Abstract
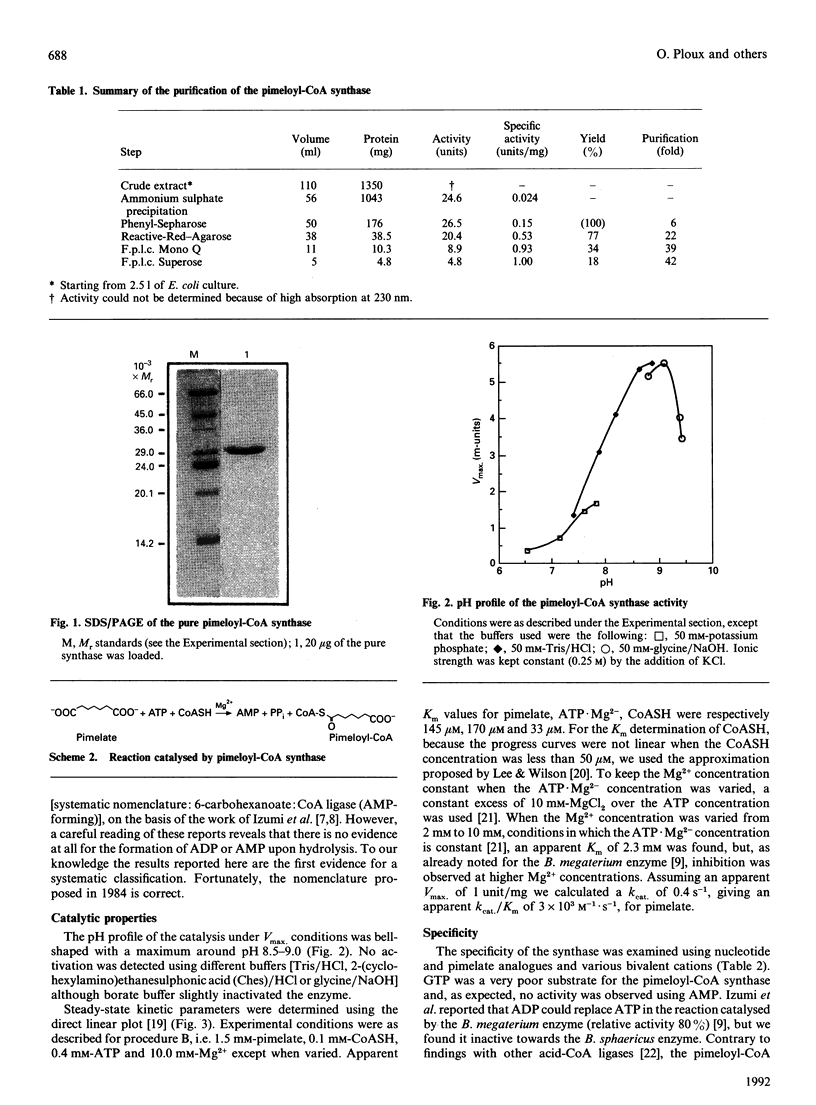
The pimeloyl-CoA synthase from Bacillus sphaericus has been purified to homogeneity from an overproducing strain of Escherichia coli. The purification yielded milligram quantities of the synthase with a specific activity of 1 unit/mg of protein. Analysis of the products showed that this enzyme catalysed the transformation of pimelate into pimeloyl-CoA with concomitant hydrolysis of ATP to AMP. Using a continuous spectrophotometric assay, we have examined the catalytic properties of the pure enzyme. The pH profile under Vmax. conditions showed a maximum around 8.5. Apparent Km values for pimelate, CoASH, ATP.Mg2- and Mg2+ were respectively 145 microM, 33 microM, 170 microM and 2.3 mM. The enzyme was inhibited by Mg2+ above 10 mM. This acid-CoA ligase exhibited a very sharp substrate specificity, e.g. neither GTP nor pimelate analogues (di- or mono-carboxylic acids) were processed. The bivalent metal ion requirement was also investigated: Mn2+ (73%) and Co2+ (32%) but not Ca2+ could replace Mg2+. The enzyme was inhibited by metal chelators such as 1,10-phenanthroline and EDTA. The synthase was a homodimer with a 28,000-M(r) subunit. N-Terminal sequencing definitely proved that this enzyme was encoded by the bioW gene. A careful study of pimelate uptake by B. sphaericus, E. coli and Pseudomonas dentrificans showed that this metabolite crossed the membrane of these microorganisms by passive diffusion, ruling out the involvement of the bioX gene product as pimelate carrier.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Campbell A. Deletion and complementation analysis of biotin gene cluster of Escherichia coli. J Bacteriol. 1972 Nov;112(2):830–839. doi: 10.1128/jb.112.2.830-839.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M. A., Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968 Oct;96(4):1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloeckler R., Ohsawa I., Speck D., Ledoux C., Bernard S., Zinsius M., Villeval D., Kisou T., Kamogawa K., Lemoine Y. Cloning and characterization of the Bacillus sphaericus genes controlling the bioconversion of pimelate into dethiobiotin. Gene. 1990 Mar 1;87(1):63–70. doi: 10.1016/0378-1119(90)90496-e. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Morita H., Sato K., Tani Y., Ogata K. Synthesis of biotin-vitamers from pimelic acid and coenzyme A by cell-free extracts of various bacteria. Biochim Biophys Acta. 1972 Mar 30;264(1):210–213. doi: 10.1016/0304-4165(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Wilson I. B. Enzymic parameters: measurement of V and Km. Biochim Biophys Acta. 1971 Sep 22;242(3):519–522. doi: 10.1016/0005-2744(71)90144-6. [DOI] [PubMed] [Google Scholar]
- Nozaki H., Sasaki Y., Sato C., Suzuki M., Okuaki A. [Cardiac output. 1. Changes in cardiac output, organ blood flow and blood flow distribution ratio in endotoxin shock]. Masui. 1973 Nov;22(12):1335–1340. [PubMed] [Google Scholar]
- Pai C. H., McLaughlin G. E. Uptake of pimelic acid by Escherichia coli and Pseudomonas denitrificans. Can J Microbiol. 1969 Jul;15(7):809–810. doi: 10.1139/m69-140. [DOI] [PubMed] [Google Scholar]
- Ploux O., Marquet A. The 8-amino-7-oxopelargonate synthase from Bacillus sphaericus. Purification and preliminary characterization of the cloned enzyme overproduced in Escherichia coli. Biochem J. 1992 Apr 15;283(Pt 2):327–331. doi: 10.1042/bj2830327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



