Abstract
Background
Ventilation management may differ between COVID–19 ARDS (COVID–ARDS) patients and patients with pre–COVID ARDS (CLASSIC–ARDS); it is uncertain whether associations of ventilation management with outcomes for CLASSIC–ARDS also exist in COVID–ARDS.
Methods
Individual patient data analysis of COVID–ARDS and CLASSIC–ARDS patients in six observational studies of ventilation, four in the COVID–19 pandemic and two pre–pandemic. Descriptive statistics were used to compare epidemiology and ventilation characteristics. The primary endpoint were key ventilation parameters; other outcomes included mortality and ventilator–free days and alive (VFD–60) at day 60.
Results
This analysis included 6702 COVID–ARDS patients and 1415 CLASSIC–ARDS patients. COVID–ARDS patients received lower median VT (6.6 [6.0 to 7.4] vs 7.3 [6.4 to 8.5] ml/kg PBW; p < 0.001) and higher median PEEP (12.0 [10.0 to 14.0] vs 8.0 [6.0 to 10.0] cm H2O; p < 0.001), at lower median ΔP (13.0 [10.0 to 15.0] vs 16.0 [IQR 12.0 to 20.0] cm H2O; p < 0.001) and higher median Crs (33.5 [26.6 to 42.1] vs 28.1 [21.6 to 38.4] mL/cm H2O; p < 0.001). Following multivariable adjustment, higher ΔP had an independent association with higher 60–day mortality and less VFD–60 in both groups. Higher PEEP had an association with less VFD–60, but only in COVID–ARDS patients.
Conclusions
Our findings show important differences in key ventilation parameters and associations thereof with outcomes between COVID–ARDS and CLASSIC–ARDS.
Trial registration
Clinicaltrials.gov (identifier NCT05650957), December 14, 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-02910-2.
Keywords: Acute respiratory distress syndrome, ARDS, COVID–19, Critical care, Mechanical ventilation, Ventilation management
Background
The high numbers of patients who needed invasive ventilation early in the unprecedented pandemic of coronavirus disease 2019 (COVID–19) has led to numerous studies of epidemiology, ventilation management and outcomes in patients with acute respiratory distress syndrome (ARDS) related to an infection with SARS–CoV–2. COVID–19 ARDS would differ from ARDS before the pandemic (CLASSIC–ARDS) in several aspects [1, 2], and different phenotypes have even been suggested [3, 4].
The number of studies that directly compared ventilation management of COVID–ARDS with CLASSIC–ARDS is limited [5, 6]. It remains uncertain whether practice of invasive ventilation in COVID–ARDS patients really differed from that in CLASSIC–ARDS patients. It is also unknown whether associations of certain aspects of ventilation with outcomes found in CLASSIC–ARDS also exist in COVID–ARDS. This would have serious implications on how to set the ventilator in the two patient groups, as then certain recommendations in guidelines for ventilation in CLASSIC–ARDS may not apply in COVID–ARDS [7].
We performed an analysis of a conveniently–sized database that pooled the data of individual patients of six observational ventilation studies, four of which were conducted in the COVID–19 pandemic and two pre–pandemic, to compare epidemiology, ventilator management and associations of ventilation characteristics and outcome between COVID–ARDS and CLASSIC–ARDS patients. To have comparable patient groups, we only selected patients with ARDS from a respiratory infection from the two pre–pandemic studies. We hypothesized that key ventilator parameters would be different between the two groups, and used multivariable analyses to determine associations with outcomes.
Methods
Study design and participants
This is a meta–analysis using the individual patient data of patients in six preselected large observational studies focusing on a diverse representation of epidemiological features and ventilation management in both COVID–19 and pre–pandemic ARDS. The six studies were selected because they all contained detailed data on epidemiological features, ventilation data, and outcomes, originating from various regions worldwide, both in resource–limited and resource–rich settings.
The corresponding authors of the original studies accepted the invitation, after which the data dictionaries of the studies were compared to check whether the data could be harmonized. Then, the databases were transferred after local approval and agreement on the analysis plan of the current investigation.
The two pre–pandemic studies were the national ‘Epidemiology of Respiratory Insufficiency in Critical Care’ study (ERICC) conducted in 2011 in Brazil [8], and the international ‘Large Observational Study to UNderstand the Global Impact of Severe Acute Respiratory FailurE’ study (LUNG SAFE) conducted in 2014 in 50 countries worldwide [9]. All four studies were conducted during the COVID–19 pandemic, ranging from March 2020 to 2021 and included: the national ‘Practice of Ventilation in COVID–19 patients’ study (PRoVENT–COVID) from The Netherlands [10], the national ‘EPIdemiology of Critical COVID–19’ study (EPICCoV) from Brazil [11, 12], the national ‘Centro de Investigación Biomédica en Red Enfermedades Respiratorias COVID–19 study’ (CIBERESUCICOVID) from Spain [13], and the national ‘Sociedad Argentina de Terapia Intensiva–COVID–19 study’ (SATI–COVID–19) from Argentina [14].
The study protocols of the original studies were approved by Institutional Review Boards if applicable, and need for individual patient informed consent was waived for all studies due to their observational designs. Details of all studies can be found in the original publications [8–10, 12–14]. We invited the corresponding investigators of the original studies to provide us the case report forms and data dictionaries, and the data of all patients. The creation of the pooled database did not require additional ethical approval. The databases of the original studies were harmonised using the case report forms and data dictionaries, and finally merged. This current analysis is registered at clinicaltrials.gov (study identifier NCT05650957), and its statistical analysis plan was finalized before cleaning and closing of the database.
Patients in the merged database were eligible for participation in this current analysis if: (1) aged 18 years or higher; (2) having received invasive ventilation within the first 48 h of ICU admission, regardless of its duration; and (3) fulfilling the Berlin definition of ARDS. We excluded CLASSIC–ARDS patients when ARDS was reported not to be caused by a respiratory infection.
Data available for merging
The following baseline and demographic variables were available for merging into the new database—sex, age, body weight and height, comorbidities including hypertension and cardiac failure, chronic obstructive pulmonary disease (COPD), diabetes mellitus, kidney failure, liver failure, and cancer, date of hospital and intensive care unit (ICU) admission, and disease severity scores, including the Simplified Acute Physiology Score (SAPS) II at ICU admission and a daily Sequential Organ Failure Assessment (SOFA) scores.
Collected ventilation variables were––mode of ventilation, tidal volume (VT), positive end–expiratory pressure (PEEP), fraction of inspired oxygen (FiO2), respiratory rate (RR), peak pressure (Ppeak) in volume–controlled ventilation and plateau pressure (Pplat) in pressure–controlled ventilation, blood gas analyses results, and adjunctive therapies to improve oxygenation in case of refractory hypoxaemia. The first available measurement of the day was used. If multiple measurements were taken on the same day, we selected earliest one.
The dynamic driving pressure (ΔP) was calculated by subtracting PEEP from the maximum airway pressure [15, 16]. Respiratory system compliance (Crs) was calculated by dividing VT by ΔP. MP was calculated using the power Eq. (17), wherein MP (J/min) = 0.098 * VT * RR * (Ppeak − 0.5 * ΔP) [17]; a modified power equation was used if no Ppeak was available 0.098 * VT * RR * (Pplat − 0.5 * ΔP) [16]. The ventilatory ratio was calculated as (minute ventilation * PaCO2)/(predicted bodyweight * 100 * 37.5) [18]. The number of ventilator–free days at day 60 (VFD–60) was calculated by subtracting the number of calendar days a patient received invasive ventilation up to the day of successful extubation from 60, similar to the method used for calculating VFD–28. Patients that died before or at day 60 received zero VFD–60 [19, 20].
The following follow–up data were available for merging—last day of ventilation, tracheostomy use, last day in ICU and hospital, and life status at day 60.
Endpoints
The primary endpoint of this analysis was a combination of the following key ventilation characteristics as done before [10]—VT, PEEP, ΔP, and Crs. Secondary outcomes were other ventilator parameters, the use of prone positioning, muscle paralysis or extracorporeal membrane oxygenation, and 60–day mortality and the number of VFD–60.
Power analysis
We did not perform a formal power analysis; instead, the number of available patients served as the sample size.
Statistical analysis
Baseline demographics were compared using Fisher’s exact tests for categorical variables and Wilcoxon rank–sum tests for continuous variables. Continuous distributed variables are presented as medians and interquartile ranges, categorical variables are presented as frequencies and proportions.
The first day a patient received invasive ventilation and the first full calendar day were combined into ‘day 1’, the next day was designated as ‘day 2’. Information on missing values for each ventilation parameters and other variable can be found in the Supplementary Material (eTable 1). Only SOFA scores were available for all patients, therefore, we chose to only report these instead of other severity scores.
To compare ventilation characteristics between COVID–ARDS and CLASSIC–ARDS patients, a Wilcoxon rank–sum test was used. Cumulative distribution plots were constructed to visualize cumulative distribution frequencies of each ventilation variable or parameter, wherein vertical dotted lines represent broadly accepted safety cutoffs for each variable, and horizontal dotted lines show the respective proportion of patients reaching that cutoff.
As a post–hoc analysis to identify whether VT, PEEP and ΔP have independent associations with 60–day mortality and the number of VFD–60, a multivariable mixed–effects model with centre as random effect was performed. A linear mixed–effects model was used for the number of VFD–60 and a logistic mixed–effects model for 60–day mortality.
The following covariates, with a known or suspected association with these two outcomes were included in the model, based on clinical relevance: (1) PaO2/FiO2; and (2) demographic variables, including sex, age, BMI, history of heart failure, COPD, diabetes mellitus, kidney failure, liver failure and cancer.
In this mixed model analysis, when a covariate exhibited more than 10% missing data, we utilized multiple imputation techniques implemented through the MICE package in R. The model was checked for collinearity using variance–inflation factors, wherein a variance–inflation factor < 5 was deemed acceptable. The variance–inflation factor was < 2 for all included variables in our model.
The estimate refers to the average effect of the ventilation parameter, i.e., VT, PEEP or ΔP on the outcome of interest, i.e., 60–day mortality and VFD–60 while controlling for the other variables in the model. A positive estimate indicates that an increase in the predictor variable tends to lead to a corresponding increase in the response variable, indicating a proportional relationship between them. Conversely, a negative estimate suggests that an increase in the predictor variable tends to result in a decrease in the response variable, indicating an inverse proportional relationship between them.
All analyses were conducted in R v.4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A p value < 0.05 was considered statistically significant.
Results
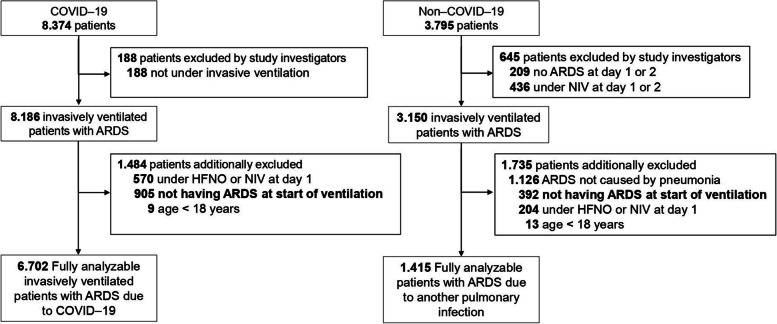
We received the individual data of a total of 8374 COVID–ARDS patients and 3795 CLASSIC–ARDS patients (Fig. 1). After exclusion of patients that did not fulfil the Berlin definition of ARDS, patients that did not receive invasive ventilation on the first and second day in the study, and patients included in the two pre–pandemic studies who did not have a respiratory infection as the cause for ARDS, we had 6702 fully–analysable COVID–ARDS patients and 1415 fully–analysable CLASSIC–ARDS. COVID–ARDS patients were more often male, had higher median BMI, a history of diabetes more often, and a history of COPD or chronic kidney disease less often (Table 1). COVID–ARDS patients had lower median SOFA scores, and ARDS severity was more often classified as moderate or severe.
Fig. 1.
Flowchart of included studies. Abbreviations: ARDS = acute respiratory distress syndrome; COVID–19 = coronavirus disease 2019
Table 1.
Patient demographics, Baseline Characteristics and ARDS Severity
|
COVID–ARDS N = 6.702 |
CLASSIC–ARDS N = 1.415 |
p | |
|---|---|---|---|
| Demographics | |||
| age, years, median [IQR] | 64.0 [55.0 to 71.0] | 63.0 [50.0 to 74.0] | 0.361 |
| height, cm, median [IQR] | 170.0 [163.0 to 175.0] | 168.0 [160.0 to 175.0] | < 0.001 |
| weight, kg, median [IQR] | 82.0 [74.0 to 95.0] | 74.0 [62.0 to 86.0] | < 0.001 |
| male gender, n/N (%) | 4.655 (69.5) | 859 (60.7) | < 0.001 |
| BMI, kg/m2, median [IQR] | 28.5 [25.6 to 32.4] | 25.8 [22.5 to 30.1] | < 0.001 |
| SOFA score, median [IQR] | 6.0 [4.0 to 8.0] | 10.0 [7.0 to 12.0] | < 0.001 |
| Comorbidities | |||
| heart failure, N (%) | 633 (9.4) | 143 (10.1) | 0.472 |
| COPD, N (%) | 688 (10.3) | 323 (22.8) | < 0.001 |
| diabetes, N (%) | 1.906 (28.4) | 322 (22.8) | < 0.001 |
| chronic kidney disease, N (%) | 402 (6.0) | 140 (9.9) | < 0.001 |
| liver failure, N (%) | 122 (1.8) | 53 (3.7) | < 0.001 |
| active neoplasm, N (%) | 253 (3.8) | 119 (8.4) | < 0.001 |
| ARDS severity categories | < 0.001 | ||
| mild, N (%) | 1.937 (28.9) | 362 (25.6) | |
| moderate, N (%) | 3.477 (51.8) | 673 (47.6) | |
| severe, N (%) | 1.288 (19.2) | 380 (26.9) | |
Abbreviations: ARDS Acute respiratory distress syndrome, IQR Interquartile range, N Number, BMI Body mass Index, SOFA Sequential organ failure assessment, COPD Chronic obstructive pulmonary disease
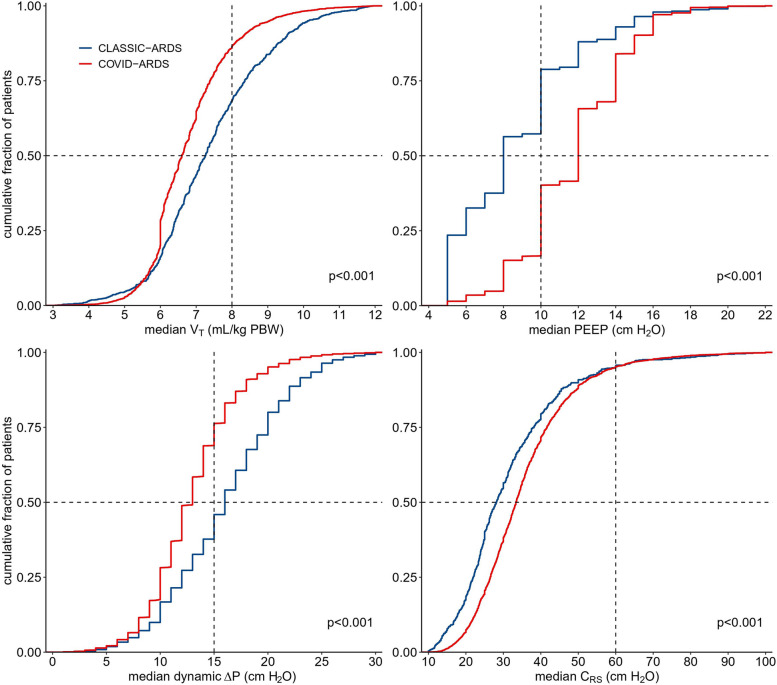
COVID–ARDS patients were ventilated with volume–controlled ventilation more often than CLASSIC–ARDS patients (Table 2) and received ventilation with lower VT (6.6 [6.0 to 7.4] vs 7.3 [6.4 to 8.5] ml/kg PBW; p < 0.001), higher PEEP (12.0 [10.0 to 14.0] vs 8.0 [6.0 to 10.0] cm H2O; p < 0.001), at lower ΔP (13.0 [10.0 to 15.0] vs 16.0 [IQR 12.0 to 20.0] cm H2O; p < 0.001) and higher Crs (33.5 [26.6 to 42.1] vs 28.1 [21.6 to 38.4] mL/cm H2O; p < 0.001) (Fig. 2) COVID–ARDS patients received higher PEEP than CLASSIC–ARDS patients at any FiO2 level (eFigure 2). Within each group, the ventilation characteristics were not different between day 1 and 2 (eTable 2 and eFigure 1 and 2).
Table 2.
Ventilation Characteristics, Adjunctive Therapies, Arterial Blood Gas Analysis and Outcomes
|
COVID–ARDS N = 6.702 |
CLASSIC–ARDS N = 1.415 |
p | |
|---|---|---|---|
| Ventilation characteristics | |||
| mode of ventilation, N (%) | < 0.001 | ||
| volume–controlled ventilation | 4.689 (70.3) | 521 (36.8) | |
| pressure–controlled ventilation | 1.403 (21.0) | 493 (34.8) | |
| pressure–support ventilation | 336 (5.0) | 143 (10.1) | |
| other | 242 (3.6) | 258 (18.2) | |
| VT, mL/kg PBW, median [IQR] | 6.6 [6.0 to 7.4] | 7.3 [6.4 to 8.5] | < 0.001 |
| < 6 ml/kg PBW | 1.464 (24.8) | 211 (16.3) | < 0.001 |
| 6–8 ml/kg PBW | 3.601 (61.2) | 645 (50.0) | < 0.001 |
| 8–10 ml/kg PBW | 698(11.9) | 324 (25.1) | < 0.001 |
| > 10 ml/kg PBW | 125 (2.1) | 102 (7.9) | < 0.001 |
| PEEP, cmH2O, median [IQR] | 12.0 [10.0 to 14.0] | 8.0 [6.0 to 10.0] | < 0.001 |
| < 8 cmH2O | 325 (4.8) | 530 (37.5) | < 0.001 |
| 8–12 cmH2O | 4.075 (60.8) | 713 (50.4) | < 0.001 |
| 12–16 cmH2O | 2.101 (31.3) | 140 (9.9) | < 0.001 |
| > 16 cmH2O | 201 (2.9) | 32 (2.3) | 0.37 |
| PMAX, cmH2O, median [IQR] | 25.5 [21.0 to 30.0] | 25.0 [22.0 to 28.0] | 0.001 |
| dynamic ΔP, cmH2O, median [IQR] | 13.0 [10.0 to 15.0] | 16.0 [12.0 to 20.0] | < 0.001 |
| CRS, mL/cmH2O, median [IQR] | 33.5 [26.6 to 42.1] | 28.1 [21.6 to 38.4] | < 0.001 |
| MP, J/min, median [IQR] | 16.6 [13.4 to 20.6] | 15.2 [11.3 to 19.2] | < 0.001 |
| FiO2, median [IQR] | 0.6 [0.5 to 0.9] | 0.6 [0.5 to 0.9] | 0.017 |
| total RR, breaths per min, median [IQR] | 22.0 [20.0 to 25.0] | 20.0 [16.0 to 25.0] | < 0.001 |
| ventilatory ratio, median [IQR] | 1.75 [1.43 to 2.20] | 1.76 [1.35 to 2.27] | 0.835 |
| Adjunctive therapies | |||
| prone positioning, N (%) | 4.615 (69.2) | 144 (10.2) | < 0.001 |
| recruitment manoeuvres, N (%) | 1.924 (39.4) | 313 (22.1) | < 0.001 |
| ECMO, N (%) | 142 (2.5) | 56 (4.0) | 0.005 |
| tracheostomy, N (%) | 2.033 (30.5) | 214 (15.1) | < 0.001 |
| neuromuscular blocking agents, N (%) | 3.838 (73.9) | 350 (24.7) | < 0.001 |
| continuous sedation, N (%) | 1.188 (84.0) | 1.785 (98.1) | < 0.001 |
| vasopressor use, N (%) | 4440 (85.5) | 1025 (72.5) | < 0.001 |
| Arterial blood gas analysis | |||
| pH, median [IQR] | 7.35 [7.28 to 7.41] | 7.34 [7.26 to 7.41] | < 0.001 |
| PaO2, mmHg, median [IQR] | 80.1 [67.5 to 98.5] | 83.6 [68.0 to 105.8] | < 0.001 |
| PaCO2, mmHg, median [IQR] | 44.25 [38.0 to 52.0] | 44.0 [37.0 to 54.0] | 0.320 |
| PaO2/FiO2, median [IQR] | 135.0 [94.0 to 184.9] | 146.0 [99.4 to 207.3] | < 0.001 |
| Outcomes | |||
| 60–day mortality, N (%) | 2963 (44.2) | 515 (36.4) | < 0.001 |
| VFD–60, median [IQR] | 11.0 [0.0 to 47.0] | 41.0 [0.0 to 54.0] | < 0.001 |
Abbreviations: ARDS Acute respiratory distress syndrome, VT Tidal volume; PBW Predicted bodyweight, IQR Interquartile range, N Number, PEEP Positive end–expiratory pressure, Pmax Maximum airway pressure, ΔP Driving pressure, MP Mechanical power, CRS Respiratory system compliance, FiO2 Fraction of inspired oxygen, RR Respiratory rate, PaO2 Partial pressure of arterial oxygen, PaCO2 Partial pressure of arterial carbon dioxide, VFD Ventilator–free days and alive
Fig. 2.
Key ventilation parameters. Cumulative frequency distribution of VT, PEEP, ΔP, and respiratory system compliance on the first calendar day for each variable. Vertical dotted lines represent broadly accepted safety cutoffs for each variable, and horizontal dotted lines show the respective proportion of patients reaching that cutoff. Abbreviations: VT = tidal volume; PBW = predicted bodyweight; PEEP = positive end–expiratory pressure; ΔP = driving pressure; CRS = respiratory system compliance
Prone positioning and neuromuscular blocking agents were more often used in COVID–ARDS patients than in CLASSIC–ARDS patients (Table 2). COVID–ARDS patients received a tracheostomy more often than CLASSIC–ARDS patients.
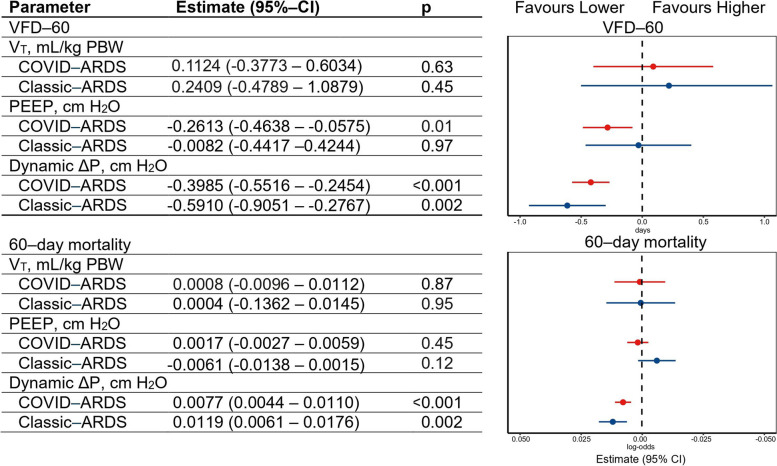
Mortality at day 60 was higher in COVID–ARDS patients compared to CLASSIC–ARDS patients (Table 2 and Fig. 3), and COVID–ARDS patients had significantly less VFD–60. Following multivariable adjustment, higher ΔP had an association with higher 60–day mortality and less VFD–60 in both groups. Higher PEEP also had an association with less VFD–60, but only in COVID–ARDS patients and not in CLASSIC–ARDS patients. In both groups, VT neither had an association with 60–day mortality nor with VFD–60 (eFigure 3 and eFigure 4).
Fig. 3.
Mortality and ventilator–free days and Alive at day–60, and associations with ventilator parameters. The estimate is the average effect of the predictor variable on the response variable, while controlling for the other variables in the model. A positive estimate suggests a proportional effect, whereas a negative estimate suggests an inversely proportional effect. Abbreviations: ARDS = acute respiratory distress syndrome; VFD = ventilator–free days and alive; IQR = interquartile range; N = number; CI = confidence interval; VT = tidal volume; PBW = predicted bodyweight; PEEP = positive end–expiratory pressure; ΔP = driving pressure
Discussion
We pooled the individual data of patients from six observational studies of ventilation and compared ventilation characteristics and associations with outcomes between COVID–ARDS with CLASSIC–ARDS. The main findings were: (1) compared to CLASSIC–ARDS patients, COVID–ARDS patients were ventilated with lower VT and higher PEEP, at lower ΔP and higher Crs, however with a higher MP; (2) 60–day mortality was not different between COVID–ARDS and CLASSIC–ARDS, but COVID–ARDS patients had less VFD–60; (3) higher ΔP had an association with higher 60–day mortality and less VFD–60 in COVID–ARDS and CLASSIC–ARDS; and (4) higher PEEP also had an association with less VFD–60, but only in COVID–ARDS.
Our findings add to the current understanding of differences and similarities between COVID–19 ARDS patients and pre–COVID ARDS patients. The international design of our study increases the generalizability of the findings across diverse healthcare systems, both in ARDS patients caused by COVID–19 and in patients with ARDS due to pneumonia from before the pandemic. The large sample size and high quality of the collected data allowed for sophisticated analyses of epidemiology, respiratory support strategies, and outcomes. Additionally, we found associations between key ventilator settings and patient outcomes.
Several studies have compared COVID–19 ARDS with pre–COVID ARDS. The epidemiological differences between COVID–19 ARDS and pre–COVID ARDS patients in our study align with previous findings [21]. As with other studies [22, 23], we also found significant differences in ventilator variables like VT, PEEP, and ΔP, and in the use of adjunctive therapies. Our study contributes by demonstrating these differences specifically among ARDS patients and comparing COVID–19 ARDS to pre–COVID ARDS due to respiratory infections. Differences in outcomes found in our study are, at least in part, in line with prior research findings [21, 23]. Our findings confirm that there are differences in mortality and the number of VFD–60 between COVID–19 ARDS and pre–COVID ARDS patients. However, these difference disappeared after propensity matching. This is important as it shows that, at least when comparing outcomes in ARDS patients from an infectious cause, outcomes are not different, opposite to what was thought at the start of the pandemic.
We observed more frequent use of lower VT in COVID–ARDS compared to CLASSIC–ARDS. Indeed, proportions of COVID–ARDS patients that received ventilation with a VT < 6 or between 6 and 8 ml/kg PBW was higher than in CLASSIC–ARDS patients. This finding can be explained in several ways––e.g., it could be that the use of lung–protective ventilation with a lower VT has improved in the last decade [15]. It is also conceivable that, at least early in the pandemic care for COVID–ARDS patients was provided by inexperienced ICU staff which could have been more adherent to existing guidelines for management of patients with ARDS [10, 24]. It is also possible that use of low VT in COVID–ARDS is easier to control––these patients were often deeply sedated and paralyzed allowing a stricter adherence to lower VT. Of note, especially in those patients, ventilation with a lower VT might be more beneficial than in spontaneous breathing patients [25].
Higher PEEP was more often used in COVID–ARDS patients than in CLASSIC–ARDS patients, at any FiO2 level. Indeed, proportions of COVID–ARDS patients that received ventilation with a PEEP between 8 and 12 cmH2O and even between 12 and 16 cmH2O was higher than in CLASSIC–ARDS patients. This finding can also be explained in several ways––e.g., a preference for use of higher PEEP in COVID–ARDS patients may have been triggered by the severity of ARDS, as COVID–ARDS was more often classified as moderate or severe, and more severe hypoxaemia naturally triggers the use of higher PEEP if PEEP/FiO2 tables are used. It is also possible that higher PEEP was used in the assumption that lung lesions with COVID–ARDS are more recruitable than in CLASSIC–ARDS. This may at least explain the lower ΔP and higher Crs in COVID–ARDS patients.
In COVID–ARDS patients, mechanical power exceeded that of CLASSIC–ARDS, even though the driving pressure was lower. This observation marks the significance of considering factors beyond driving pressure, such as respiratory rate and PEEP, when evaluating the protective nature of invasive ventilation. These findings emphasize the complexity of respiratory management in COVID–ARDS and the need for a comprehensive approach to optimize lungprotective ventilation strategies.
COVID–ARDS patients received prone positioning more often than CLASSIC–ARDS patients. Before the pandemic, prone positioning remained underused, probably because it was more considered a rescue therapy for refractory hypoxaemia [26]. While we cannot rule out that use of prone positioning increased already before the pandemic, we favour the idea that the higher use of prone positioning in COVID–ARDS patients was triggered by the more severe hypoxaemia in COVID–ARDS patients.
Our analysis found several associations between ventilation parameters and outcome. The association of higher ΔP with higher 60–day mortality and less VFD–60 is in line with previous studies [27–29]. The association of higher PEEP with worse outcome confirms the findings of earlier studies [30, 31]. Of note, this association was only found for COVID–ARDS. This may have been caused by the more frequent use of higher PEEP in COVID–ARDS than in CLASSIC–ARDS. One reason for the association between higher PEEP and worse outcome may be that sicker patients, with a higher chance of dying and prolonged ventilation, received higher PEEP than patients that were less sick. Nonetheless, a high PEEP is suggested to have detrimental effects [32], emphasizing the need to determine the optimal PEEP level based on lung recruitability rather than hypoxemia alone. Actually, one analysis of PRoVENT–COVID suggested worse outcomes if patients received ventilation according to a higher PEEP/lower FiO2 table as compared to ventilation according to a lower PEEP/higher FiO2 [30]. A post–hoc Bayesian analysis of a randomised clinical study, named the ‘Alveolar Recruitment for ARDS Trial’ (ART), wherein patients were randomized to receive ventilation with PEEP titrated to the best Crs and aggressive recruitment manoeuvres versus ventilation with a low PEEP strategy, suggested that higher PEEP with recruitment manoeuvres worsens the outcome of ARDS from pneumonia, while it may be beneficial in ARDS from another cause [33]. A posthoc analysis of a randomised clinical study named ‘Lung Imaging for Ventilator Setting in ARDS trial’ (LIFE), suggest that higher PEEP worsens outcomes in patients with ARDS with lesions that may not be recruitable with higher PEEP [34].
The findings of this pooled analysis extend the existing knowledge of the epidemiology, management of invasive ventilation and outcomes in COVID–ARDS. Our study shows that lung–protective ventilation was applied well in COVID–ARDS, and was comparable to best practice used in management for patients with CLASSIC–ARDS. Additionally, the effect of PEEP on major outcomes may have implications for care. At least it should trigger new studies that directly compare different PEEP strategies. Meanwhile, it could be more attractive to not use higher PEEP by default.
Our study has several strengths. We managed to receive and merge the datasets of four large observational studies of ventilation conducted in the COVID–19 pandemic with two well–performed pre–pandemic observational studies of ventilation––these six studies all focused on ventilation management and reported outcomes of invasively ventilated ARDS patients, allowing a robust analysis of ventilation management and the impact of certain ventilation parameters on outcome. While the COVID–19 studies were all national investigations, they are from different regions worldwide and were conducted in different types of hospitals, which increases the generalizability of our findings. The datasets from the original studies were rich and comprehensive, encompassing baseline and demographic data, granular ventilator settings and ventilation variables, and key clinical outcomes. All data could be harmonized and merged into one database.
We had an analysis plan in place before cleaning and closing of the new database, and this plan was strictly followed. The large numbers of patients allowed us to perform sophisticated statistical analyses of associations with outcomes.
This study has limitations. First, individual data was obtained from observational studies, which limits the ability to establish causality. Additionally, the willingness of data sharing could have led to selection bias towards the inclusion of ICUs with an interest in invasive ventilation and management of ARDS in the original studies. Second, studies in COVID–ARDS were conducted early in the COVID–19 pandemic, during which inexperienced staff and resource limitations could have influenced clinical decision making. Third, data was collected early in the pandemic when patient care took priority over data collection, resulting in more missing data than in previous studies. This affects the completeness and may impact the accuracy of our analysis. Fourth, we only reported on ventilation characteristics on day 1 and 2, because not all studies collected ventilation data beyond this timepoint. Therefore we were not able to compare ventilation management beyond day 2. Nevertheless, previous studies have shown ventilation characteristics don’t significantly change in the first four days after initiation of invasive ventilation [10]. Fifth, it is imperative to acknowledge the temporal distance between comparator cohorts. For the pre–COVID ARDS group we used patients of which data was collected between seven to nine years before the pandemic. We cannot exclude temporal differences, for instance due to studies that showed the importance of limiting liberal use of oxygen, and reducing the intensity of ventilation, e.g., by targeting a low driving pressure or a low mechanical power of ventilation, as well as the importance of early use of prone positioning. Sixth, is the lack of detailed subgroup analyses, particularly in patients with chronic respiratory comorbidities such as COPD. Although recent findings from a post–hoc analysis of the PRoVENT–COVID study by Tripipitsiriwat et al. [35] indicated that ventilation parameters did not show significant differences between COPD and non–COPD patients, it could be interesting to explore these subgroups. However, it was beyond the scope of our primary endpoint. Conducting such detailed subgroup investigations would require careful consideration to ensure the data from all included studies are appropriate for this type of analysis.
Finally, all COVID–19 ARDS patients, by definition, had a viral pneumonia, while patients in the classic ARDS group had respiratory infections of which the pathogen was not collected. This is an important limitation, as ARDS from a viral respiratory infection may differ from ARDS due to bacterial pneumonia. Consistent with other studies comparing COVID–19 ARDS to ARDS caused by other viruses, we found that the duration of ventilation was longer, and mortality was higher [21, 36, 37].
Conclusions
Epidemiology and key ventilation characteristics were different in patients with COVID–ARDS compared to CLASSIC–ARDS, also ΔP was lower in COVID––ARDS patients. ΔP had an independent association with outcome in both groups, whereas PEEP had an independent association with outcome only in COVID–ARDS patients.
Supplementary Information
Acknowledgements
for the ERICCa–,LUNG SAFEb–, PRoVENT–COVIDc–, EPICCoVd–, CIBERESUCICOVIDe– and SATI–COVID–19f–investigators
aERICC, ‘Epidemiology of Respiratory Insufficiency in Critical Care’
bLUNG SAFE, ‘Large Observational Study to UNderstand the Global Impact of Severe Acute Respiratory FailurE’
cPRoVENT–COVID, ‘Practice of Ventilation in COVID–19 patients’
dEPICCoV, EPIdemiology of Critical COVID–19
eCIBERESUCICOVID, ‘Centro de Investigación Biomédica en Red Enfermedades Respiratorias COVID–19’
fSATI–COVID–19, ‘Sociedad Argentina de Terapia Intensiva–COVID–19’
Authors’ contributions
Author contribution: FV, SB, MS, FP and DM had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors Acquisition, analysis, or interpretation of data: FV, SB, MS, FP and DM Drafting of the manuscript: FV, SB, MS, FP and DM Critical revision of the manuscript for important intellectual content: All authors Statistical analysis and data verification: FV, SB, and DM Obtained funding: Not applicable; the original studies were performed with funding as stated in the original reports. Administrative, technical, or material support: LA, GB, MB, EE, JF, JL, TP, AT Supervision: MS, FP and DM.
Funding
No additional funding was received for this analysis.
Availability of data and materials
Data sharing: A de–identified dataset can be made available upon request to the corresponding authors one year after publication of this study, but only after permission of the principal investigators of all original studies. The request must include a statistical analysis plan.
Declarations
Ethics approval and consent to participate
The creation of the pooled database did not require additional ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fleur–Stefanie L. I. M. van der Ven and Siebe G. Blok contributed equally to this work.
References
- 1.Chiumello D, Busana M, Coppola S, Romitti F, Formenti P, Bonifazi M, Pozzi T, Palumbo MM, Cressoni M, Herrmann P, Meissner K, Quintel M, Camporota L, Marini JJ, Gattinoni L. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46:2187–96. 10.1007/s00134-020-06281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L, Rizzuto C, Zanella A, Scaravilli V, Pizzilli G, Grieco DL, Di Meglio L, de Pascale G, Lanza E, Monteduro F, Zompatori M, Filippini C, Locatelli F, Cecconi M, Fumagalli R, Nava S, Vincent JL, Antonelli M, Slutsky AS, Pesenti A, Ranieri VM. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–8. 10.1016/S2213-2600(20)30370-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. 10.1186/s13054-020-02880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Pape M, Besnard C, Acatrinei C, Guinard J, Boutrot M, Genève C, Boulain T, Barbier F. Clinical impact of ventilator-associated pneumonia in patients with the acute respiratory distress syndrome: a retrospective cohort study. Ann Intensive Care. 2022;12:24. 10.1186/s13613-022-00998-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutsogiannis DJ, Alharthy A, Balhamar A, Faqihi F, Papanikolaou J, Alqahtani SA, Memish ZA, Brindley PG, Brochard L, Karakitsos D. Mortality and Pulmonary Embolism in Acute Respiratory Distress Syndrome From COVID-19 vs. Non-COVID-19. Front Med (Lausanne). 2022;9:800241. 10.3389/fmed.2022.800241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L, van Haren F, Heunks LM, Kurahashi K, Laake JH, Larsson A, McAuley DF, McNamee L, Nin N, Qiu H, Ranieri M, Rubenfeld GD, Thompson BT, Wrigge H, Slutsky AS, Pesenti A. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–76. 10.1007/s00134-016-4571-5 [DOI] [PubMed] [Google Scholar]
- 8.Azevedo LC, Park M, Salluh JI, Rea-Neto A, Souza-Dantas VC, Varaschin P, Oliveira MC, Tierno PF, dal-Pizzol F, Silva UV, Knibel M, Nassar AP Jr, Alves RA, Ferreira JC, Teixeira C, Rezende V, Martinez A, Luciano PM, Schettino G, Soares M. Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: a multicenter, prospective, cohort study. Crit Care. 2013;17:R63. 10.1186/cc12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 10.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, Dongelmans DA, Hollmann MW, Horn J, Vlaar APJ, Schultz MJ, Neto AS, Paulus F. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9:139–48. 10.1016/S2213-2600(20)30459-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira JC, Ho YL, Besen B, Malbuisson LMS, Taniguchi LU, Mendes PV, Costa ELV, Park M, Daltro-Oliveira R, Roepke RML, Silva JM Jr, Carmona MJC, Carvalho CRR, Hirota A, Kanasiro AK, Crescenzi A, Fernandes AC, Miethke-Morais A, Bellintani AP, Canasiro AR, Carneiro BV, Zanbon BK, Batista B, Nicolao BR, Besen B, Biselli B, Macedo BR, Toledo CMG, Pompilio CE, Carvalho CRR, Mol CG, Stipanich C, Bueno CG, Garzillo C, Tanaka C, Forte DN, Joelsons D, Robira D, Costa ELV, Silva EMJ, Regalio FA, Segura GC, Marcelino GB, Louro GS, Ho YL, Ferreira IA, Gois JO, Silva JMJ, Reusing JOJ, Ribeiro JF, Ferreira JC, Galleti KV, Silva KR, Isensee LP, Oliveira LS, Taniguchi LU, Letaif LS, Lima LT, Park LY, Chaves LN, Nobrega LC, Haddad L, Hajjar L, Malbouisson LM, Pandolfi MCA, Park M, Carmona MJC, Andrade M, Santos MM, Bateloche MP, Suiama MA, Oliveira MF, Sousa ML, Louvaes M, Huemer N, Mendes P, Lins PRG, Santos PG, Moreira PFP, Guazzelli RM, Reis RB, Oliveira RD, Roepke RML, Pedro RAM, Kondo R, Rached SZ, Fonseca SRS, Borges TS, Ferreira T, Cobello VJ, Sales VVT, Ferreira WSC. Characteristics and outcomes of patients with COVID-19 admitted to the ICU in a university hospital in São Paulo. Brazil - study protocol Clinics (Sao Paulo). 2020;75:e2294. 10.6061/clinics/2020/e2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira JC, Ho YL, Besen B, Malbouisson LMS, Taniguchi LU, Mendes PV, Costa ELV, Park M, Daltro-Oliveira R, Roepke RML, Silva-Jr JM, Carmona MJC, Carvalho CRR. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A, Arguimbau M, Bermejo-Martín J, Campo R, Ceccato A, Fernandez-Barat L, Ferrer R, Jarillo N, Lorente-Balanza J, Menéndez R, Motos A, Muñoz J, Peñuelas Rodríguez Ó, Pérez R, Riera J, Rodríguez A, Sánchez M. Barbe F [CIBERESUCICOVID: A strategic project for a better understanding and clinical management of COVID-19 in critical patients]. Arch Bronconeumol. 2021;57:1–2. 10.1016/j.arbres.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estenssoro E, Loudet CI, Ríos FG, KanooreEdul VS, Plotnikow G, Andrian M, Romero I, Piezny D, Bezzi M, Mandich V, Groer C, Torres S, Orlandi C, RubattoBirri PN, Valenti MF, Cunto E, Sáenz MG, Tiribelli N, Aphalo V, Reina R, Dubin A. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9:989–98. 10.1016/S2213-2600(21)00229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuijt MTU, Hol L, Nijbroek SG, Ahuja S, van Meenen D, Mazzinari G, Hemmes S, Bluth T, Ball L, Gama-de Abreu M, Pelosi P, Schultz MJ, Serpa NA. Associations of dynamic driving pressure and mechanical power with postoperative pulmonary complications-posthoc analysis of two randomised clinical trials in open abdominal surgery. EClinicalMedicine. 2022;47:101397. 10.1016/j.eclinm.2022.101397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Meenen DMP, Algera AG, Schuijt MTU, Simonis FD, van der Hoeven SM, Neto AS, Abreu MG, Pelosi P, Paulus F, Schultz MJ. Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: An analysis of three randomised clinical trials. Eur J Anaesthesiol. 2023;40:21–8. 10.1097/EJA.0000000000001778 [DOI] [PubMed] [Google Scholar]
- 17.Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–75. 10.1007/s00134-016-4505-2 [DOI] [PubMed] [Google Scholar]
- 18.Sinha P, Fauvel NJ, Singh S, Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br J Anaesth. 2009;102:692–7. 10.1093/bja/aep054 [DOI] [PubMed] [Google Scholar]
- 19.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of Ventilator-Free Days in Critical Care Research. Am J Respir Crit Care Med. 2019;200:828–36. 10.1164/rccm.201810-2050CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Meenen DMP, van der Hoeven SM, Binnekade JM, de Borgie C, Merkus MP, Bosch FH, Endeman H, Haringman JJ, van der Meer NJM, Moeniralam HS, Slabbekoorn M, Muller MCA, Stilma W, van Silfhout B, Neto AS, Ter Haar HFM, Van Vliet J, Wijnhoven JW, Horn J, Juffermans NP, Pelosi P, Gama de Abreu M, Schultz MJ, Paulus F. Effect of On-Demand vs Routine Nebulization of Acetylcysteine With Salbutamol on Ventilator-Free Days in Intensive Care Unit Patients Receiving Invasive Ventilation: A Randomized Clinical Trial. Jama. 2018; 319:993–1001. [DOI] [PMC free article] [PubMed]
- 21.Richards-Belle A, Orzechowska I, Gould DW, Thomas K, Doidge JC, Mouncey PR, Christian MD, Shankar-Hari M, Harrison DA, Rowan KM. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46:2035–47. 10.1007/s00134-020-06267-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjoding MW, Admon AJ, Saha AK, Kay SG, Brown CA, Co I, Claar D, McSparron JI, Dickson RP. Comparing Clinical Features and Outcomes in Mechanically Ventilated Patients with COVID-19 and Acute Respiratory Distress Syndrome. Ann Am Thorac Soc. 2021;18:1876–85. 10.1513/AnnalsATS.202008-1076OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolley EP, Sahetya SK, Hochberg CH, Hossen S, Hager DN, Brower RG, Stuart EA, Checkley W. Outcomes Among Mechanically Ventilated Patients With Severe Pneumonia and Acute Hypoxemic Respiratory Failure From SARS-CoV-2 and Other Etiologies. JAMA Netw Open. 2023;6:e2250401. 10.1001/jamanetworkopen.2022.50401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown-Brumfield D, DeLeon A. Adherence to a medication safety protocol: current practice for labeling medications and solutions on the sterile field. Aorn j. 2010;91:610–7. 10.1016/j.aorn.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, Mercat A, Meade M, Morais CCA, Goligher E, Carvalho CRR, Amato MBP. Ventilatory Variables and Mechanical Power in Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2021;204:303–11. 10.1164/rccm.202009-3467OC [DOI] [PubMed] [Google Scholar]
- 26.Guérin C, Beuret P, Constantin JM, Bellani G, Garcia-Olivares P, Roca O, Meertens JH, Maia PA, Becher T, Peterson J, Larsson A, Gurjar M, Hajjej Z, Kovari F, Assiri AH, Mainas E, Hasan MS, Morocho-Tutillo DR, Baboi L, Chrétien JM, François G, Ayzac L, Chen L, Brochard L, Mercat A. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44:22–37. 10.1007/s00134-017-4996-5 [DOI] [PubMed] [Google Scholar]
- 27.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- 28.van Meenen DMP, SerpaNeto A, Paulus F, Merkies C, Schouten LR, Bos LD, Horn J, Juffermans NP, Cremer OL, van der Poll T, Schultz MJ. The predictive validity for mortality of the driving pressure and the mechanical power of ventilation. Intensive Care Med Exp. 2020;8:60. 10.1186/s40635-020-00346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urner M, Jüni P, Hansen B, Wettstein MS, Ferguson ND, Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8:905–13. 10.1016/S2213-2600(20)30325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valk CMA, Tsonas AM, Botta M, Bos LDJ, Pillay J, SerpaNeto A, Schultz MJ, Paulus F. Association of early positive end-expiratory pressure settings with ventilator-free days in patients with coronavirus disease 2019 acute respiratory distress syndrome: A secondary analysis of the Practice of VENTilation in COVID-19 study. Eur J Anaesthesiol. 2021;38:1274–83. 10.1097/EJA.0000000000001565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalcanti AB, Suzumura É A, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, Romano ER, Regenga MM, Taniguchi LNT, Teixeira C, Pinheiro de Oliveira R, Machado FR, Diaz-Quijano FA, Filho MSA, Maia IS, Caser EB, Filho WO, Borges MC, Martins PA, Matsui M, Ospina-Tascón GA, Giancursi TS, Giraldo-Ramirez ND, Vieira SRR, Assef M, Hasan MS, Szczeklik W, Rios F, Amato MBP, Berwanger O, Ribeiro de Carvalho CR. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. Jama. 2017;318:1335–1345. [DOI] [PMC free article] [PubMed]
- 32.Tsolaki V, Zakynthinos GE, Makris D. The ARDSnet protocol may be detrimental in COVID-19. Crit Care. 2020;24:351. 10.1186/s13054-020-03081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampieri FG, Costa EL, Iwashyna TJ, Carvalho CRR, Damiani LP, Taniguchi LU, Amato MBP, Cavalcanti AB. Heterogeneous effects of alveolar recruitment in acute respiratory distress syndrome: a machine learning reanalysis of the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Br J Anaesth. 2019;123:88–95. 10.1016/j.bja.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 34.Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, Ferrandière M, Grelon F, Seguin P, Ichai C, Veber B, Souweine B, Uberti T, Lasocki S, Legay F, Leone M, Eisenmann N, Dahyot-Fizelier C, Dupont H, Asehnoune K, Sossou A, Chanques G, Muller L, Bazin JE, Monsel A, Borao L, Garcier JM, Rouby JJ, Pereira B, Futier E. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–80. 10.1016/S2213-2600(19)30138-9 [DOI] [PubMed] [Google Scholar]
- 35.Tripipitsiriwat A, Suppapueng O, van Meenen DMP, Paulus F, Hollmann MW, Sivakorn C, Schultz MJ. Epidemiology, Ventilation Management and Outcomes of COPD Patients Receiving Invasive Ventilation for COVID-19-Insights from PRoVENT-COVID. J Clin Med. 2023;12. [DOI] [PMC free article] [PubMed]
- 36.Brinkman S, Termorshuizen F, Dongelmans DA, Bakhshi-Raiez F, Arbous MS, de Lange DW, de Keizer NF. Comparison of outcome and characteristics between 6343 COVID-19 patients and 2256 other community-acquired viral pneumonia patients admitted to Dutch ICUs. J Crit Care. 2022;68:76–82. 10.1016/j.jcrc.2021.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virk S, Quazi MA, Nasrullah A, Shah A, Kudron E, Chourasia P, Javed A, Jain P, Gangu K, Cheema T, DiSilvio B, Sheikh AB. Comparing Clinical Outcomes of COVID-19 and Influenza-Induced Acute Respiratory Distress Syndrome: A Propensity-Matched Analysis. Viruses. 2023;15. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing: A de–identified dataset can be made available upon request to the corresponding authors one year after publication of this study, but only after permission of the principal investigators of all original studies. The request must include a statistical analysis plan.