Abstract
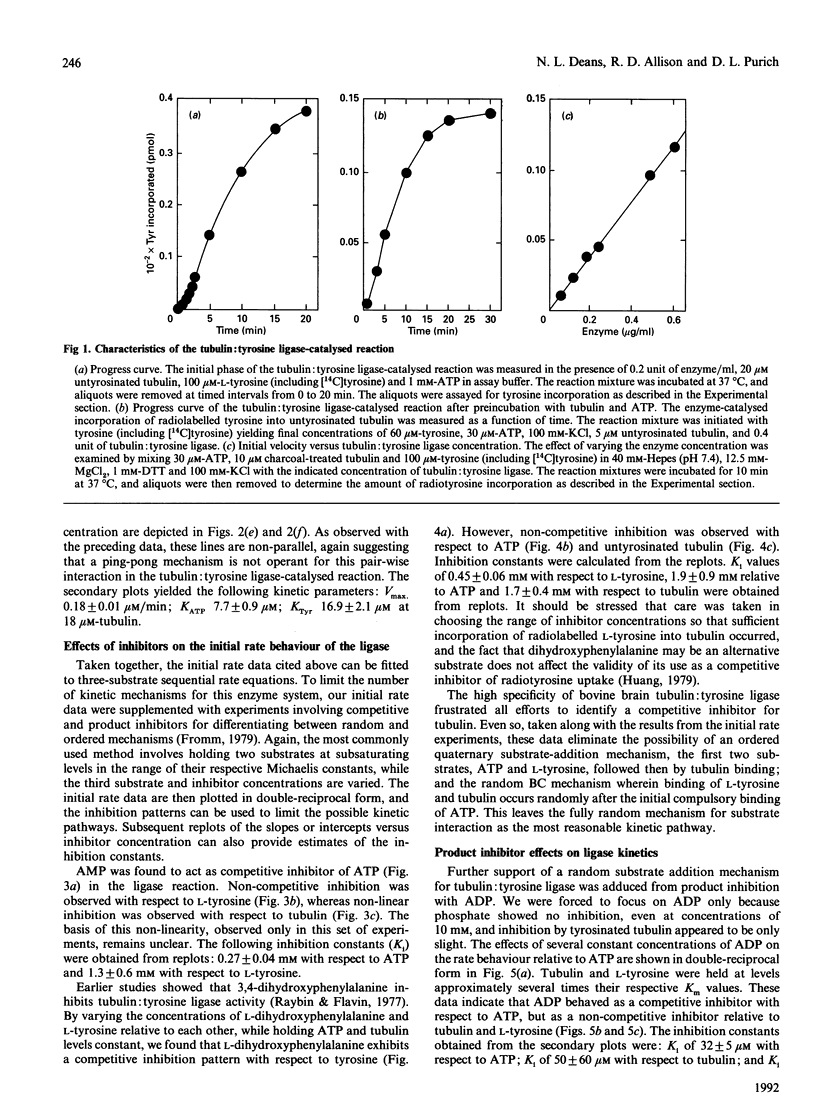
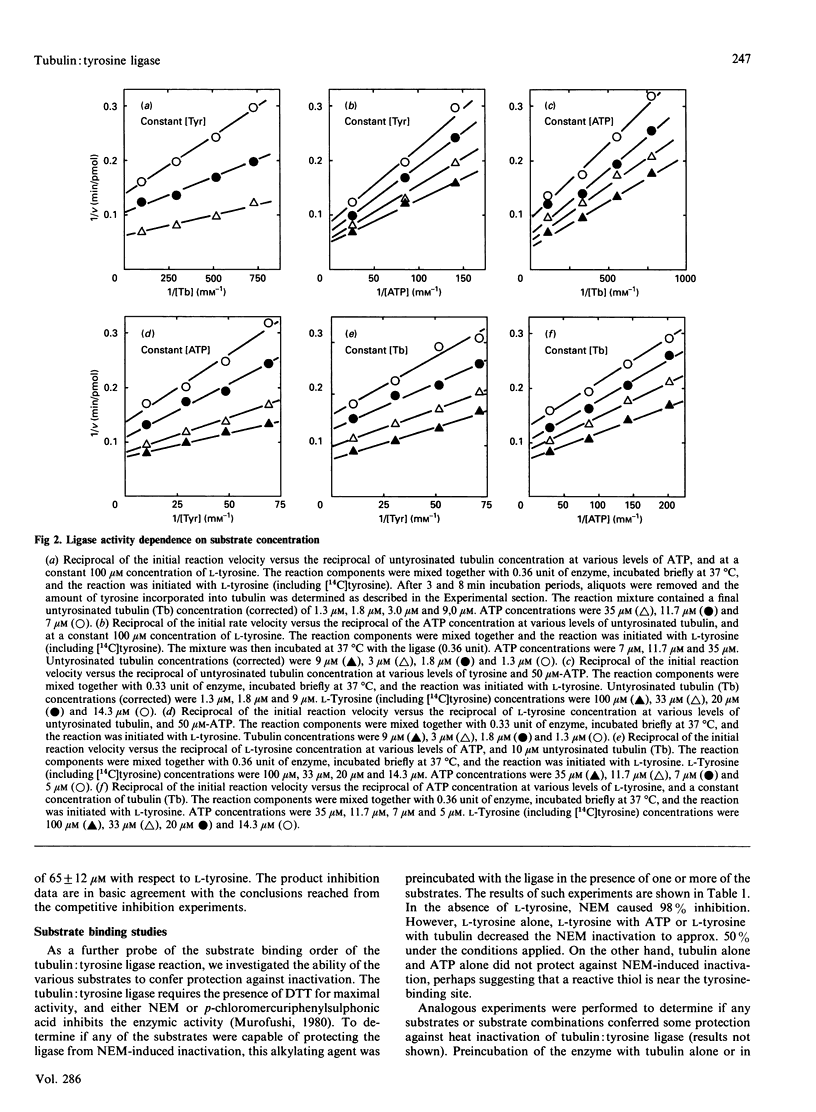
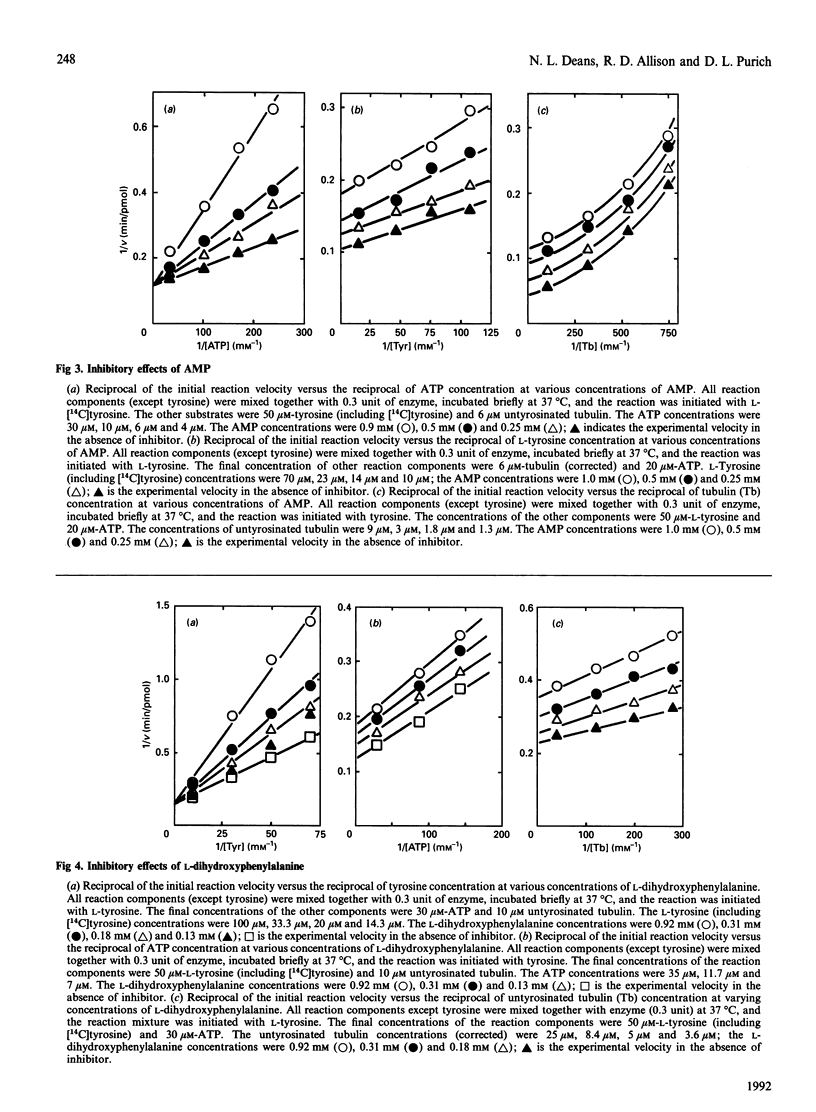
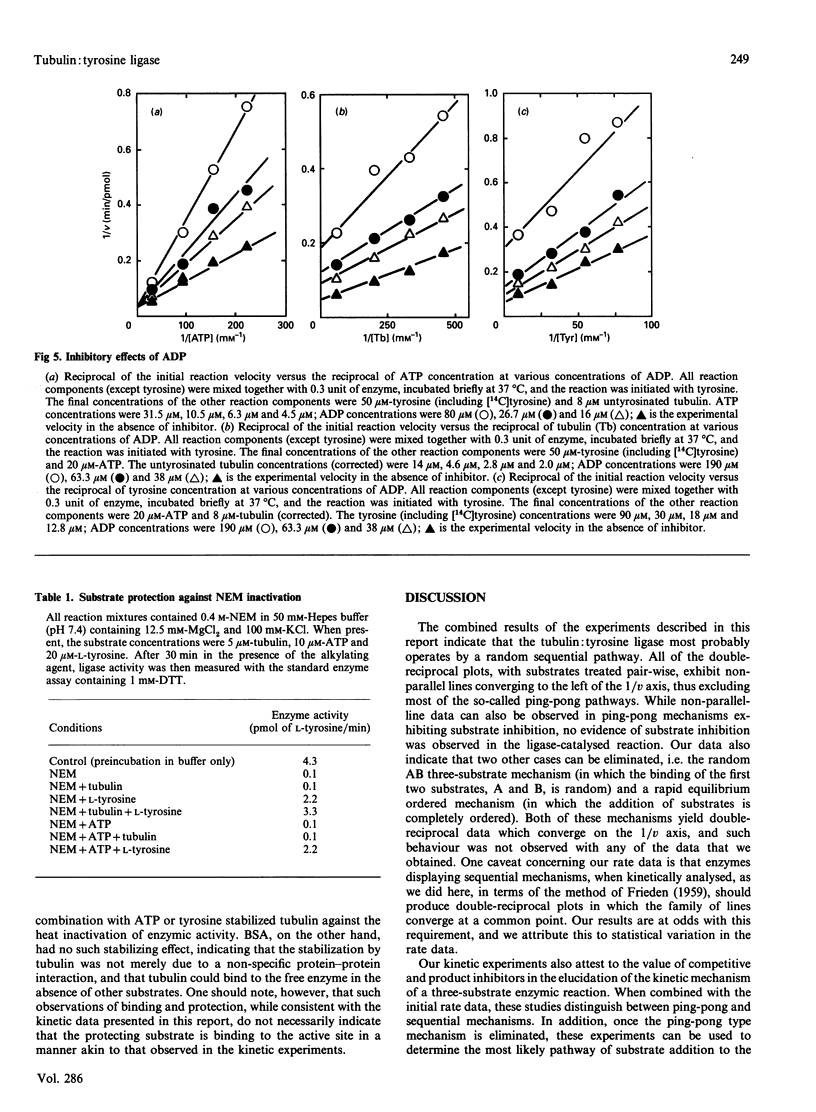
The ATP-dependent resynthesis of tubulin from tyrosine and untyrosinated tubulin was examined to establish the most probable steady-state kinetic mechanism of the tubulin: tyrosine ligase (ADP-forming). Three pair-wise sets of initial rate experiments, involving variation of two substrates pair-wise with the third substrate held at a high (but non-saturating) level, yielded convergent-line data, a behaviour that is diagnostic for sequential mechanisms. Michaelis constants were 14 microM, 1.9 microM and 17 microM for ATP, untyrosinated tubulin and L-tyrosine respectively, and the maximal velocity was 0.2 microM/min. AMP was a competitive inhibitor with respect to ATP, and a non-competitive inhibitor versus either tubulin or tyrosine. Likewise, L-dihydroxyphenylalanine acted competitively relative to tyrosine and non-competitively with respect to either ATP or tubulin. These findings directly support a random sequential mechanism. Product inhibition patterns with ADP were also consistent with this assignment; however, inhibition studies were not practical with either orthophosphate or tyrosinated tubulin because both were very weak inhibitors. Substrate protection of the enzyme against alkylation by N-ethylmaleimide and thermal inactivation, along with evidence of enzyme binding to ATP-Sepharose and tubulin-Sepharose, also supports the idea that this three-substrate enzyme reaction exhibits a random substrate addition pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Todhunter J. A., Purich D. L. Steady state and equilibrium exchange kinetic studies of the sheep brain glutamine synthetase reaction. J Biol Chem. 1977 Sep 10;252(17):6046–6051. [PubMed] [Google Scholar]
- Arce C. A., Rodriguez J. A., Barra H. S., Caputo R. Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem. 1975 Nov 1;59(1):145–149. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Argarana C. E., Barra H. S., Caputto R. Tubulinyl-tyrosine carboxypeptidase from chicken brain: properties and partial purification. J Neurochem. 1980 Jan;34(1):114–118. doi: 10.1111/j.1471-4159.1980.tb04628.x. [DOI] [PubMed] [Google Scholar]
- Argaraña C. E., Barra H. S., Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978 Feb 24;19(1):17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- Bachelard H. S. Allosteric activation of brain hexokinase by magnesium ions and by magnesium ion--adenosine triphosphate complex. Biochem J. 1971 Nov;125(1):249–254. doi: 10.1042/bj1250249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra H. S., Arce C. A., Rodríguez J. A., Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1384–1390. doi: 10.1016/0006-291x(74)90351-9. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blosser J. C., Wells W. W. Studies on amino acid levels and protein metabolism in the brains of galactose-intoxicated chicks. J Neurochem. 1972 Jan;19(1):69–79. doi: 10.1111/j.1471-4159.1972.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- Flavin M., Kobayashi T., Martensen T. M. Tubulin-tyrosine ligase from brain. Methods Cell Biol. 1982;24:257–263. doi: 10.1016/s0091-679x(08)60659-7. [DOI] [PubMed] [Google Scholar]
- Flavin M., Murofushi H. Tyrosine incorporation in tubulin. Methods Enzymol. 1984;106:223–237. doi: 10.1016/0076-6879(84)06024-9. [DOI] [PubMed] [Google Scholar]
- Fromm H. J. The use of competitive inhibitors in studying the mechanism of action of some enzyme systems utilizing three substrates. Biochim Biophys Acta. 1967 Jul 11;139(2):221–230. doi: 10.1016/0005-2744(67)90026-5. [DOI] [PubMed] [Google Scholar]
- Fromm H. J. Use of competitive inhibitors to study substrate binding order. Methods Enzymol. 1979;63:467–486. doi: 10.1016/0076-6879(79)63020-3. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol. 1986 Mar;102(3):1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986 Dec;42(2):288–294. [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987 Jul;105(1):251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977 Feb 1;73(2):147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry. 1984 Aug 28;23(18):4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- Huang C. Y. Use of alternative substrates to probe multisubstrate enzyme mechanisms. Methods Enzymol. 1979;63:486–500. doi: 10.1016/0076-6879(79)63021-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDermott A. E., Creuzet F., Griffin R. G., Zawadzke L. E., Ye Q. Z., Walsh C. T. Rotational resonance determination of the structure of an enzyme-inhibitor complex: phosphorylation of an (aminoalkyl)phosphinate inhibitor of D-alanyl-D-alanine ligase by ATP. Biochemistry. 1990 Jun 19;29(24):5767–5775. doi: 10.1021/bi00476a018. [DOI] [PubMed] [Google Scholar]
- Murofushi H. Purification and characterization of tubulin-tyrosine ligase from porcine brain. J Biochem. 1980 Mar;87(3):979–984. doi: 10.1093/oxfordjournals.jbchem.a132828. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Ning J., Purich D. L., Fromm H. J. Studies on the kinetic mechanism and allosteric nature of bovine brain hexokinase. J Biol Chem. 1969 Jul 25;244(14):3840–3846. [PubMed] [Google Scholar]
- Paturle-Lafanechère L., Eddé B., Denoulet P., Van Dorsselaer A., Mazarguil H., Le Caer J. P., Wehland J., Job D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry. 1991 Oct 29;30(43):10523–10528. doi: 10.1021/bi00107a022. [DOI] [PubMed] [Google Scholar]
- Purich D. L., Allison R. D. Isotope exchange methods for elucidating enzymic catalysis. Methods Enzymol. 1980;64:1–46. [PubMed] [Google Scholar]
- Raybin D., Flavin M. An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1088–1095. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Modification of tubulin by tyrosylation in cells and extracts and its effect on assembly in vitro. J Cell Biol. 1977 May;73(2):492–504. doi: 10.1083/jcb.73.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Litwin S. Determination of the rate of hexokinase-glucose dissociation by the isotope-trapping method. J Biol Chem. 1974 Aug 25;249(16):5163–5168. [PubMed] [Google Scholar]
- Rudolph F. B., Fromm H. J. Kinetic studies of the adenosine 5'-triphosphatase activity of yeast hexokinase and its relationship to the mechanism of action of the enzyme. J Biol Chem. 1970 Aug 25;245(16):4047–4052. [PubMed] [Google Scholar]
- Rudolph F. B. Product inhibition and abortive complex formation. Methods Enzymol. 1979;63:411–436. doi: 10.1016/0076-6879(79)63018-5. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys B. J., Borisy G. G. Polymerization of tubulin in vivo: direct evidence for assembly onto microtubule ends and from centrosomes. J Cell Biol. 1985 May;100(5):1682–1689. doi: 10.1083/jcb.100.5.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wehland J., Weber K. Tubulin-tyrosine ligase has a binding site on beta-tubulin: a two-domain structure of the enzyme. J Cell Biol. 1987 Apr;104(4):1059–1067. doi: 10.1083/jcb.104.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]


