Abstract
Recent studies have suggested that the decline in protein synthesis that occurs in rat liver and brain during development and aging is associated with a decrease in the activity of eukaryotic initiation factor 2 (eIF-2). One way in which eIF-2 activity could be decreased in tissue extracts would be through a decrease in the activity of the GDP exchange factor, eIF-2B. In the present study, the activity of eIF-2B was measured in tissue extracts and was found to be less in older than in younger rats. Thus a decrease in eIF-2B activity could account for part of the decrease in protein synthesis that occurs during aging. Another way in which eIF-2 activity could be decreased would be through a decrease in amount of the protein. Therefore the amount of eIF-2 in various tissues was quantified by protein immunoblot analysis. We found that the amount of eIF-2 relative to total protein tended to fall with increasing age. Furthermore, eIF-2 content was directly proportional to the rate of protein synthesis in the tissues examined. Finally, slot-blot analysis of polyadenylated RNA revealed no significant change in the relative abundance of eIF-2 alpha mRNA with age. The last-mentioned experiments suggest that the synthesis of eIF-2 may be regulated through changes in the deficiency of translation of eIF-2 alpha mRNA rather than through changes in gene transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bylund-Fellenius A. C., Ojamaa K. M., Flaim K. E., Li J. B., Wassner S. J., Jefferson L. S. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984 Apr;246(4 Pt 1):E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Calés C., Fando J. L., Azuara C., Salinas M. Developmental studies of the first step of the initiation of brain protein synthesis, role for initiation factor 2. Mech Ageing Dev. 1986 Jan;33(2):147–156. doi: 10.1016/0047-6374(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Castañeda M., Vargas R., Galván S. C. Stagewise decline in the activity of brain protein synthesis factors and relationship between this decline and longevity in two rodent species. Mech Ageing Dev. 1986 Oct;36(2):197–210. doi: 10.1016/0047-6374(86)90020-5. [DOI] [PubMed] [Google Scholar]
- Chang J. Y., Knecht R., Braun D. G. Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography. Methods Enzymol. 1983;91:41–48. doi: 10.1016/s0076-6879(83)91009-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dholakia J. N., Mueser T. C., Woodley C. L., Parkhurst L. J., Wahba A. J. The association of NADPH with the guanine nucleotide exchange factor from rabbit reticulocytes: a role of pyridine dinucleotides in eukaryotic polypeptide chain initiation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6746–6750. doi: 10.1073/pnas.83.18.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dholakia J. N., Wahba A. J. Phosphorylation of the guanine nucleotide exchange factor from rabbit reticulocytes regulates its activity in polypeptide chain initiation. Proc Natl Acad Sci U S A. 1988 Jan;85(1):51–54. doi: 10.1073/pnas.85.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H., Duncan R. F., Hershey J. W. Cloning and sequencing of complementary DNAs encoding the alpha-subunit of translational initiation factor eIF-2. Characterization of the protein and its messenger RNA. J Biol Chem. 1987 Jan 25;262(3):1206–1212. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fando J. L., Salinas M., Wasterlain C. G. Age-dependent changes in brain protein synthesis in the rat. Neurochem Res. 1980 Apr;5(4):373–383. doi: 10.1007/BF00964226. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Peavy D. E., Everson W. V., Jefferson L. S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. I. Reduction in rates of synthesis resulting from amino acid deprivation and recovery during flow-through perfusion. J Biol Chem. 1982 Mar 25;257(6):2932–2938. [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. Protein turnover and growth of the rat brain from the foetus to old age. J Neurochem. 1988 May;50(5):1364–1368. doi: 10.1111/j.1471-4159.1988.tb03017.x. [DOI] [PubMed] [Google Scholar]
- Junghahn I., Bommer U. A. Age dependent changes in the activity of the cytosolic fraction from rat liver to stimulate polysomal protein synthesis and the role of initiation factor eIF-2. Biomed Biochim Acta. 1987;46(11):791–794. [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- Kimball S. R., Antonetti D. A., Brawley R. M., Jefferson L. S. Mechanism of inhibition of peptide chain initiation by amino acid deprivation in perfused rat liver. Regulation involving inhibition of eukaryotic initiation factor 2 alpha phosphatase activity. J Biol Chem. 1991 Jan 25;266(3):1969–1976. [PubMed] [Google Scholar]
- Kimball S. R., Everson W. V., Flaim K. E., Jefferson L. S. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am J Physiol. 1989 Jan;256(1 Pt 1):C28–C34. doi: 10.1152/ajpcell.1989.256.1.C28. [DOI] [PubMed] [Google Scholar]
- Kimball S. R., Everson W. V., Myers L. M., Jefferson L. S. Purification and characterization of eukaryotic initiation factor 2 and a guanine nucleotide exchange factor from rat liver. J Biol Chem. 1987 Feb 15;262(5):2220–2227. [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Mechanism of the inhibition of protein synthesis by vasopressin in rat liver. J Biol Chem. 1990 Oct 5;265(28):16794–16798. [PubMed] [Google Scholar]
- Kimball S. R., Rannels S. L., Elensky M. B., Jefferson L. S. Quantitation of proteins by dot-immunobinding assay. A comparison of visualization methods using eukaryotic initiation factor 2 and a monospecific antibody. J Immunol Methods. 1988 Feb 10;106(2):217–223. doi: 10.1016/0022-1759(88)90200-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Murthy M. R. Protein synthesis in growing-rat tissues. II. Polyribosome concentration of brain and liver as a function of age. Biochim Biophys Acta. 1966 Jun 22;119(3):599–613. [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Jagus R., Kemper W. M. Analysis of initiation factor function in highly fractionated and unfractionated reticulocyte lysate systems. Methods Enzymol. 1979;60:61–87. doi: 10.1016/s0076-6879(79)60008-3. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Shahbazian F. M., Jacobs M., Lajtha A. Regional and cellular differences in rat brain protein synthesis in vivo and in slices during development. Int J Dev Neurosci. 1986;4(3):209–215. doi: 10.1016/0736-5748(86)90060-2. [DOI] [PubMed] [Google Scholar]
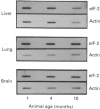
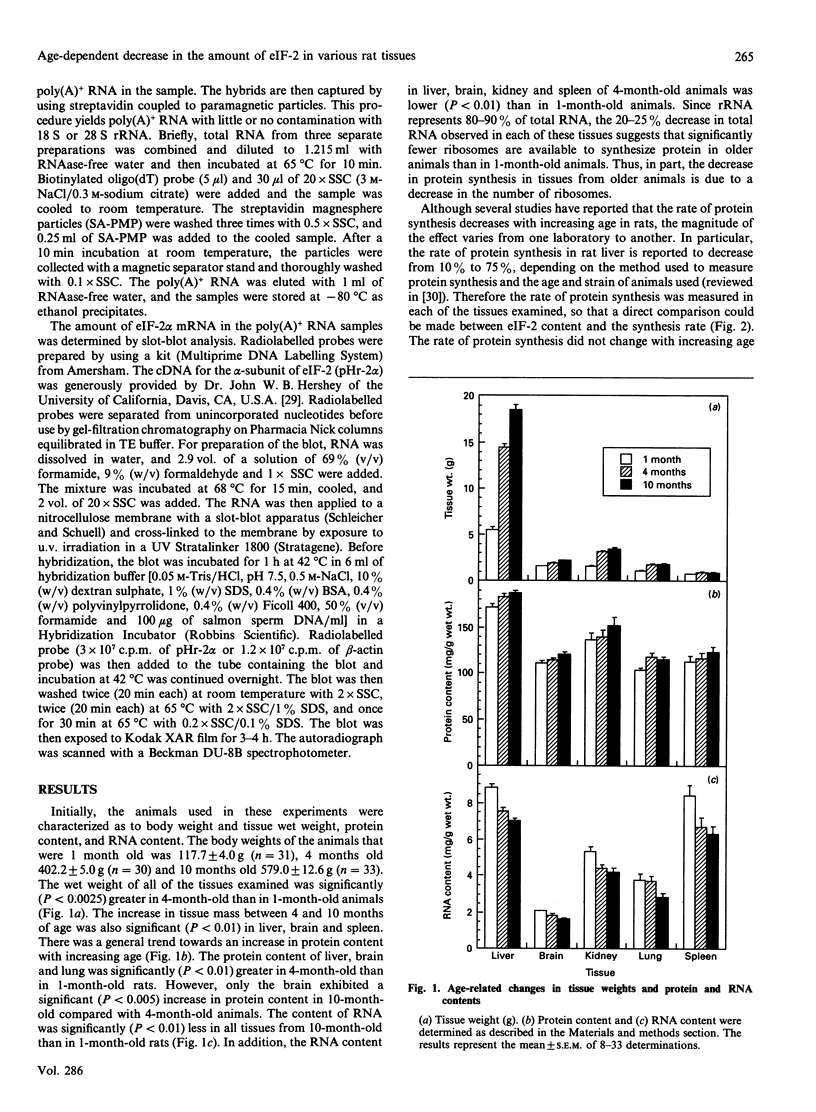
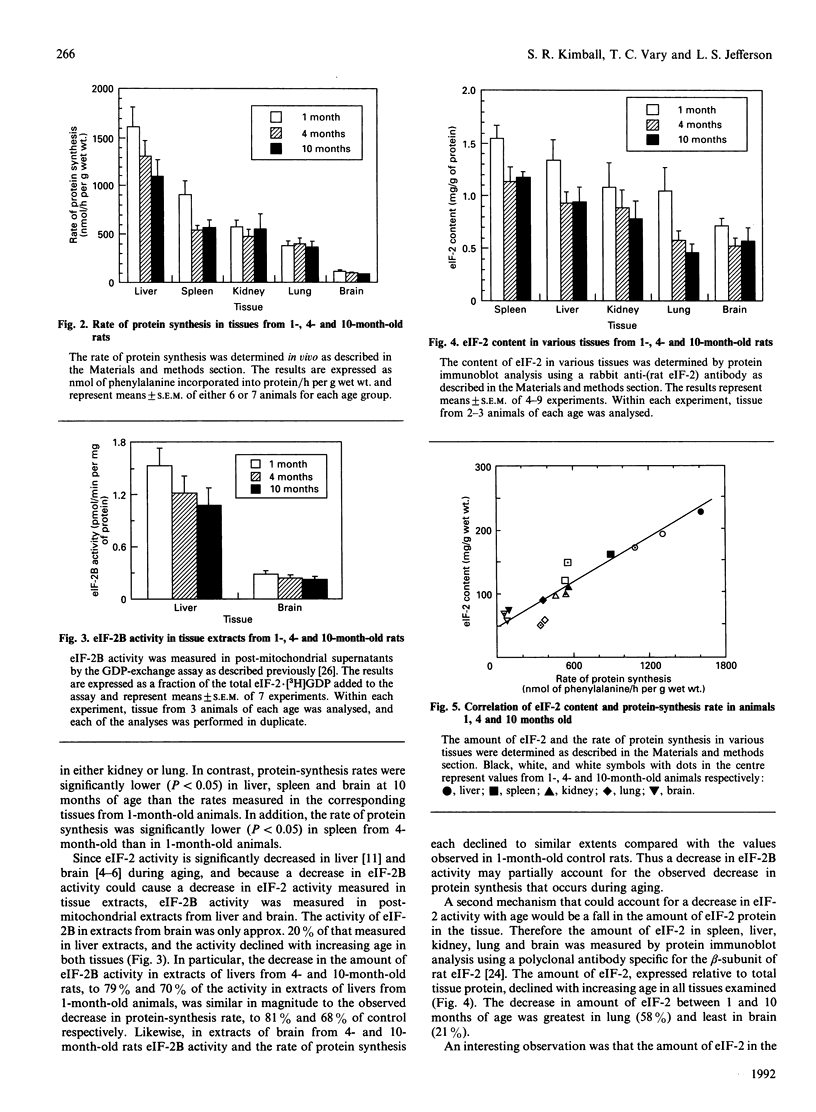
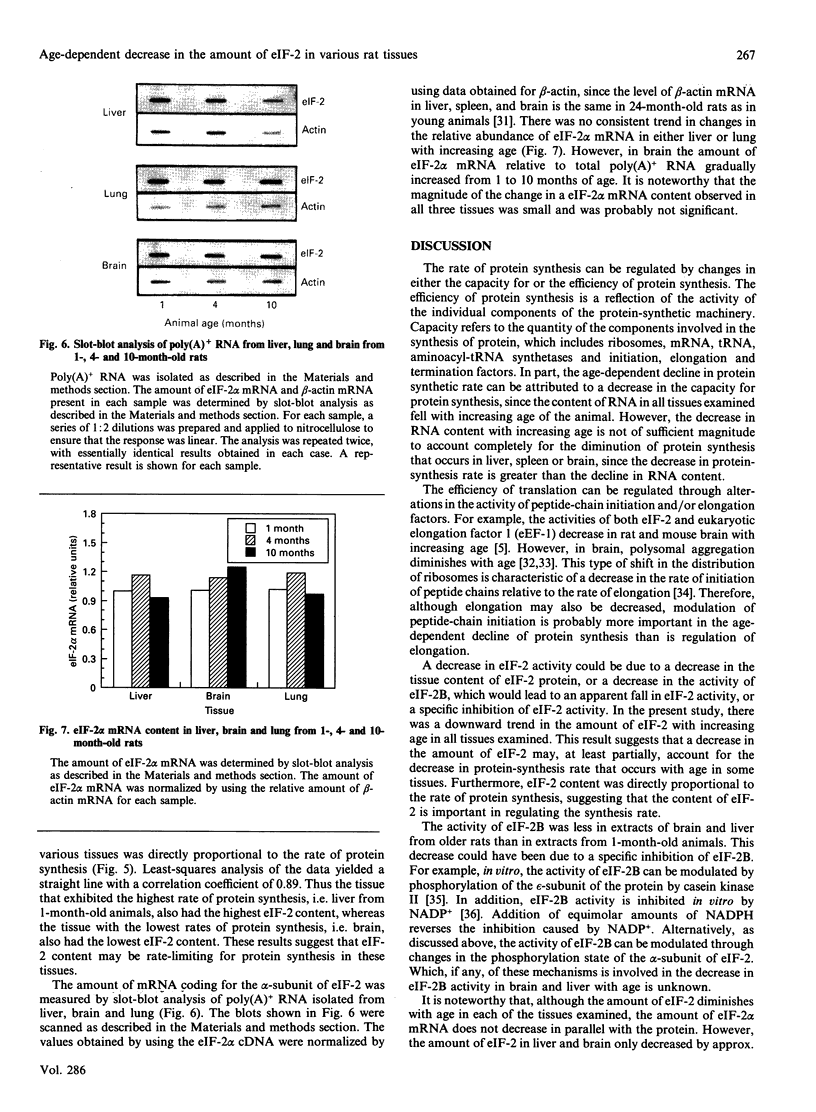
- Slagboom P. E., de Leeuw W. J., Vijg J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990 Apr 30;53(3):243–257. doi: 10.1016/0047-6374(90)90042-e. [DOI] [PubMed] [Google Scholar]
- Vargas R., Castañeda M. Age-dependent decrease in the activity of protein-synthesis initiation factors in rat brain. Mech Ageing Dev. 1983 Feb;21(2):183–191. doi: 10.1016/0047-6374(83)90073-8. [DOI] [PubMed] [Google Scholar]
- Vary T. C., Kimball S. R. Regulation of hepatic protein synthesis in chronic inflammation and sepsis. Am J Physiol. 1992 Feb;262(2 Pt 1):C445–C452. doi: 10.1152/ajpcell.1992.262.2.C445. [DOI] [PubMed] [Google Scholar]
- Ward W., Richardson A. Effect of age on liver protein synthesis and degradation. Hepatology. 1991 Nov;14(5):935–948. doi: 10.1002/hep.1840140529. [DOI] [PubMed] [Google Scholar]
- Zomzely C. E., Roberts S., Peache S., Brown D. M. Cerebral protein synthesis. 3. Developmental alteratons in the stability of cerebral messenger ribonucleic acid-ribosome complexes. J Biol Chem. 1971 Apr 10;246(7):2097–2103. [PubMed] [Google Scholar]