Abstract
The ongoing worldwide effort to reduce animal numbers in research often omits the issue of pre-weaning mortality in mouse breeding. A conservative estimate of 20% mortality would mean approximately 1.1 M mice die annually in the EU before scientific use. We hypothesize that pre-weaning mortality in laboratory mouse breeding is associated with cage social and macro/micro-environment conditions. Here we count pups from 509 C57BL/6J litters daily for accurate detection of mortality, and monitor cage micro-environment for 172 C57BL/6J litters. Probability of pups to die increases with the increase in dam age, number and age of older pups in the cage (of overlapped/cohabitating litters), and in small (<6 pups) and large (>11 pups) focal litters. Higher temperatures (>23.6 °C) and nest scores (>3.75) compensate for some of the socially-associated risks for pup death. These findings can be implemented in strategies for reducing pre-weaning mouse mortality, a more welfare-friendly and sustainable approach for science.
Subject terms: Animal behaviour, Ethics
A laboratory mouse breeding study suggests that new pups born in the presence of older pups and older dams are more likely to die before weaning, but these social risks can be alleviated by proper thermal and light environment.
Introduction
Despite the ambition in legislation and policy (e.g. Directive 2010/63/EU, Article 4 and 13), there is no clear evidence of an overall reduction of animals used for research purposes over the past years. In 2015, the reported number of animals used in the EU, Norway and UK was 9,590,3791, while figures for 2022 were 9,897,6072, with mice consistently accounting for over 50% of all animals used. Breeding of healthy laboratory animals is not covered in much statistical reporting and is therefore often overlooked in the discussion of animal numbers.
When attempting to reduce the mice required for research, a problem remaining largely unaddressed is the issue of pup mortality in routine laboratory mouse breeding. The main supplier for C57BL/6J mice, one of the most widely used strains in biomedical research3, reported a mean weaned-born ratio of ~92.5% for this strain, i.e. 7.5% pre-weaning mortality4. However, higher and greatly variable mortality levels have been reported for the same strain used in scientific studies, from <10%5,6 to 49%7 often reaching over 20%. In our previous studies, using experimental8 (109 litters, UK), retrospective9 (344 litters, Germany), and breeding-software10 (34,949 litters, UK) data, we found total litter losses (litters with 100% mortality rate) in 6–31%10, 32%9, and 33%8 of the litters, with overall mortality of 14–39%10, and 52% in trio (one male plus two female mice)-bred mice8, suggesting that pre-weaning mortality in practice is often much higher than indicated by supplier reference data. The fact that most deaths occur within the first 48 h post-partum, and the cadavers are often cannibalized, makes the detection of the number of pups born quite challenging without daily counting, leading to underestimation of number of pups born, and therefore underestimation of mortality levels11.
High pre-weaning mortality rates would necessitate a substantial number of pups being born in mouse colonies to compensate for these deaths and supply the demand for use in science. Considering an average of 5.6 million mice used in EU research yearly (based on data from 20151 to 20221,2,12) and assuming a mortality level of 20% (a conservative estimate), up to 1.4 million mice may have been dying annually, before weaning and being available for use in science. Some animals with genetic modifications may be at particular risk, such as those with impaired maternal behaviour or more vulnerable to cold stress13,14. Furthermore, the more pups that are bred so that the survivors can be used in science, the more breeding adults, infrastructure and husbandry resources are needed. Therefore, laboratory mouse pre-weaning mortality is not only an animal welfare matter but also a worldwide scientific, economic, logistic, and sustainability issue.
Pre-weaning mortality, reproductive, and welfare parameters have previously been linked with micro-environmental factors, such as cage temperature and light intensity6,15–17, cage enrichment (e.g. nest material and amount6,14,18,19), bedding material20, animal-related factors, such as dam handling and parity21,22, and more recently, litter overlap (i.e. cohabitating litters resulting when new pups are born in the presence of older pups already in the cage)8,10,11. Litter overlap happens in any housing configuration in which males and females co-habit to allow for uninterrupted breeding. To our knowledge, many breeding facilities house mice continuously either in pairs (one male and one female) or cages with a male and multiple females. In the latter configuration, litter overlap is a normal consequence of the set-up. For pairs, it is rare but does happen, particularly if females become pregnant again by being mated to the post-partum oestrus, and the weaning of their previous litter is delayed.
In an experimental study with 109 litters (55 in trio-cages), we found risks for entire litter loss and individual pup loss increased by multiples of 2.3 and 1.8, respectively, compared to non-overlapped litters8. In our data analysis of mortality records from two UK breeding facilities for 34,949 C57BL/6 (Babr and NTac) litters, we also found that litter overlap is one of the main drivers of the increase in pup probability to die in trio-cages. The higher the age gap between overlapped litters and the higher the number of older pups present in the cage, the higher the probability of the newborns dying. Increased dam age and small or large litter sizes also negatively impacted the pups' probability of surviving10.
The present study aimed to evaluate pup survival as a function of cage social factors (Dam Age, Litter Overlap, Age and Size of the Older Litter in the Cage when the focal litter is born, and Number of Pups Born in the Focal Litter), cage micro-environment (Cage Temperature, Light Intensity, Vibration, frequency and duration of human Motion Events near the cage, and Nest Score), and cage macro-environment (Season and Weekday of Birth of the Focal Litter).
A total of 509 litters were randomly selected to be observed daily from birth to four days post-partum, and once again at ~21 days post-partum, prior to weaning. The cage micro-environment was monitored for a subset of 172 litters with the use of data loggers and daily checks. This study demonstrated that litter overlap, increased number of older pups in the cage, small (<6 pups) and large (>11 pups) litter sizes, and advanced age of older pups and dam are risk factors for new-born mortality, some of which could potentially be alleviated by adjusting the cage’s micro-environment. Knowledge generated from this work is intended to aid the development of effective strategies to reduce pre-weaning mortality in the context of laboratory mouse breeding.
Results
Pre-weaning mortality
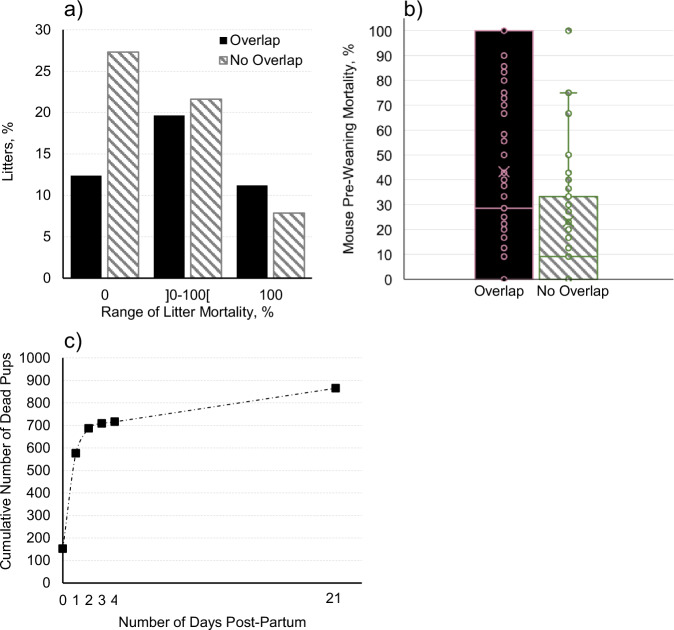
Overall pup pre-weaning mortality was 25.6%. Death of the entire litter occurred in 19.1% of all studied litters, while 39.7% of litters had no mortality. Figure 1a, b depicts pre-weaning mortality rates, considering both categories of overlapped and non-overlapped litter. On a per-litter basis, litters lost 31.9 ± 39.8% of their pups (ranging from 0% to 100%). Most of the deaths (66.6% or 576 of the 865 dead pups) happened within the first 24–48 h post-partum (Fig. 1c).
Fig. 1. Overall mortality and pup probability of death.
a Percentage of litters by range of litter mortality with or without the presence of older pups in the cage (Litter Overlap), based on raw data considering the total number of litters (n = 509). The open bracket next to a number designates a non-inclusive endpoint. b Pre-weaning mortality rates with or without Litter Overlap, based on raw data considering the total number of litters (n = 509). Line and cross within the box-plot depict the median and mean, respectively. c Cumulative number of pups that went missing from Focal Litters (likely eaten by a cage mate11) and/or found dead over time before weaning at 21 days of age.
Results considering the whole dataset (Supplementary Table 1) revealed that pup probability of death was affected by litter overlap while interacting with size of focal litter (t(1, 2893) = −2.34, P = 0.019), size of focal litter in a quadradic fashion (t(1, 2893) = 4.21, P <0.001), and dam age (t(1, 2893) = 4.64, P < 0.001). When analysing only the overlapped litters, pup probability of death was affected by number of older pups (t(1, 1144) = 4.54, P < 0.001), age of older pups in a quadratic fashion (t(1, 1144) = −3.33, P < 0.001), size of focal litter in a quadratic fashion (t(1, 1144) = 2.16, P = 0.031), and Dam Age (t(1, 1144) = 2.87, P = 0.004, Supplementary Table 2).
Cage social factors
Dam age
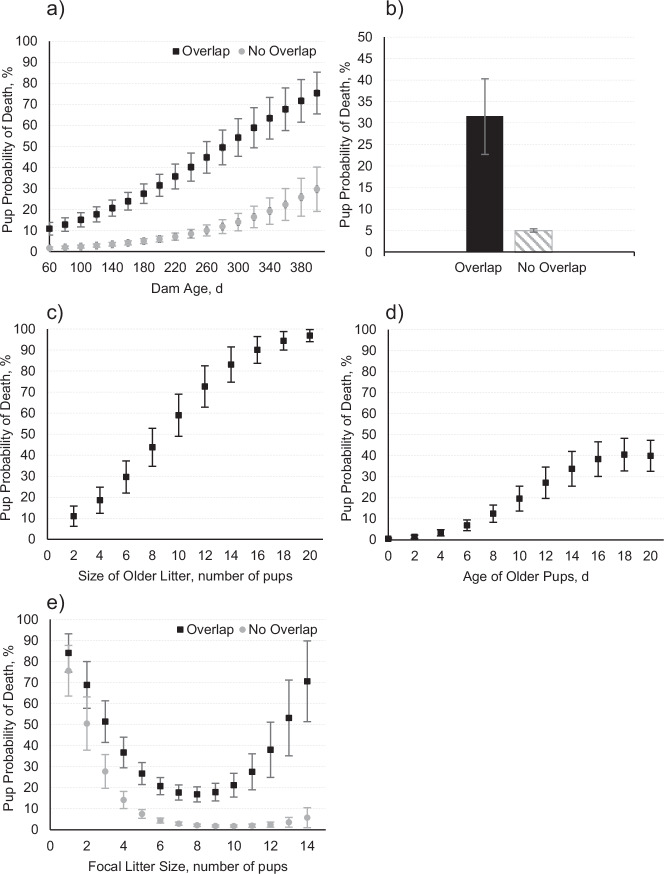
Dams were 174 ± 73 days old on average (range 58–493 days; Supplementary Note 1.1). Pup probability of death increased with the increase in dam age (P < 0.001) in a linear fashion (Fig. 2a) and was higher in overlapped litters compared with non-overlapped litters for all categories of Dam Age.
Fig. 2. Pup probability of death and social factors.
Predicted probability of pup death (least-squares means with standard error bars) as a function of a Dam Age. b Presence of older pups in the cage at birth (Litter Overlap). c Number of Older Pups. d Age of Older Pups, and e Number of pups born (Focal Litter Size). Predicted means were plotted considering a fixed mean Dam Age of 120 days, a mean Age of Older Litter of 15 days, and a mean Size of Focal and Older Litter of seven pups, where applicable.
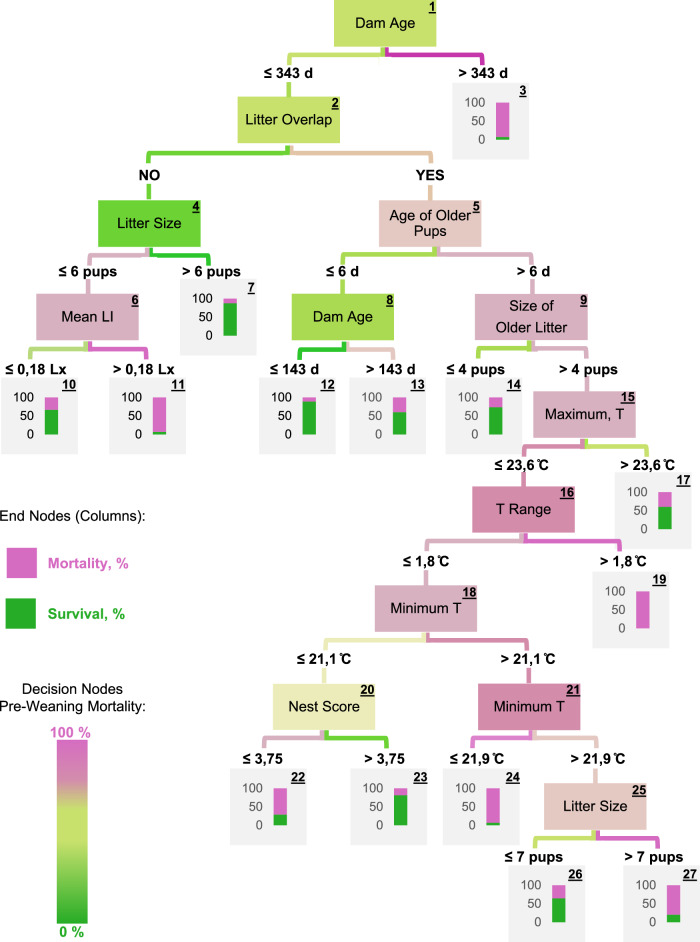
The results of the Decision Tree (Fig. 3), obtained with a balanced subset of 172 micro-environmentally monitored litters, are in agreement with those described above (obtained with the entire dataset and overlapped litters). On the Decision Tree, Dam Age represented the first and thus most important attribute (factor), splitting the data at a Dam Age threshold of 343 days, meaning that for dams above the threshold, only 7.4% of the 68 pups survived (Fig. 3, Node 3), compared to 59.7% of the 1411 pups born from dams younger than 343 days of age.
Fig. 3. Decision tree.
Description of pup death by using a total of 10 social (Dam Age, Litter Overlap, Age of Older Pups, Size of Older Litter, Size of Focal Litter) and micro-environment (Mean Light Intensity, Cage Temperature Range, Maximum and Minimum Cage Temperature, and Nest Score, on Day 0) attributes (factors) with an overall accuracy of 84.1% and a rate of correctly classified instances (TP-rate) of 80.9%. A total of 1479 instances were used in the balanced dataset and the Confusion Matrix is presented in Supplementary Table 4. T temperature, LI light intensity.
Litter overlap and older pups
Overlapped litters, i.e. those born in the presence of older pups leading to cohabitating litters in a cage, occurred in 63.1% and 11.3% of all trio- (n = 314) and pair-housed (n = 195) litters, respectively. The older litters had, on average, 6.7 ± 3.1 pups that were 15.0 ± 7.7 days old on the day that the focal litter was born (Day 0, Supplementary Note 1.2).
Overlapped litters (n = 220) had 12.1 percentage points higher occurrence of total litter loss (litter dying entirely) and 19.5 percentage points lower occurrence of no mortality compared to non-overlapped litters (n = 289; Fig. 1a). The estimated probability of pup death was 26.5 percentage points higher (P < 0.001) in overlapped compared to non-overlapped litters (Fig. 2b).
Pup Probability of Death increased (P < 0.001) as the Size (Fig. 2c) and Age (Fig. 2d) of Older Litter was higher on the day of the focal litter’s birth. On the Decision Tree (Fig. 3), Litter Overlap appears as the second node, nearly as relevant as Dam Age for describing pup death, representing 95.4% of the micro-environmentally monitored dataset (Fig. 3, Node 2). ~79.0% of all the 695 pups born to younger dams in non-overlapped litters survived.
On the right side of the Decision Tree, ~59.1% of the 716 pups of younger dams in overlapped litters died. When five older pups or more (≥7-day-old) were present in the cage, 66.4% of 581 newborn pups died (Fig. 3, Node 9). Overall, the left upper side of the Decision Tree (green portion of Fig. 3) depicts a path of pup survival.
Size of the focal litter
On average, 6.6 ± 2.8 pups were born per litter (range 1–14 pups), and 4.9 ± 3.0 pups were weaned per litter on day 21 post-partum (Supplementary Note 1.3). In overlapped litters, Pup Probability of Death increased in focal litters with either below six or above 11 pups born, in a quadratic fashion (P = 0.031, see Fig. 2e). Pup Probability of Death was higher in overlapped litters compared with non-overlapped litters for all categories of Size of the Focal Litter.
In the Decision Tree, the Size of Focal Litter appears to be relevant after Node 4 (Fig. 3), in which pups were born to dams of 343 days of age or less in non-overlapped litters. 87.4% of 585 pups of this group, which were born in litters greater than six pups survived (Fig. 3, Node 7), whereas only 34.5% of 110 pups which were born in litters of six pups or less survived.
The increased mortality of larger focal litters, initially found by analysing the entire dataset, only appeared in the very last nodes of the Decision Tree, in that 79.7% of (64) pups born in litters of over seven pups died (Fig. 3, Nodes 27). Independently of the Size of the Focal Litter, these pups (Fig. 3, Node 25) were already at high risk for pre-weaning mortality for being in overlapped litters (Fig. 3, Node 2), with over four older pups (Fig. 3, Node 9) which were older than 6 d (Fig. 3, Node 5), i.e. the bottom right side of the tree.
Cage macro- and micro-environment
There were no effects of cage macro-environment (Season and Weekday) on Pup Probability of Death (P < 0.05). Therefore, these terms were removed from the final statistical models. Cage Vibration and frequency and duration of Motion Events did not appear in the Decision Tree describing pup death. Details about the Frequency and Duration of Motion Events and Cage Vibration experienced by litters on Day 0 are summarized in Supplementary Notes 2.1 and 2.2, respectively.
Cage Light Intensity
All litters experienced complete or near complete darkness (0.00–2.00 lx) for at least 60% of Day 0 (Supplementary Note 2.3). In the Decision Tree, Light Intensity appears to be relevant as the last decision node of the left side of the tree, in which pups were born to younger dams (≤343 days of age, Fig. 3, Node 1), in overlapped litters (Fig. 3, Node 2) of six new-born pups or less (Fig. 3, Node 4). ~66.7% of the 51 pups under these conditions and exposed to an average of 0.18 lx or less on Day 0, survived (Fig. 3, Node 10), whereas only 6.8% of 59 pups in similar conditions, except for exposure to a Light Intensity of over 0.18 lx, survived.
Cage temperature
The most recurrent temperature range measured inside the cages was 22.0–22.9 °C, within which 52.3% of the litters spent at least half of their Day 0. Nevertheless, there was substantial temperature variation during Day 0 inside some cages, from 18.8 to 24.7 °C. On average, new-born pups experienced <21.0 °C or over 23.0 °C for 8.3 ± 20.6% and 7.2 ± 15.9% of Day 0, respectively (Supplementary Note 2.4).
On the Decision Tree, the temperature-related attributes that appeared to be relevant for pup survival were Temperature Range (i.e. temperature fluctuation), and Maximum and Minimum Temperature experienced by new-borns within Day 0 (Fig. 3, Nodes 16, 15, and 18 respectively). Thirty-eight (60.3%) of 63 pups that experienced a maximum recorded temperature of over 23.6 °C on Day 0 survived (Fig. 3, Node 17). Conversely, only 23.8% of the 450 pups which experienced a maximum temperature below 23.6 °C survived (Fig. 3, Node 16). All 75 pups that experienced a temperature fluctuation of over 1.8 °C during Day 0 died. Most (79.7%) of the 64 pups that experienced a Minimum Temperature above 21.9 °C and were born in larger litters of above seven pups died (Fig. 3, Node 27).
Nest score
Mean nest score across all 172 litters on Day 0 was 2.45 ± 1.52, within a range of 0–5 (Supplementary Note 2.5). The majority (81.4%) of the 45 pups that experienced a minimum temperature of 21.9 °C or below on Day 0 but had a nest score above 3.75, survived (Fig. 3, Node 23).
Discussion
Pup Probability of Death was higher in overlapped litters, in small (<6 pups) and large (>11 pups) focal litters, and increased with the increase in Dam Age. In overlapped litters, the Pup Probability of Death also increased with the increase in the Number and Age of Older Pups. Some of these risks were alleviated in cages with reduced temperature variability, higher temperatures (if litters were ≤7 pups), reduced exposure to light, and in those with high-scoring nests. The obtained overall pre-weaning mortality of 25.6% is in agreement with the literature6,7 and with our own previous pre-weaning mortality findings ranging from 14% to 39% in an analysis involving over 34,000 litters10.
The reduced pup survivability as dams reach higher parities and/or older age found in this and our previous8,10 studies is in line with other studies reporting reduced survivability in older dams8–10,23 but contradicts to some extent the received wisdom that first-parity females are less successful due to inexperience, a view which finds some limited support in literature showing reduced survivability of litters from first-parity and/or younger female mice18.
Dam age and parity are confounded in this study as higher parity dams are also older dams. Furthermore, interpretation of results related to Dam Age should be done with caution, for being confounded with Sire Age in this study. It is known that the presence of the sire can facilitate pup survival24, but not much has been done to evaluate the effect of Sire Age on paternal care.
The increase in the probability of pup death in overlapped litters found in this study is consistent with our previous work, recorded in both experimental (n = 109)8 and observational (n > 34,000) litters10. However, the differences in the probability of pup death between overlapped and non-overlapped litters found in the studies of 2019 (72.3% vs. 40.0%, respectively)8 and the present study (31.5% vs. 5.0%, respectively) were substantially higher than differences found in the historical breeding records in 2020 (27.9% vs. 21.4% and 5.0% vs. 2.9%, in two collaborators)10.
Such differences in magnitude may have been at least partially related to the increased accuracy in estimating mouse pre-weaning mortality by counting pups daily, as in the present work and in Brajon et al.8. Conversely, the historical breeding records corresponded to data manually entered into the breeding software by animal caretakers, whose routine protocol involved counting pups only once either weekly or biweekly10. In a study with 193 C57BL/6 litters, we demonstrated that a pup-counting method of daily inspecting breeding trios through the transparent cage wall (occasionally opening the lid when necessary) led to an underestimation of the number of pups that were born by 35% and those that died by 102% relative to data from daily inspections combined with video observations11. Studies in which pup counting is not conducted rigorously from the day of birth may underestimate pre-weaning mortality even more, and events of total litter loss may go entirely unrecorded due to pup cadavers being cannibalized.
Parental care is essential for the survival of mouse pups, which are born with nearly no mobility, hairless, blind, and with limited ability to produce heat by non-shivering thermogenesis25, thus dependent on their dams for meeting their nutritional and thermal needs. Therefore, anything that potentially disturbs parental care will likely affect the pups’ probability of survival. Breeding female and male mice, which were required to run a wheel to obtain food reduced the amount of parental care and had fewer and lighter surviving pups compared to controls24. Lactating female mice repeatedly exposed to an acute social stressor showed less nursing-, sniffing-, and licking contact with pups, more fragmented and disorganized maternal care and lower pup-survival rates compared with controls26.
While there were no experimental stressors in the present study, the higher stocking densities (animals per unit of cage-floor area) in cages with overlapped litters may have decreased the cage air and bedding quality. Increased urine in the bedding material leads to higher ammonia concentration, resulting from urea decomposition. In breeding pairs, increasing the floor area from 300 to 676 cm2 reduced ammonia levels from well above to almost at the 25-ppm general accepted level for human health27. Given that ammonia has been demonstrated to harm mice’ health28, adults of overlapped cages may have invested more energy into coping with a harsher micro-environment, and less in parental care. Brajon et al.8 found that the combined total amount of time spent in parental care by trio-housed C57BL/6 adult-breeding mice was 20% less in overlapped compared to non-overlapped litters.
Dams of overlapped litter may have more complications during parturition, leading to stillbirths. Parturition length has been reported to be as long as 20 h in dams of overlapped litters8. Long parturition has been associated with changes in maternal behaviour, offspring survival, and entire-litter loss in mice29, something also observed in other mammalian species30,31. Our preliminary data from pup post-mortem analyses suggest that the majority of C57BL/6 pups found dead in trio- and pair-cages are either stillborn or die before having been able to suckle32,33.
Older pups are more mobile and heavier, which may make access to teats more challenging for new-borns through competition and increase the probability of pup death. Older pups may displace new-borns from the dams’ teats and consume the post-partum iron-rich milk of dams. Lactating female mice are able to increase their milk production if more pups other than those from her own litter start consuming its milk soon after parturition. However, the increase in the milk’s nutritional contents is not necessarily proportional to the total number of additional pups, while dams will nurse pups indiscriminately34,35.
Moreover, dams do not seem to be able to increase their milk production and quality at late lactation when new pups are added to a communal nest35,36. This may explain the reduced pup survival in an overlapped litter with large age gaps: under these circumstances, mate females will probably not increase their milk production when new pups are born. With increased stocking density and competition for access to milk in cages with overlapped litters, new-borns may also be at higher risk for trampling by the older cage mates. This may also explain why the increased age gap between overlapped litters and the increased Size of the Older Litter (number of pups) enhanced the probability of new-borns to die.
A decreased number of pups weaned was found in a study in which female mice nested communally in a barn, compared to females raising pups solitarily37. The authors hypothesized that female infanticide may be one of the mechanisms explaining pup mortality in communal nests. However, previous research from our group revealed that infanticide is quite rare among C57BL/6 laboratory mice8,11,38, although once dead, pups are often entirely or partially cannibalized.
The quadratic effect of the Size of the Focal Litter on Pup Probability of Death is in agreement with the results of our study of breeding records10, which showed an increase in the probability of pup death in both small (<4 pups) and large (>11 pups) litters. In the present study, the effect of the focal litter being large (>11 pups) was substantially more pronounced in overlapped litters, while small litter sizes affected the Pup Probability of Death in both overlapped and non-overlapped litters similarly.
The reasons for increased risks for pup death in large overlapped focal litters are probably similar to the reasons why a large number of older pups in the cage affect focal-Pup Probability of Death regarding cage stocking density and access to milk. Interestingly, König et al.35 reported that average-sized litters of 7.3 pups demanded the least amount of energy per unit of weight for metabolism and growth during days 5–16 post-partum, compared to litters of 6 and 12 pups. This is the period in which the pups obtain energy, mainly from the dam’s milk before starting to eat solid food. Knowing that pups of small litters generally have higher weaning weight than larger litters35,36, our finding of a reduced risk for pup death in average-sized litters suggests that litter size of approximately seven pups may be optimal in the trade-off between number of pups and their size.
Increased probability of pup death in small litters could also be related to reduced maternal investment in small litters. Female mice were demonstrated to invest less in defending their litter against one male intruder when litters were of four pups compared to eight pup litters39. If laboratory mice invest in maternal care proportionally to the expected benefits, small litters in our study may have received less maternal care needed for pup survival29,40. This is in line with our previous findings in an experimental study where video-recorded litters that were born small (2–7 pups) had reduced survival compared to litters of 8–14 pups8.
The results depicted by the Decision Tree (Fig. 3) are in agreement with the logistic-regression models, in which the most important variables were the social ones: Dam Age, Litter Overlap, Size of Focal Litter, Number and Age of Older Pups, representing the most relevant attributes (upper decision nodes of the Decision Tree, Fig. 3).
Cage Vibration did not appear to be relevant for describing pup death in this study. However, vibration resulting from cage disturbance is confounded with vibration from mice’s own movement in the cage, making it difficult to assess the effects of cage manipulation on pup survival. Similarly, Human Motion Events near the cage on Day 0 did not explain pup death. Although the ability of mice to conceal signs of pain is accentuated in the presence of olfactory cues emanating from male humans41, it is well established that mice, as nocturnal animals, possess limited visual acuity42, and the extent to which mice perceive humans moving outside their cage remains unclear.
Nevertheless, it is also possible that the mice were habituated to non-threatening sensory stimuli in the macro-environment, which may be small compared to all the sensorial stimuli from the inside of the cage and mice in neighbouring cages.
Cage Light Intensity only appeared to be relevant for litters of less than six pups (Fig. 3, Node 6). Light Intensity could be confounded with cage location within the rack and possibly with cage handling, as a light-intensity assessment without mice in the cages, prior to the study, showed a variation from 5.00 lx (near the bottom of the rack) to 550.00 lx at the top of the rack and approximately 980.00 lx at the transfer station, where cages were routinely (Supplementary Table 3) cleaned. However, cages of this study were randomly assigned to slots avoiding top-, bottom-, and side-rows of the rack, probably explaining why all the studied litters experienced complete or near complete darkness (0.00–2.00 lx) for most of Day 0.
Nevertheless, momentary high levels of Light Intensity were recorded among cages during Day 0. The recommended maximum Light Intensity of 325.00 lx43 was only exceeded in ~3.5% of the litters, probably during pup counting procedures, in which cages were placed with their lid open on the transfer station while with the fans operating and working lights on. The relative low light levels found in the present work were in agreement with that reported by Barabas et al.44.
Despite the breeding rooms’ automatic temperature control being set for 21.0 °C, a variation in Cage Temperature of 4.0 °C (within the cage) to 6.0 °C (between cages) was found during Day 0 (Supplementary Note 2.4). As the cages used in this study did not have individual ventilation, variation in room temperature could have driven cage temperature variation. Cage stocking density and animal activity may also have affected Cage Temperature. Additional linear regressions performed for Mean-, Minimum-, and Maximum Cage Temperatures on Day 0 as functions of total estimated pup mass in the cage (considering data on C57BL/6 pup weight gain from the Mouse Phenome Database at the Jackson Laboratory) indicated that for every increase in 1 g of pup mass, Mean-, Minimum-, and Maximum- Cage Temperatures would significantly have increased by 0.0033, 0.0029, and 0.0069 °C, respectively.
Regarding the increased survival at cage maximum temperatures above 23.6 °C (Node 7, Fig. 3), it is possible that the probability of pups dying in risky situations (when born in the presence of more than four older pups of at least 7 days of age) was somewhat counter-balanced when the maximum registered temperature during the first few hours post-partum was above 23.6 °C.
There is increasing evidence in the literature that laboratory mice are cold-stressed at the commonly set room temperatures of 20.0–22.0 °C. When provided with a temperature gradient, 2-month-old mice chose a minimum of 24.0 °C when highly active, to 30.0 °C when levels of activity were low, whereas aged mice (11-month-old or older) chose a minimum temperature level of 26.0 °C when active16. Overall, mice spent more than twice and five times as much time in cages with 30.0 °C than in 25.0 and 20.0 °C cages, respectively. Mice showed evidence of cold stress with altered organ development even at ambient temperatures of 22.0 °C45.
Kgwatalala et al.46 found that survivability of laboratory mice is significantly greater at ambient temperature of 31.0 °C (98.6%), compared to 21.0 °C (91.9%). Simons et al.47 demonstrated that despite the decrease in milk production by vole (Microtus arvalis) dams at an ambient temperature of 30.0 °C, pup survivability was significantly higher than at 21.0 °C.
Tsubota et al.48 demonstrated that mouse and hamster pups fully develop homoeothermy at around day 14 post-partum and thermogenesis of Brown Adipose Tissue (BAT) is essential for thermoregulation of pups even in non-cold environments (23.0 °C). Thus, new-born pups in higher temperatures may have experienced more favourable thermal conditions for their survival, while those which were exposed to lower maximum temperatures and to a wider range of temperature variation (>1.8 °C on Day 0) were more likely to die (Fig. 3, Node 19).
One way to give breeding mice some control over their micro-environment is to provide nest material. Nude (hairless) mice provided with nesting material built higher-scoring nests, had more pups born and weaned, and needed less food to produce 1 g of the weaned pup, in comparison to controls, suggesting that the energy was redirected from producing heat to reproduction14.
Another study reported a 30.0% mortality rate for pups of C57BL/6 pairs that did not receive nest material while those receiving nest material had mortality rates of <5.0%6. Nest building and use have also been associated with pup survival. Dams with surviving litters were observed to spend less time outside the nest and more time building the nest prior to parturition than dams losing their litter29.
Providing BALB/c mice with nesting material reduced the expression of mRNA for UCP1 protein, indicating a reduced use of brown adipose tissue to generate heat49. Mice are even willing to work by transferring nesting material from a control cage to a warmer cage to achieve an optimal combination of nest quality and micro-environment temperature50.
However, these temperature-related ending nodes represent only 4.3–7.3% of the data used to build the Decision Tree, with a very specific set of social characteristics (see Fig. 3, Nodes 2, 5, and 9). Cage micro-environmental variation seemed to contribute far less to explaining pup mortality than the social factors, probably partly because the temperature and light ranges were relatively narrow and within accepted EU regulatory limits for mouse breeding.
Pup Probability of Death generally increased with the increase in Dam Age, with small (≤6 pups) and large (>11 pups) litters, and numerous older pups (>4 pups and/or within 7–25 days of age) in overlapped litters. Effects of cage temperature and light intensity were limited, probably because these factors only varied within narrow ranges: this does not imply that the currently accepted ranges are optimal. No effect of cage vibration or human proximity resulting from husbandry routines was detected. Litter overlap has been demonstrated to substantially and significantly affect the Pup Probability of Death. Whether litter overlap increases pup death through mechanisms involving reduced parental care, issues related to high stocking densities, sibling competition for access to milk, or even gestation disturbance requires further research. Studies should also focus on creating practical husbandry routines to either avoid litter overlap or reduce its negative effects on pup survivability.
Methods
Animals and housing
Data were collected in the AAALAC-accredited Animal Breeding Facility of the Institute for Research and Innovation in Health (i3S) at the University of Porto, from May 2017 to May 2019. A total of 3380 C57BL/6J mice were counted daily from newborn to Day 4 post-partum. Mice were from a total of 509 litters from randomly selected cages in three breeding rooms.
The studied mice were housed in trios (two breeding females and one male, n = 313), and pairs (one breeding female and one male, n = 196), as part of the i3S’s normal mouse housing protocol, in Type III and Type II Tecniplast Filter-Top Static cages, respectively. Trio- and pair-housing mice are common housing configurations for routine breeding mice in laboratory animal facilities. The unbalanced number of trio- and pair-litters in this study was due to the unbalanced trio- and pair-C57BL/6J mouse cages available across the breeding rooms where this pup-counting study was conducted. All counted mice were part of the i3S’s breeding programme to produce mice for experimental purposes, thus no new animals were generated solely for the purposes of this study. This study was part of the “Alive Pup” project under licence ID “DGAV 15188/2017-06-30” issued by the national Directorate-General for Food and Veterinary, with approval (ID “2016-10”) of the i3S’s Institutional Animal Care and Use Committee. We have complied with all relevant ethical regulations for animal use. Supplementary Table 3 presents a summary of the main routine practices and cage characteristics corresponding to the studied mice.
Study pup counting routine
Cages were checked twice daily for the presence of new-born litters at 09.00 h and once at 16.00 h (end of normal work day). Pup counting was performed when litters were found (Day 0) once a day at 09.00 h for the following four days (Day 1–Day 4) and once again prior to weaning at Day 21. The exact time of birth was unknown. Day 0 represents the day a litter was found born, but birth could have happened from zero to 17 h before a litter was found (if found at 09.00 h) or from zero to 6 h (if found at 16.00 h).
Pups were counted by placing each cage singly on the transfer station (Tecniplast CS5, Buguggiate, VA, Italy), opening its lid, and counting pups with minimal handling. When pups were obscured by the nest and/or were too numerous and piled on each other, the designated pup counter (a mouse-welfare trained scientist at 09.00 h or the facility’s designated animal care-taker at 16.00 h) would lightly touch the pups with the tip of a finger, while wearing latex gloves to allow for better visibility. After pups were counted, the nest was put together as found (when touching pups was needed), the lid was replaced, and the cage returned to its original slot in the rack. During this process, any missing pups or pups which were found dead were coded as “dead”. The date when they were found dead was recorded, and dead pups were removed from the cage and properly disposed of.
Cage micro-environment monitoring
Cage temperature and light intensity
Cage micro-environment was monitored in 172 randomly selected litters (82 overlapped and 90 not overlapped litters). Cage temperature and light Intensity were automatically recorded once every 10 min by temperature/light loggers (HOBO® UA-002-xx, ©2009-2017 Onset Computer Corporation, USA) placed on the inside upper front part of the cages’ lids, above the metal grid to prevent mice from having access to the loggers. This placement of the temperature/light intensity loggers was intended to record a close approximation of the temperature inside each cage and the light intensity reaching the front of the cage. Ambient room temperature was obtained from the room climate control system once a day. The room temperature sensor was fixed near a wall next to the transfer station (approximately 0.8 and 1.5 m distant from the first and last study cage slots, respectively). All breeding rooms were ventilated with a temperature-controlled and filtered air supply to achieve 15–20 air changes per hour
Given the importance of supplying nest material for breeding mice14, nests were daily scored according to their height and closure of the walls surrounding the nest cavity. Nest scores were used as an indication of nest quality, by using a scale of 0 (undisturbed nesting material) to 5 (dome shape, completely enclosed nest)51.
Cage Acceleration was automatically recorded once every 30 s by acceleration loggers (HOBO® UA-004-64, ©2009–2014 Onset Computer Corporation, USA) placed on the outside mid (vertical axis), right (horizontal axis), and front (facing corridor between racks) parts of the same randomly selected cages as for the temperature/light intensity monitoring. The placement of the acceleration loggers was intended to provide information on Cage Acceleration (to estimate Cage Vibration), while not being accessible to the mice and not obstructing the cages’ inside view from the outside of the rack.
Rack-corridor proximity monitoring
A proximity logger (HOBO® UX90-006x, ©2012–2015 Onset Computer Corporation, USA) was placed on a wall facing the corridor between the racks that had experimental cages. The proximity logger was automatically activated every time there was movement on the outside of the experimental cages. Recorded data corresponding to Day 0 on the number of times one or more person were moving in front of the cage (Motion Events), and the duration of this movement, were retrieved for each litter. The rack proximity monitoring was intended to indicate the frequency and duration of the possible mice’s visual, olfactory, and/or auditory exposure to humans.
Statistical and reproducibility
This study yielded three different dataset configurations that required three distinct statistical approaches to optimize data evaluation:
The complete dataset accounting for all counted pups (n = 3380 pups in 509 litters);
A subset of the complete dataset, comprising only pups from overlapped litters (n = 1377 pups in 220 litters) in which the influence of the older litter on the focal pups could be further explored;
A subset of the complete dataset (micro-environmentally monitored dataset), comprising the 172 randomly selected litters (of which 82 were overlapped and 90 non-overlapped litters) with data on Nest Score, Cage Temperature, Light Intensity, Motion Events, and Vibration (cage micro-environment, n = 1181 pups).
Analysis of the complete and overlapped datasets—a mixed logistic regression approach
In both datasets (entire and subset), pups that died before weaning were coded as 1 (died), while those that survived were coded as 0 (survived), to be used as the response variable. Macro-environmental and social factors were hypothesized to affect pup survivability. Independent variables representing macro-environmental factors originally considered for analysis were Season (Winter, Spring, Summer, Autumn), Month (as an alternative predictor to Season), Weekday (of birth), Year (of birth), and Breeding Room (Rooms 1–3). Independent variables for social factors included Dam Age, Sire Age, Number of Pups Born in the Focal Litter, Litter Overlap (whether or not an older litter was present in the cage at the time of birth of the focal litter), Housing Type (pairs, with one female and one male, vs. trios, with two females and one male in the cage), Age and Size (number of pups) of the Older Litter in the cage on the day that the Focal Litter was born (Day 0). Multicollinearity among independent variables was checked by regressing each independent variable as a function of the remaining ones in a numeric format. Variables Year, Month, Housing Configuration (trio vs. pair), Breeding Room, and Sire Age were excluded from the analysis for being moderate to strongly correlated with one or more of the remaining predictor variables.
The risk of pup death was modelled by mixed logistic regression, using the GLIMMIX procedure on SAS (2018 University Edition, SAS Institute Inc., USA). Two separate analyses were run: one on the complete dataset (Model 1) and one on a subset of the data containing only pups born in situations of litter overlap (Model 2). Model 1 aimed to evaluate the effects of Litter Overlap on the probability of pup death, accounting for macro-environmental effects, Litter Size (number of pups born in the focal litter), and Dam Age. Model 2 aimed to explain the variation in Pup Probability of Death with the variation in the Age and Size (number of pups) of Older Litters, which are only present in overlapped litters, also considering macro-environmental effects and the social factors of Litter Size and Dam Age. In both models, litter identity was included as a random factor to account for clustering, as each pup was considered to be the experimental unit. The models were built by adding one independent variable of interest at a time (Dam Age, Litter Size, and Litter Overlap, YES or NO in the case of Model 1, or Age and Size of the Older Litter, in the case of Model 2), in a stepwise process with bidirectional elimination. Independent variables with P ≤ 0.050 were kept in the model.
The macro-environmental factors Weekday, Season, and Breeding Room were then tested one at a time as confounders, followed by possible two- and three-way interactions and higher order terms, eliminated from the models when not significant (P ≤ 0.050). Least-square means were compared for Litter Overlap (Model 1) considering a 95% confidence interval, and probability predictions for pup death were obtained from Model 2.
Analysis of the micro-environmentally monitored dataset—a data mining approach
Cage micro-environment (Nest Score, Cage Temperature, Light Intensity, Cage Vibration, and Motion Events) was monitored for Day 0 of each studied litter, the most critical period for pup mortality, in addition to the aforementioned macro-environmental and social data. A Data Mining approach was used to evaluate the effects of all measured conditions of cage micro-environment and social factors.
All macro- and micro-environmental data were organized in one single spreadsheet with the respective social factors. Cage Vibration was calculated by the root mean square of the Acceleration values obtained both on the vertical direction alone and on the three directions together during Day 0. Cage Temperature, Light Intensity, and Vibration were averaged for Day 0 and organized into ranges of distinct magnitude. Percentage of Day 0 spent within each range was calculated for each of the studied litters and considered for the data mining approach.
A Chi-Squared Attribute Evaluation was used on WEKA software (version 3.8.6, Waikato Environment for Knowledge Analysis, the University of Waikato, Hamilton, NZ)52 to select the most relevant attributes (factors), based on the attributes’ chi-square statistical values, which represented the worth of each attribute with respect to its class. Attributes with a chi-squared value of zero were removed from the initial spreadsheet. The attributes with chi-squared values above zero were selected as input attributes for the data mining procedure.
Due to the unbalanced nature of the environmentally monitored data, a supervised Resample Filter was applied with a 0.9 bias on WEKA52 to balance the data prior to generating the Decision Tree. The Resample Filter produced a random sub-sample of the data in which the number of pups that died corresponded to ~90% of the number of pups that survived (replacing the original 0.4 bias). The resample technique allowed for the random removal of some of the instances with pups that survived and the addition of closely related non-survival instances. The balancing of the data was performed so that an accurate Decision Tree was possible, able to distinguish pups that died from those that survived. The classifier J-48 was used with a minimum of 40 objects per leaf to classify the balanced data. The Tree’s hyperparameters were tuned by using a 10-fold cross-validation method. Model selection was based on accuracy, precision, and sensibility parameters. The experimental data that support the findings of this study and the numerical source data for graphs presented in this study are available in the respective Microsoft Excel datafiles openly deposited in Figshare with the identifier 10.6084/m9.figshare.25040630)53.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank the i3S’s animal facility team and Jorge Ferreira for their help with data collection, and Professor Luiz Antunes Rodrigues for providing technical input for the data mining process. This work was funded by National Funds through Fundação para a Ciência e a Tecnologia (FCT), under the project UIDB/04293/2020 and by Fundo Europeu de Desenvolvimento (FEDER), Regional funds through the COMPETE 2020, Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by FCT/ Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the projects PTDC/CVT-WEL/1202/2014 (POCI-01-0145-FEDER-016591) and PTDC/CVT-CVT/3248/2021.
Author contributions
G.M.M. contributed to the study conceptualization, methodology, data collection and curation, formal analysis, investigation, project administration, supervision, and writing of the original manuscript draft. S.C.-P. contributed to data collection and curation, writing, reviewing and editing manuscripts. S.B. contributed to the investigation, review, and editing of the manuscript. S.L. contributed to data collection, investigation, review, and editing of the manuscript. I.M.L. contributed to data curation and formal statistical analysis. C.G. and I.A.S.O. contributed to study conceptualization, data curation, funding acquisition, investigation, writing, reviewing, and editing of the manuscript. I.A.S.O. also contributed to project coordination and supervision.
Peer review
Peer review information
Communications Biology thanks Marcell D. Cadney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres. A peer review file is available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06654-z.
References
- 1.European Commission. Summary Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union and Norway in 2019 (European Commission, Brussels, 2023).
- 2.European Commission. Summary Report on the statistics on the use of animals for scientific purposes in the Member States of the European Union and Norway. (European Commission, Brussels, 2022) https://circabc.europa.eu/ui/welcome.
- 3.Festing, M. F. W. Evidence should trump intuition by preferring inbred strains to outbred stocks in preclinical research. ILAR J.55, 399–404 (2014). 10.1093/ilar/ilu036 [DOI] [PubMed] [Google Scholar]
- 4.Mouse Phenome Database at the Jackson Laboratory. Phenotype Measure: Jax5 wean_born_ration (Mouse Phenome Database at the Jackson Laboratory, Bar Harbor, 2020).
- 5.Reeb-Whitaker, C. K. et al. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim.35, 58–73 (2001). 10.1258/0023677011911381 [DOI] [PubMed] [Google Scholar]
- 6.Gaskill, B. N. et al. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS ONE8, e74153 (2013). 10.1371/journal.pone.0074153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inglis, C. A., Campbell, E. R., Auciello, S. L. & Sarawar, S. R. Effects of Enrichment Devices on Stress-related Problems in Mouse Breeding. In Final report for the Animal Welfare Enhancement Award. 1–9 (Johns Hopkins Center for Alternatives to Animal Testing, 2004).
- 8.Brajon, S. et al. Social environment as a cause of litter loss in laboratory mouse: a behavioural study. Appl. Anim. Behav. Sci.218, 104827 (2019). 10.1016/j.applanim.2019.06.008 [DOI] [Google Scholar]
- 9.Weber, E. M., Algers, B., Wurbel, H., Hultgren, J. & Olsson, I. A. S. Influence of strain and parity on the risk of litter loss in laboratory mice. Reprod. Domest. Anim.48, 292–296 (2013). 10.1111/j.1439-0531.2012.02147.x [DOI] [PubMed] [Google Scholar]
- 10.Morello, G. M. et al. High laboratory mouse pre-weaning mortality associated with litter overlap, advanced dam age, small and large litters. PLoS ONE15, 1–27 (2020). 10.1371/journal.pone.0236290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brajon, S. et al. All the pups we cannot see: cannibalism masks perinatal death in laboratory mouse breeding but infanticide is rare. Animals11, 2327 (2021). 10.3390/ani11082327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Commission. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017 (European Commission, Brussels, 2020).
- 13.Nasu, M. et al. Deficient maternal behavior in multiparous Pou3f2⊿ mice is associated with an impaired exploratory activity. Behav. Brain Res.427, 113846 (2022). 10.1016/j.bbr.2022.113846 [DOI] [PubMed] [Google Scholar]
- 14.Gaskill, B. N., Winnicker, C., Garner, J. P. & Pritchett-corning, K. R. The naked truth: breeding performance in nude mice with and without nesting material. Appl. Anim. Behav. Sci.143, 110–116 (2013). 10.1016/j.applanim.2012.10.009 [DOI] [Google Scholar]
- 15.González, M. M. C. Dim light at night and constant darkness: two frequently used lighting conditions that jeopardize the health and well-being of laboratory rodents. Front. Neurol.9, 10.3389/fneur.2018.00609 (2018). [DOI] [PMC free article] [PubMed]
- 16.Gordon, C. J., Becker, P. & Ali, J. S. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol. Behav.65, 255–262 (1998). 10.1016/S0031-9384(98)00148-6 [DOI] [PubMed] [Google Scholar]
- 17.Bedrosian, T. A., Vaughn, C. A., Weil, Z. M. & Nelson, R. J. Behaviour of laboratory mice is altered by light pollution within the housing environment. Anim. Welf.22, 483–487 (2013). 10.7120/09627286.22.4.483 [DOI] [Google Scholar]
- 18.Whitaker, J. W., Moy, S. S., Pritchett-Corning, K. R. & Fletcher, C. Effects of enrichment and litter parity on reproductive performance and behavior in BALB/c and 129/Sv mice. J. Am. Assoc. Lab. Anim. Sci.55, 387–399 (2016). [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskill, B. N. & Pritchett-Corning, K. R. The effect of cage space on behavior and reproduction in Crl:CD1(Icr) and C57BL/6NCrl laboratory mice. PLoS ONE10, 1–22 (2015). 10.1371/journal.pone.0127875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potgieter, F. J. & Wilke, P. I. Effect of different bedding materials on the reproductive performance of mice. J. S. Afr. Vet. Assoc.68, 8–15 (1997). 10.4102/jsava.v68i1.858 [DOI] [PubMed] [Google Scholar]
- 21.Hull, M. A., Reynolds, P. S. & Nunamaker, E. A. Effects of non-aversive versus tail-lift handling on breeding productivity in a C57BL/6J mouse colony. PLoS ONE17, e0263192 (2022). 10.1371/journal.pone.0263192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarín, J. J. et al. Delayed motherhood decreases life expectancy of mouse offspring. Biol. Reprod.72, 1336–1343 (2005). 10.1095/biolreprod.104.038919 [DOI] [PubMed] [Google Scholar]
- 23.Brown, R. E., Mathieson, W. B., Stapleton, J. & Neumann, P. E. Maternal behavior in female C57BL/6J and DBA/2J inbred mice. Physiol. Behav.67, 599–605 (1999). 10.1016/S0031-9384(99)00109-2 [DOI] [PubMed] [Google Scholar]
- 24.Wright, S. L. & Brown, R. E. Maternal behavior, paternal behavior, and pup survival in CD-1 albino mice (Mus musculus) in three different housing conditions. J. Comp. Psychol.114, 183–192 (2000). 10.1037/0735-7036.114.2.183 [DOI] [PubMed] [Google Scholar]
- 25.Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev.84, 277–359 (2004). 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 26.Rosinger, Z. J., Mayer, H. S., Geyfen, J. I., Orser, M. K. & Stolzenberg, D. S. Ethologically relevant repeated acute social stress induces maternal neglect in the lactating female mouse. Dev. Psychobiol.63, e22173 (2021). 10.1002/dev.22173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eveleigh, J. R. Murine cage density: cage ammonia levels during the reproductive performance of an inbred strain and two outbred stocks of monogamous breeding pairs of mice. Lab. Anim.27, 156–160 (1993). 10.1258/002367793780810432 [DOI] [PubMed] [Google Scholar]
- 28.Vogelweid, C. M. et al. Effects of a 28-day cage-change interval on intracage ammonia levels, nasal histology, and perceived welfare of CD1 mice. J. Am. Assoc. Lab. Anim. Sci.50, 868–878 (2011). [PMC free article] [PubMed] [Google Scholar]
- 29.Weber, E. M., Hultgren, J., Algers, B. & Olsson, I. A. S. Do laboratory mouse females that lose their litters behave differently around parturition? PLoS ONE11, e0161238 (2016). 10.1371/journal.pone.0161238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarvis, S. et al. The responsiveness of sows to their piglets in relation to the length of parturition and the involvement of endogenous opioids. Appl. Anim. Behav. Sci.63, 195–207 (1999). 10.1016/S0168-1591(99)00013-1 [DOI] [Google Scholar]
- 31.Langendijk, P. & Plush, K. Parturition and its relationship with stillbirths and asphyxiated piglets. Animals9, 10.3390/ani9110885 (2019). [DOI] [PMC free article] [PubMed]
- 32.Capas-Peneda, S., Morello, G. M., Lamas, S., Olsson, I. A. S. & Gilbert, C. Causes of death in newborn C57BL/6J mice. Preprint at bioRxiv10.1101/2020.02.25.964551 (2020).
- 33.Capas-Peneda, S., Saavedra Torres, Y., Prins, J.-B. & Olsson, I. A. S. From mating to milk access: a review of reproductive vocal communication in mice. Front. Behav. Neurosci.16, 10.3389/fnbeh.2022.833168 (2022). [DOI] [PMC free article] [PubMed]
- 34.Ferrari, M., Lindholm, A. K. & König, B. A reduced propensity to cooperate under enhanced exploitation risk in a social mammal. Proc. Biol. Sci.283, 10.1098/rspb.2016.0068 (2016). [DOI] [PMC free article] [PubMed]
- 35.König, B., Riester, J. & Markl, H. Maternal care in house mice (Mus musculus): II. The energy cost of lactation as a function of litter size. J. Zool.216, 195–210 (1988). 10.1111/j.1469-7998.1988.tb02425.x [DOI] [Google Scholar]
- 36.Johnson, M. S., Thomson, S. C. & Speakman, J. R. Limits to sustained energy intake: I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol.204, 1925–1935 (2001). 10.1242/jeb.204.11.1925 [DOI] [PubMed] [Google Scholar]
- 37.Ferrari, M., Lindholm, A. K. & König, B. Fitness consequences of female alternative reproductive tactics in house mice (Mus Musculus domesticus). Am. Nat.193, 106–124 (2019). 10.1086/700567 [DOI] [PubMed] [Google Scholar]
- 38.Weber, E. M., Algers, B., Hultgren, J. & Olsson, I. A. S. Pup mortality in laboratory mice–infanticide or not? Acta Vet. Scand.55, 83 (2013). 10.1186/1751-0147-55-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maestripieri, D. & Alleva, E. Litter defence and parental investment decision rules in house mice. Behav. Process.23, 223–230 (1991). 10.1016/0376-6357(91)90052-2 [DOI] [PubMed] [Google Scholar]
- 40.Weber, E. M. & Olsson, I. A. S. Maternal behaviour in Mus musculus sp.: an ethological review. Appl. Anim. Behav. Sci.114, 1–22 (2008). 10.1016/j.applanim.2008.06.006 [DOI] [Google Scholar]
- 41.Sorge, R. E. et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods11, 629–632 (2014). 10.1038/nmeth.2935 [DOI] [PubMed] [Google Scholar]
- 42.Peirson, S. N., Brown, L. A., Pothecary, C. A., Benson, L. A. & Fisk, A. S. Light and the laboratory mouse. J. Neurosci. Methods300, 26–36 (2018). 10.1016/j.jneumeth.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council. Guide for the Care and Use of Laboratory Animals Vol. 46 (The National Academies Press, Washington, DC, 2011).
- 44.Barabas, A. J., Darbyshire, A. K., Schlegel, S. L. & Gaskill, B. N. Evaluation of ambient sound, vibration, and light in rodent housing rooms. J. Am. Assoc. Lab Anim. Sci.61, 660–671 (2022). 10.30802/AALAS-JAALAS-22-000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon, C. J. et al. Behaviorally mediated, warm adaptation: a physiological strategy when mice behaviorally thermoregulate. J. Therm. Biol.44, 41–46 (2014). 10.1016/j.jtherbio.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 46.Kgwatalala, P. M. & Nielsen, M. K. Performance of mouse lines divergently selected for heat loss when exposed to different environmental temperatures. II. Feed intake, growth, fatness, and body organs. J. Anim. Sci.82, 2884–2891 (2004). 10.2527/2004.82102884x [DOI] [PubMed] [Google Scholar]
- 47.Simons, M. J. P. et al. Ambient temperature shapes reproductive output during pregnancy and lactation in the common vole (Microtus arvalis): a test of the heat dissipation limit theory. J. Exp. Biol.214, 38–49 (2011). 10.1242/jeb.044230 [DOI] [PubMed] [Google Scholar]
- 48.Tsubota, A. et al. Role of brown adipose tissue in body temperature control during the early postnatal period in Syrian hamsters and mice. J. Vet. Med. Sci.81, 1461–1467 (2019). 10.1292/jvms.19-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaskill, B. N. et al. Impact of nesting material on mouse body temperature and physiology. Physiol. Behav.110, 87–95 (2013). 10.1016/j.physbeh.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 50.Gaskill, B. N. et al. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS ONE7, 1–11 (2012). 10.1371/journal.pone.0032799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hess, S. E. et al. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J. Am. Assoc. Lab. Anim. Sci.47, 25–31 (2008). [PMC free article] [PubMed] [Google Scholar]
- 52.Frank, E., Hall, M., Trigg, L., Holmes, G. & Witten, I. H. Data mining in bioinformatics using Weka. Bioinformatics20, 2479–2481 (2004). 10.1093/bioinformatics/bth261 [DOI] [PubMed] [Google Scholar]
- 53.Morello, G. M. et al. Dataset. Proper micro-environment alleviates mortality in laboratory mouse breeding induced by litter overlap and older dams. figshare 10.6084/m9.figshare.25040630 (2024). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.