day 7 to be 0.64, 1.80, and 1.87, respectively. All the other systems met the criteria for both bacteria and fungi, even one consisted of no traditional preservatives at all.
Table 2.
Preservative efficacy of preservative systems.
| No | System composition (% content, w/w) | Log reduction at day 7 | Day 28 | Passed (Yes/No) | |
|---|---|---|---|---|---|
| Bacteria | Fungi | ||||
| 1 | Pentylene glycol (< LQD); 1,2-Hexanediol (1.23) | > 5.75 | 0.64 | NI | No |
| 2 | Sodium benzoate (0.084); Potassium sorbate (0.079) | 1.04 | 0.91 | NI | No |
| 3 | Phenoxyethanol (0.51); Caprylyl glycol (0.11); Ethylhexylglycerin (0.05) | > 5.75 | 1.80 | NI | No |
| 4 | Phenoxyethanol (0.48) | > 5.75 | > 4.64 | NI | Yes |
| 5 | Sodium citrate (< LQD); Benzyl alcohol (0.65); Phenoxyethanol (0.46) | > 5.75 | 2.09 | NI | Yes |
| 6 | Benzyl alcohol (0.59) | > 5.75 | > 4.64 | NI | Yes |
| 7 | Sodium benzoate (0.52); p-Anisic acid (0.12) | > 5.75 | > 4.64 | NI | Yes |
| 8 | Sodium benzoate (0.29); Phenoxyethanol (0.51) | > 5.75 | > 4.64 | NI | Yes |
| 9 | Sodium benzoate (0.59); Benzyl alcohol (< LQD) | > 5.75 | > 4.64 | NI | Yes |
| 10 | Sodium benzoate (0.53); Phenoxyethanol (< LQD) | > 5.75 | > 4.64 | NI | Yes |
| 11 | Sodium benzoate (0.11); Phenoxyethanol (0.54); Benzyl alcohol (0.43) | > 5.75 | > 4.64 | NI | Yes |
| 12 | Butylene glycol (4.28); Propylene glycol (3.23); p-Anisic acid (0.12); Caprylyl glycol (0.19) | > 5.75 | 2.74 | NI | Yes |
| 13 | Pentylene glycol (1.34); Caprylyl glycol (0.28); Phenoxyethanol (0.19) | > 5.75 | 1.87 | NI | No |
| 14 | Butylene glycol (1.54); Methylparaben (0.29) | > 5.75 | > 4.64 | NI | Yes |
NI, no increase from previous counting.
Discussion
The present study investigated the trend in use and measured the contents of conventional preservatives and multifunctional ingredients with antimicrobial properties that have not been legislated as preservatives in cosmetics intended for infants and sensitive population. Our data indicates that multifunctional ingredients with antimicrobial properties like glycols are more frequently used than conventional preservatives in products for sensitive population, while the latter still dominate in infant cosmetics. Phenoxyethanol and caprylyl glycol were the top selected conventional preservative and multifunctional ingredient with antimicrobial properties, respectively, in products for both infants and sensitive skin. The plant derived p-anisic acid was rarely selected but showed sufficient efficacy when used in combination with other preservatives. Ethylhexylglycerin was substantially used in combination with traditional preservatives and/or glycols due to its preservative boosting effects. At last, the preservative challenge test showed that failure to pass the test could occur irrespective of the preservative system composition, suggesting that other factors may also have an impact in deciding preservative efficacy. In summary, our study provides insights into selection of preservative systems for cosmetics intended for consumer population that have special needs for mildness.
In the present study, though traditional preservatives were still prevalent especially in infant cosmetics, the dominant choices have become preservatives with relatively low allergenic potential like phenoxyethanol and naturally derived chemicals including organic acids (i.e., benzoic acid and sorbic acid) and aromatic alcohols (i.e., benzyl alcohol). Other traditional preservatives that have been well-recognized to cause sensitization like formaldehyde and formaldehyde releasers, isothiazolinones, and iodopropynyl butylcarbamate were not identified in any of the products for infants or sensitive skin. To our surprise, we still found products containing parabens, which, despite relatively low sensitizing potential, have been gradually abandoned by the industry due to their possible estrogenic activities23,24. It was within our prediction that phenoxyethanol has become the most frequently used traditional preservative considering its well-known safety profile. The European Scientific Committee on Consumer Safety (SCCS) has published an opinion suggesting that phenoxyethanol is safe for all consumers, including children of 0–3 years of age, when used as a preservative in cosmetic products at a concentration lower than 1%25. Therefore, we suggest that phenoxyethanol should be granted the highest priority when considering to use traditional preservatives in cosmetics. However, it is worth noting that even for preservatives with low allergenic potential like phenoxyethanol, organic acids, and aromatic alcohols, the concentration used in cosmetics need to be strictly controlled. One percent of phenoxyethanol, 5% benzoic acid, 10% benzyl alcohol, 5% sodium benzoate, and 2% sorbic acid have all been patch tested positive for allergenicity and are listed in the ACDS Core Allergen Series26.
Glycols are a group of chemicals with amphiphilic character and medium molecular size playing versatile roles including as humectants and viscosity modulators27. The antimicrobial properties depend on their emulsifier-like structure, which disrupts the cellular integrity and in turn increases the permeability of cell membrane. The antimicrobial potency increases from propylene glycol to caprylyl glycol, but decreases rapidly from there due to limited water solubility of longer chain glycols28,29. Mounting evidence has proven the antimicrobial efficacy of caprylyl glycol when used alone or in combination with other antimicrobial ingredients30–33. This, in addition to their demonstrated safety profiles and multifunctional properties, has considerably driven the use of glycols in cosmetics and personal care products34. However, the viscosity modulating effects of caprylyl glycol have been correlated to the compromise of stability of formulations33. Therefore, blends containing decylene glycol instead of caprylyl glycol have also been developed in case of stability or viscosity issues in emulsions35. In consistent with this trend, we also identified and detected decylene glycol in 2 infant cosmetics. However, no decylene glycol was identified in products for sensitive skin. Regarding the allergenic potential of glycols, while only sparse case reports have been reported for other glycols, studies on propylene glycol have correlated it with both allergic and irritant patch test reactions in a dose-dependent manner36,37. Corresponding to these findings, propylene glycol (30% w/w in aqua) has been listed in the American Contact Dermatitis Society (ACDS) Core Allergen Series and the North American Contact Dermatitis Group (NACDG) series for screening of contact allergens26. Moreover, a newly proposed European cosmetic series for contact allergen has also suggested to include 20% (w/w in aqua) of propylene glycol due to its sensitization prevalence > 0.3%38. Thus, we suggest that the concentration of propylene glycol should be carefully controlled to below 20% (w/w) in cosmetics.
In the present study, ethylhexylglycerin was detected in 23 infant products and 2 products for sensitive skin with concentrations ranged between 0.0079–0.55%, at which when used in combination with phenoxyethanol, glycols, or methylparaben, ethylhexylglycerin has proven to significantly enhance the antimicrobial activity of those preservatives39–42. This preservative boosting action of ethylhexylglycerin has been attributed to its surfactant-like structure, which allows more preservatives to penetrate through cell membrane. Thus, addition of a small amount of ethylhexylglycerin into traditional preservatives has emerged in the modern design of preservative systems for cosmetics. However, even ethylhexylglycerin is generally considered to pose low risks, cases of sensitization have been reported occasionally43. The allergenic potential of 5% (w/w in petroleum) of ethylhexylglycerin has been well recognized that it is listed in the ACDS Core Allergen Series and the NACDG series for screening of contact allergens, and is proposed to be included in the European cosmetic series26,38. Therefore, routine testing of the sensitization potential has been suggested by the Belgian Contact and Environmental Dermatitis Group to its members43. Moreover, ethylhexylglycerin has been suggested to have the possibility to be officially categorized as preservatives by the EU authorities considering its application scenarios43. Thus, we suggest the industry to closely follow the research and regulatory developments in this field and make appropriate changes in the practice of using ethylhexylglycerin accordingly.
Though the use of p-anisic acid was rare, it is worth to point out that it was one of the botanically derived ingredient with antimicrobial property in the present study. p-Anisic acid has shown antioxidant, anti-inflammatory, and anti-tumor functions in addition to preservation of cosmetics44. Acting as an organic acid, its effectiveness has been suggested to be determined by pH, which determines the concentration of formed undissociated acids45. Moreover, the growth of Gram- bacteria is discouraged at pH < 5.5, a limit needed for p-anisic acid to be active46. Technically, p-anisic acid is suggested to be applied in combination with alcohols, such as phenoxyethanol or benzyl alcohol, which complement its weakness against bacteria. In our study, all the preservative systems containing p-anisic acid passed the challenge test, indicating at least in part its effectiveness in preservation of cosmetics. To follow the trend of reducing or replacing traditional preservatives and to claim a ‘green’ preservation, p-anisic acid is progressively studied and employed in the preservation of cosmetics and food sectors39.
At last, it is worth noting that preservation efficacy depends not only on preservatives but also on other factors, including pH, temperature of storage, the water content of the formulation, the presentation of other ingredients, such as proteins and amino-acids, and the possible interactions between preservatives and containers9,10,29,48. Our study demonstrates that even the preservative systems were formulated with solely traditional preservatives, failure to pass the preservative challenge test could still occur, indicating other factors may have impacts on the efficacy. Theoretically, a product may be truly preservative-free if it is completely free of water, which is extremely difficult to achieve. Nonetheless, following some practical recommendations, which are now referred to as the “hurdle” technology, can reduce the risk of microbial contamination to a certain extent and significantly reduce the use of preservatives and multifunctional ingredients with antimicrobial properties, which in turn reduce the potential of inducing ACD.
Methods
Materials
Reference standards used for quantification of phenoxyethanol, sodium benzoate, potassium sorbate, p-anisic acid, methylparaben, propylene glycol, butanediol, 1,2-pentanediol, 1,2-hexanediol, caprylyl glycol, aminomethyl propanol, ethylhexylglycerin, and were purchased from Sigma-Aldrich (Darmstadt, Germany). Methanol and acetonitrile of HPLC grade were obtained from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and were purchased from Sinopharm (Shanghai, China).
Sample cosmetics
Samples were collected mainly from the online market by searching for products with claims of suitable for infants or sensitive skin. The labels of searched products were further reviewed for phrases like “suitable for babies”, “suitable for whole family members”, “suitable for 0–3 years”, “suitable for infants”, and “suitable for sensitive skin”, before they were purchased. A total of 83 cosmetic products that are currently present in mainland China market, consisting of 70 products intended for infants and younger children and 13 skincare products intended for sensitive population, were collected from local or online suppliers. Information on preservatives used in each product was reviewed based on the 53 preservatives for cosmetic products approved by the European Union and Association of Southeast Asian Nations, plus 5 additional preservatives used in the US. Furthermore, information on ingredients with anti-microbial functions was collected based on literature review. Samples were stored at room temperature per instructions before being subjected to analyses. No signs of deterioration of any products were observed during time of investigation, though no chemical investigation of the stability of the preservatives were performed.
High-performance liquid chromatography (HPLC) analysis
The contents (w/w) of 7 traditional preservatives, including phenoxyethanol, benzoic acid and its salts, benzyl alcohol, sorbic acid and its salts, methylparaben, propylparaben, and MIT, and 3 multifunctional ingredients with antimicrobial properties, including p-anisic acid, caprylhydroxamic acid, and hydroxyacetophenone, were measured by HPLC. All of the sample products containing at least one of these substances were subjected to the analysis. One gram of sample was weighed and dissolved in 5 mL of anhydrous ethanol by mixing thoroughly, followed by bathing in 50 °C water for 5 min. The sample solution was then ultrasonically extracted for 20 min and was added with anhydrous ethanol to a final volume of 10 mL after cooling to room temperature. Finally, sample solution was centrifuged at 5000 r/min for 5 min and the supernatant was filtered through a 0.45 μm membrane. The measurement was conducted on an Alliance 2695 HPLC system (Waters, Milford, MA) coupled with a quaternary solvent delivery system, an auto-sampler, and a UV detector. The column used was a ZORBAX SB-C18 (4.6 mm i.d. × 250 mm with 5 μm particle size) (Agilent Technologies, Santa Clara, CA). Isocratic elution was realized by three mobile phases composed of methanol, acetonitrile, and phosphate buffer (pH 3.2 ± 0.1) at a ratio of 30:10:60. The flow rate was kept at 1.0 mL/min and the injection volume of sample was 10 μL. UV absorption was measured at 275 nm. The limit of quantification (LOQ) for chemicals subjected to HPLC were listed as follows: benzyl alcohol, 0.03 mg/mL; sorbic acid/potassium sorbate, 0.002 mg/mL; phenoxyethanol, 0.02 mg/mL; MIT, 0.0006 mg/mL; benzoic acid, 0.005 mg/mL; caprylhydroxamic acid, 0.015 mg/mL; p-anisic acid, 0.006 mg/mL; methylparaben, 0.001 mg/mL; ethylparaben, 0.0025 mg/mL; hydroxyacetophenone, 0.005 mg/mL; propylparaben, 0.0025 mg/mL; butylparaben, 0.003 mg/mL.
Gas chromatography (GC) analysis
The contents (w/w) of polyols including 6 glycols, ethylhexylglycerin, and aminomethyl propanol were measured by gas chromatography. All of the sample products containing at least one of these substances were subjected to the analysis. One gram of sample was weighed and dissolved in water to a final volume of 100 mL. After vortex for 1 min, 10 mL of sample solution was transferred to a 20 mL headspace bottle, which was then added with 1 g of NaCl (baked in a muffle furnace at 550 °C overnight) and sealed with an aluminum cap. Measurements were carried out using an Agilent 6890N gas chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a flame ionization detector and an HP-5 capillary column (0.32 mm i.d. × 0.25 μm × 30 m) (Agilent Technologies, Santa Clara, CA). The oven temperature was started and held at 80 °C for 5 min prior to being increased to 200 °C at a rate of 6 °C/min, followed by being increased to 280 °C at a rate of 20 °C/min. The temperature was kept at 250 °C for both the injector and the detector. The carrier gas was nitrogen with a flow rate of 1.0 mL/min. The split ratio was at 20:1 and the injected volume was set to 1 μL. The quantity of polyols was determined by an internal standard method with n-butyl alcohol and n-decyl alcohol used as reference standards. The LOQ for chemicals subjected to GC were listed as follows: 1,2-propylene glycol, 0.2 mg/mL; butylene glycol, 0.3 mg/mL; pentylene glycol, 0.2 mg/mL; 1,3-propylene glycol, 0.4 mg/mL; 1,2-Hexanediol, 0.2 mg/mL; caprylyl glycol, 0.2 mg/mL; ethylhexylglycerin, 0.2 mg/mL; aminomethyl propanol, 0.2 mg/mL.
Preservative challenge test
The efficacy of selected preservative systems against microbial challenge was evaluated in accordance with the recommendations inspired by the U.S. pharmacopoeia with minor modifications. Briefly, five specific strains of microorganisms were obtained from Shanghai Institute of Materia Medica (Shanghai, China), including 3 strains of bacteria (Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa) and 2 strains of fungi (Aspergillus niger and Candida albicans). Mixtures of 3 strains of bacteria and of 2 strains of fungi were prepared separately, and were inoculated into the required quantity of test products in such a way that the final concentration of the microorganisms in each product was 1 × 106 and 1 × 105 colony-forming units (CFU) per milliliter for bacteria and fungi, respectively. Upon inoculation, samples were mixed thoroughly to ensure homogeneous distribution, and 1 g of the mixed sample was removed immediately and diluted to an appropriate concentration for colony counting in sterile Letheen broth. Those diluted samples were then transferred to petri dishes containing solidified Tryptose soya agar (TSA) or molten Sabouraud dextrose agar (SDA) for enumeration of bacterial and fungal colonies, respectively. For determination of bacterial and fungal colonies, the petri dishes were incubated at 36 ± 1 °C for 48 h and at 25 ± 1 °C for 5–7 days, respectively. The inoculated sample products were evaluated for microbial load every 7 days up to 28 days from the day of inoculation. Product was considered passing the test if the bacterial and fungal loads reduced by 3 and 2 logs within 7 days from day of challenge, respectively, and with no further growth observed by day 28. In the present study, products with weakly acidic pH (4–6.5) and packed in plastic squeeze tubes were subjected to the test, in order to control the potential influences of pH and packaging styles on the preservation efficacy.
Acknowledgements
Dr. Tian Chen was supported by the Shanghai Professional Technical Service Platform Construction Project (grant number 22DZ2292100), the Project of the Professional Committee of Cosmetics Regulatory Research of CSDR (grant number 2022-Z-H-002), National Disease Control and Prevention Administration Talent Training Project for Public Health (2023), and the Key Projects in the Three-Year Plan of Shanghai Municipal Public Health System (2023-2025) (GWVI-4).
Author contributions
T.C.: Conceptualization, Methodology, Investigation, Writing- Original draft preparation. H.C.: Conceptualization, Data curation, Visualization, Writing- Reviewing and Editing, Supervision.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products. Off. J. Eur. Union2009, 59–209 (2009).
- 2.Basketter, D. A. et al. Preservatives and skin sensitization quantitative risk assessment. Dermatitis19, 20–27. 10.2310/6620.2008.07018 (2008). 10.2310/6620.2008.07018 [DOI] [PubMed] [Google Scholar]
- 3.Wetter, D. A. et al. Results of patch testing to personal care product allergens in a standard series and a supplemental cosmetic series: an analysis of 945 patients from the Mayo Clinic Contact Dermatitis Group, 2000–2007. J. Am. Acad. Dermatol.63, 789–798. 10.1016/j.jaad.2009.11.033 (2010). 10.1016/j.jaad.2009.11.033 [DOI] [PubMed] [Google Scholar]
- 4.Gimenez-Arnau, A. M. et al. Contact allergy to preservatives: ESSCA* results with the baseline series, 2009–2012. J. Eur. Acad. Dermatol. Venereol.31, 664–671. 10.1111/jdv.14063 (2017). 10.1111/jdv.14063 [DOI] [PubMed] [Google Scholar]
- 5.Goossens, A. et al. Adverse cutaneous reactions to cosmetic allergens. Contact Dermatitis40, 112–113. 10.1111/j.1600-0536.1999.tb06004.x (1999). 10.1111/j.1600-0536.1999.tb06004.x [DOI] [PubMed] [Google Scholar]
- 6.Schalock, P. C. et al. American contact dermatitis society core allergen series. Dermatitis24, 7–9. 10.1097/DER.0b013e318281d87b (2013). 10.1097/DER.0b013e318281d87b [DOI] [PubMed] [Google Scholar]
- 7.Schalock, P. C. et al. American contact dermatitis society core allergen series: 2017 update. Dermatitis28, 141–143. 10.1097/DER.0000000000000261 (2017). 10.1097/DER.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 8.Schalock, P. C. et al. American contact dermatitis society core allergen series: 2020 update. Dermatitis31, 279–282. 10.1097/DER.0000000000000621 (2020). 10.1097/DER.0000000000000621 [DOI] [PubMed] [Google Scholar]
- 9.Halla, N. et al. Cosmetics preservation: A review on present strategies. Molecules23, 1. 10.3390/molecules23071571 (2018). 10.3390/molecules23071571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman, A. Antimicrobial ingredients as preservative booster and components of self-preserving cosmetic products. Curr. Microbiol.76, 744–754. 10.1007/s00284-018-1492-2 (2019). 10.1007/s00284-018-1492-2 [DOI] [PubMed] [Google Scholar]
- 11.Papageorgiou, S., Varvaresou, A., Tsirivas, E. & Demetzos, C. New alternatives to cosmetics preservation. J. Cosmet. Sci.61, 107–123 (2010). [PubMed] [Google Scholar]
- 12.Bom, S. et al. Replacing synthetic ingredients by sustainable natural alternatives: A case study using topical O/W emulsions. Molecules25, 4887 (2020). 10.3390/molecules25214887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misery, L., Loser, K. & Stander, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol.30(Suppl 1), 2–8. 10.1111/jdv.13532 (2016). 10.1111/jdv.13532 [DOI] [PubMed] [Google Scholar]
- 14.Berardesca, E., Farage, M. & Maibach, H. Sensitive skin: an overview. Int. J. Cosmet. Sci.35, 2–8. 10.1111/j.1468-2494.2012.00754.x (2013). 10.1111/j.1468-2494.2012.00754.x [DOI] [PubMed] [Google Scholar]
- 15.Willis, C. M. et al. Sensitive skin: an epidemiological study. Br. J. Dermatol.145, 258–263. 10.1046/j.1365-2133.2001.04343.x (2001). 10.1046/j.1365-2133.2001.04343.x [DOI] [PubMed] [Google Scholar]
- 16.Berardesca, E., Cespa, M., Farinelli, N., Rabbiosi, G. & Maibach, H. In vivo transcutaneous penetration of nicotinates and sensitive skin. Contact Dermatitis25, 35–38. 10.1111/j.1600-0536.1991.tb01770.x (1991). 10.1111/j.1600-0536.1991.tb01770.x [DOI] [PubMed] [Google Scholar]
- 17.Ludriksone, L., Garcia Bartels, N., Kanti, V., Blume-Peytavi, U. & Kottner, J. Skin barrier function in infancy: A systematic review. Arch Dermatol Res306, 591–599. 10.1007/s00403-014-1458-6 (2014). 10.1007/s00403-014-1458-6 [DOI] [PubMed] [Google Scholar]
- 18.Dumycz, K., Kunkiel, K. & Feleszko, W. Cosmetics for neonates and infants: haptens in products’ composition. Clin. Transl. Allergy9, 15. 10.1186/s13601-019-0257-8 (2019). 10.1186/s13601-019-0257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low, K. Y. & Wallace, M. Prevalence of potential contact allergens in baby cosmetic products. Clin. Experim. Dermatol.44, 411–413. 10.1111/ced.13767 (2019). 10.1111/ced.13767 [DOI] [PubMed] [Google Scholar]
- 20.Bonchak, J. G., Prouty, M. E. & de la Feld, S. F. Prevalence of contact allergens in personal care products for babies and children. Dermatitis29, 81–84. 10.1097/DER.0000000000000348 (2018). 10.1097/DER.0000000000000348 [DOI] [PubMed] [Google Scholar]
- 21.Bernauer, U. et al. The SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation, 11th revision, 30–31 March 2021, SCCS/1628/21. Regulat. Toxicol. Pharmacol.127, 102. 10.1016/j.yrtph.2021.105052 (2021). 10.1016/j.yrtph.2021.105052 [DOI] [PubMed] [Google Scholar]
- 22.Chang, H. & Chen, T. A compositional analysis of preservative systems in cosmetics intended for infants. Dermatitis34, 462–463. 10.1097/der.0000000000000878 (2023). 10.1097/der.0000000000000878 [DOI] [PubMed] [Google Scholar]
- 23.Routledge, E. J., Parker, J., Odum, J., Ashby, J. & Sumpter, J. P. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol.153, 12–19. 10.1006/taap.1998.8544 (1998). 10.1006/taap.1998.8544 [DOI] [PubMed] [Google Scholar]
- 24.Okubo, T., Yokoyama, Y., Kano, K. & Kano, I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem. Toxicol.39, 1225–1232. 10.1016/s0278-6915(01)00073-4 (2001). 10.1016/s0278-6915(01)00073-4 [DOI] [PubMed] [Google Scholar]
- 25.Scientific Committee on Consumer Safety. Opinion of the Scientific Committee on Consumer Safety (SCCS) - Final version of the opinion on Phenoxyethanol in cosmetic products. Regul Toxicol Pharmacol82, 156. 10.1016/j.yrtph.2016.11.007 (2016). 10.1016/j.yrtph.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 26.Yu, J., Bai, H. & Brod, B. In Contact Dermatitis (eds Jeanne Duus Johansen, Vera Mahler, Jean-Pierre Lepoittevin, & Peter J. Frosch) 709–715 (Springer International Publishing, 2021).
- 27.Kerdudo, A., Fontaine-Vive, F., Dingas, A., Faure, C. & Fernandez, X. Optimization of cosmetic preservation: water activity reduction. Int. J. Cosmet. Sci.37, 31–40. 10.1111/ics.12164 (2015). 10.1111/ics.12164 [DOI] [PubMed] [Google Scholar]
- 28.Yoo, I. K., Kim, J. I. & Kang, Y. K. Conformational preferences and antimicrobial activities of alkanediols. Comput. Theor. Chem.1064, 15–24. 10.1016/j.comptc.2015.04.007 (2015). 10.1016/j.comptc.2015.04.007 [DOI] [Google Scholar]
- 29.Varvaresou, A. et al. Self-preserving cosmetics. Int. J. Cosmet. Sci.31, 163–175. 10.1111/j.1468-2494.2009.00492.x (2009). 10.1111/j.1468-2494.2009.00492.x [DOI] [PubMed] [Google Scholar]
- 30.Rigano, L. & Leporatti, R. Systemic Constellations: With or without preservatives?. Sofw J.129, 30–43 (2003). [Google Scholar]
- 31.Janichen, J. The quest for the ideal preserving system - Reducing traditional preservatives in combination with dermosoft octiol. Euro Cosmet.12, 10–17 (2004). [Google Scholar]
- 32.Fang, B., Yu, M., Zhang, W. & Wang, F. A new alternative to cosmetics preservation and the effect of the particle size of the emulsion droplets on preservation efficacy. Int. J. Cosmet. Sci.38, 496–503. 10.1111/ics.12317 (2016). 10.1111/ics.12317 [DOI] [PubMed] [Google Scholar]
- 33.Sigg, M. & Daniels, R. Investigations on alkanediols as alternative preservatives in a nonionic hydrophilic cream. Pharmaceutics12, 1 (2020). 10.3390/pharmaceutics12111117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, W. Jr. et al. Safety assessment of 1,2-glycols as used in cosmetics. Int. J. Toxicol.31, 147s–168s. 10.1177/1091581812460409 (2012). 10.1177/1091581812460409 [DOI] [PubMed] [Google Scholar]
- 35.Schaefer, K. Broad-spectrum Alternative to Caprylyl Glycol Preservative Blends, <https://www.cosmeticsandtoiletries.com/formulating/function/preservatives/137670898.html> (2012).
- 36.Lalla, S. C. et al. Patch testing to propylene glycol: The Mayo clinic experience. Dermatitis29, 200–205. 10.1097/der.0000000000000393 (2018). 10.1097/der.0000000000000393 [DOI] [PubMed] [Google Scholar]
- 37.Bizjak, M. et al. Patch testing with the European baseline series and 10 added allergens: Single-centre study of 748 patients. Contact Dermatitis87, 439–446. 10.1111/cod.14178 (2022). 10.1111/cod.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton, E. et al. Developing a cosmetic series: Results from the ESSCA network, 2009–2018. Contact Dermatitis84, 82–94. 10.1111/cod.13690 (2021). 10.1111/cod.13690 [DOI] [PubMed] [Google Scholar]
- 39.Leschke, M. & Siegert, W. Boosting efficacy of preservatives. Personal Care, 1–4 (2008).
- 40.Leschke, M. A multifunctional ingredient for leave-on cosmetics. Cosmet. Sci. Technol. 189–195 (2006).
- 41.Leschke, M. & Wustermann, S. A reliable alternative for traditional preservative systems. Sofw J.132, 78 (2006). [Google Scholar]
- 42.Lawan, K., Kanlayavattanakul, M. & Lourith, N. Antimicrobial efficacy of caprylyl glycol and ethylhexylglycerine in emulsion. J. Health Res.23, 1–3 (2009). [Google Scholar]
- 43.Aerts, O., Verhulst, L. & Goossens, A. Ethylhexylglycerin: a low-risk, but highly relevant, sensitizer in “hypo-allergenic” cosmetics. Contact Dermatitis74, 281–288. 10.1111/cod.12546 (2016). 10.1111/cod.12546 [DOI] [PubMed] [Google Scholar]
- 44.Martinka Maksymiak, M. et al. Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes. Materials (Basel)13, 4153. 10.3390/ma13184153 (2020). 10.3390/ma13184153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricke, S. C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poultry Science82, 632–639. 10.1093/ps/82.4.632 (2003). 10.1093/ps/82.4.632 [DOI] [PubMed] [Google Scholar]
- 46.Pilz, F. Sorbitan Caprylate—The preservative boosting multifunctional ingredient. Cosemt. Sci. Technol.131, 1 (2011). [Google Scholar]
- 47.Straetmans, U. D. O. et al. Concentrated, aqueous solutions of p-methoxybenzoic acid for use in cosmetic and dermatologic formulations. (2006).
- 48.Deza, G. & Gimenez-Arnau, A. M. Allergic contact dermatitis in preservatives: Current standing and future options. Curr. Opin. Allergy Clin. Immunol.17, 263–268. 10.1097/ACI.0000000000000373 (2017). 10.1097/ACI.0000000000000373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.