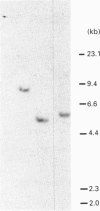
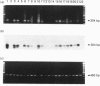
Abstract
Heparin-binding epidermal growth factor (HB-EGF) is a recently identified member of the EGF family. Mature HB-EGF is processed from a larger transmembrane precursor which can itself act as a cell-surface receptor for the internalization of diphtheria toxin into eukaryotic cells. However, to date there is no information available on the distribution of HB-EGF in mammalian tissues. We have therefore used reverse-transcription PCR to analyse the expression of HB-EGF mRNA in a wide range of tissues. HB-EGF transcripts were detected in RNA isolated from 15 of the 22 tissues obtained from adult pigs, which is consistent with the ability of diphtheria toxin to affect many body tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasband A. J., Rogers K. T., Chen X. R., Azizkhan J. C., Lee D. C. Characterization of the rat transforming growth factor alpha gene and identification of promoter sequences. Mol Cell Biol. 1990 May;10(5):2111–2121. doi: 10.1128/mcb.10.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. I., Lam R., Lakshmanan J., Fisher D. A. Transforming growth factor alpha in developing rats. Am J Physiol. 1990 Aug;259(2 Pt 1):E256–E260. doi: 10.1152/ajpendo.1990.259.2.E256. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Roberts A. B., Winkler M. E., Chen E. Y., Goeddel D. V. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell. 1984 Aug;38(1):287–297. doi: 10.1016/0092-8674(84)90550-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fukuyama R., Shimizu N. Expression of epidermal growth factor (EGF) and the EGF receptor in human tissues. J Exp Zool. 1991 Jun;258(3):336–343. doi: 10.1002/jez.1402580309. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Lau K., Besner G. E., Abraham J. A., Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992 Mar 25;267(9):6205–6212. [PubMed] [Google Scholar]
- Lee D. C., Rose T. M., Webb N. R., Todaro G. J. Cloning and sequence analysis of a cDNA for rat transforming growth factor-alpha. Nature. 1985 Feb 7;313(6002):489–491. doi: 10.1038/313489a0. [DOI] [PubMed] [Google Scholar]
- Naglich J. G., Metherall J. E., Russell D. W., Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992 Jun 12;69(6):1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Pascall J. C., Brown K. D. Structural analysis of the 5'-flanking sequence of the mouse epidermal growth factor gene. J Mol Endocrinol. 1988 Jul;1(1):5–11. doi: 10.1677/jme.0.0010005. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R. Epidermal growth factor and the nervous system. Peptides. 1991 May-Jun;12(3):653–663. doi: 10.1016/0196-9781(91)90115-6. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent S. A., Lemoine N. R. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4(1):1–24. doi: 10.1016/0955-2235(92)90002-y. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Scott J., Bell G. I., Crawford R. J., Penschow J. D., Niall H. D., Coghlan J. P. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985 Jan 17;313(5999):228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Takacs K., Coffey R. J. Transforming growth factor-alpha gene expression and action in the seminiferous tubule: peritubular cell-Sertoli cell interactions. Endocrinology. 1989 Feb;124(2):845–854. doi: 10.1210/endo-124-2-845. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan T. J., Pascall J. C., James P. S., Brown K. D. Expression of epidermal growth factor and its mRNA in pig kidney, pancreas and other tissues. Biochem J. 1991 Oct 1;279(Pt 1):315–318. doi: 10.1042/bj2790315. [DOI] [PMC free article] [PubMed] [Google Scholar]