Abstract
Hereditary deafness and retinal dystrophy are each genetically heterogenous and clinically variable. Three small unrelated families segregating the combination of deafness and retinal dystrophy were studied by exome sequencing (ES). The proband of Family 1 was found to be compound heterozygous for NM_004525.3: LRP2: c.5005A > G, p.(Asn1669Asp) and c.149C > G, p.(Thr50Ser). In Family 2, two sisters were found to be compound heterozygous for LRP2 variants, p.(Tyr3933Cys) and an experimentally confirmed c.7715 + 3A > T consensus splice-altering variant. In Family 3, the proband is compound heterozygous for a consensus donor splice site variant LRP2: c.8452_8452 + 1del and p.(Cys3150Tyr). In mouse cochlea, Lrp2 is expressed abundantly in the stria vascularis marginal cells demonstrated by smFISH, single-cell and single-nucleus RNAseq, suggesting that a deficiency of LRP2 may compromise the endocochlear potential, which is required for hearing. LRP2 variants have been associated with Donnai–Barrow syndrome and other multisystem pleiotropic phenotypes different from the phenotypes of the four cases reported herein. Our data expand the phenotypic spectrum associated with pathogenic variants in LRP2 warranting their consideration in individuals with a combination of hereditary hearing loss and retinal dystrophy.
Keywords: deafness, Donnai–Barrow syndrome, LRP2, megalin, retinal dystrophy, RNAseq, stria vascularis
1 |. INTRODUCTION
Multiple pathogenic mechanisms and numerous genes are associated with deafness or retinal dystrophy.1,2 There are also monogenic syndromes including loss of hearing and vision. Usher syndrome (USH, OMIM 276900) is one such example and is the most common deafblindness disorder. USH is genetically and clinically heterogenous and associated with variants of several different genes3 (Table S1). Atypical USH shows deviations in either retinal, auditory, or vestibular aspects.3,4 Stickler syndrome (STL) is also characterized by clinically variable vision and hearing loss as well as skeletal abnormalities.5 This study included three small families presenting with hereditary deafness and retinal dystrophy for whom we did not identify biallelic pathogenic variants in any of the USH or STL-associated genes.3,5 Exome sequencing (ES) of probands’ genomic DNA revealed biallelic compound heterozygous variants of LRP2 (OMIM 600073) encoding LRP2, the low-density lipoprotein receptor related protein 2 (a.k.a. megalin), an evolutionarily conserved multifunctional cell-surface endocytic receptor of the low-density lipoprotein receptor (LDLR) family.6
2 |. MATERIALS AND METHODS
Informed consent was obtained from participants as approved by the Combined NIH IRB for National Eye Institute (NEI) protocols 05-EI-0096 and 08-EI-0102. Extensive clinical and molecular genetic data and experimental methods are available in Data S1.
3 |. RESULTS
The Family 1 proband is the only child of unrelated, unaffected parents of European ancestry, who report no family history of hearing or vision loss. The proband has a profound sensorineural hearing loss and has two cochlear implants (Figures 1A and S1A). Vestibular areflexia was detected as bilaterally absent cervical vestibular evoked myogenic potentials and no vestibular contribution to postural stability was measured on computerized dynamic platform posturography.3 Electroretinography performed at 14 years of age was consistent with rod-cone degeneration with severely reduced rod and cone amplitudes and an electronegative scotopic combined response, myopic astigmatism, and a severe color vision deficit.
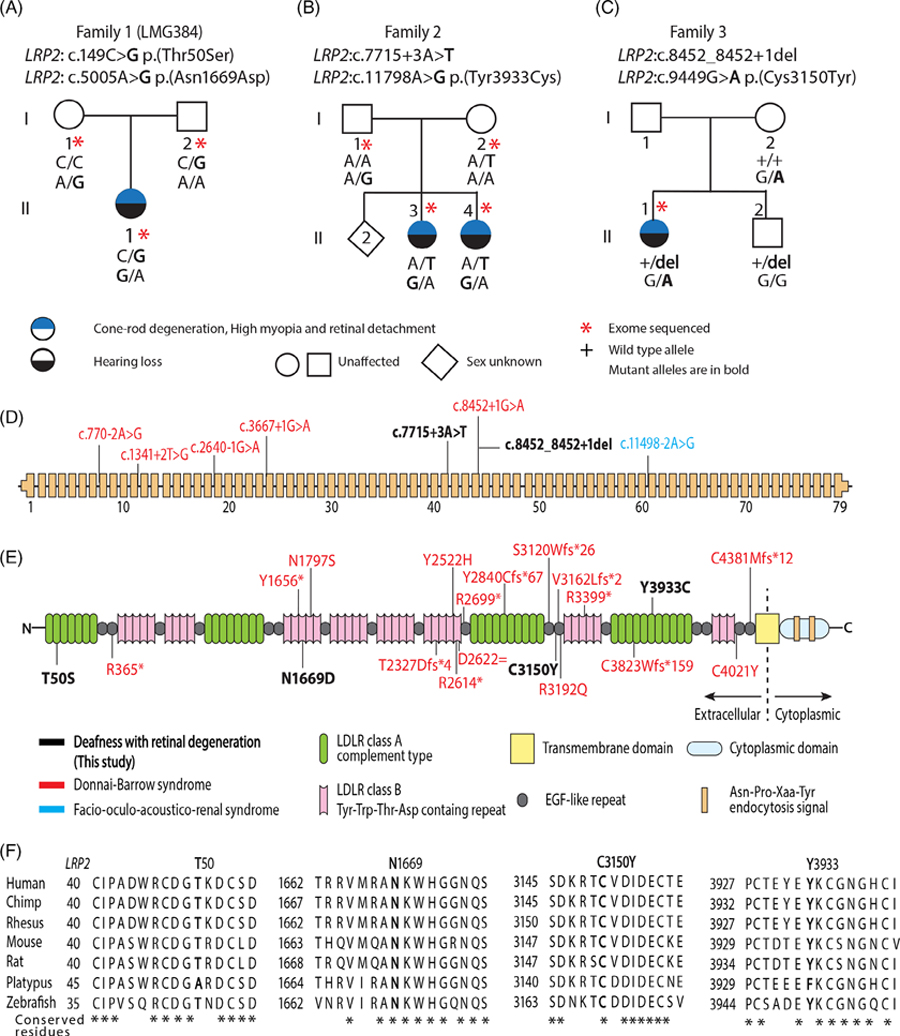
FIGURE 1.
Hearing loss and retinal dystrophy associated with LRP2 variants. (A–C) Pedigrees of Families 1, 2, and 3. (D) The 79 coding exons of LRP2 and locations of reported splice variants. (E) Protein domains of LRP2 protein (RefSeq ID NP_004516.2) with reported missense variants (modified from Kantarci et al.7). (F) Conservation of human Thr-50, Asn-1669, Cys-3150, and Tyr-3933 residues in LRP2 orthologues (RefSeq ID: Human, NP_004516.2; Chimp, XP_009441920.3; Rhesus, XP_014965780.2; Mouse, NP_001074557.1; Rat, NP_110454.2; Platypus, XP_028927340.1; Zebrafish, NP_001181916.1).
Two sisters of Family 2 were ascertained at the Dayton Children’s Hospital in Ohio (Figure 1B). The older sister’s recent examination at 12 years old noted a corrected visual acuity of 20/200 in both eyes. She was diagnosed with advanced myopic degeneration, retinal dystrophy bilaterally, and mild juvenile cataracts in both eyes. She passed newborn hearing screening but was subsequently diagnosed with sensorineural hearing loss (Figure S1). The younger sister of Family 2 was seen as a 6 years old at the Dayton Children’s Hospital and has bilateral high myopia and right eye esotropia as a 1 year old. Retinal dystrophy and advanced myopic degeneration bilaterally were also noted (Figure S2). Due to reports of proteinuria associated with variants of LRP2, renal evaluation was pursued for both siblings, which revealed persistent proteinuria.8
The proband from Family 3 was 16 years old at the time of her presentation to the NEI/NIH (Figure 1C,D). She had proteinuria and a history of high myopia since infancy and had undergone surgical correction (scleral buckle, pars plana vitrectomy, and laser retinopexy) of a left eye retinal detachment, cataract extraction and intraocular lens placement (Figure S2).
Parents and proband of Family 1 were studied by ES (Figure 1). Biallelic pathogenic variants were not detected in USH-associated genes (Tables S1 and S2) or in genes associated with nonsyndromic deafness itemized on the HHLHP (https://hereditaryhearingloss.org). However, two deleterious variants of LRP2 were identified in trans-configuration (RefSeq ID NM_004525.3: c.149C > G, p.(Thr50Ser) in exon 2 and c.5005A > G, p. (Asn1669Asp) in exon 30), confirmed by Sanger sequencing and co-segregated with the phenotype as recessive variants (Figures 1A and S1A). The p.(Asn1669Asp) variant is predicted to be damaging by multiple in silico tools (Table S3). The p.(Thr50Ser) and p.(Asn1669Asp) are located in the extracellular LDLR class A and B domains, respectively. The human Thr-50 and Asn-1669 residues are well conserved among species (Figure 1F). Perhaps these variants influence ligand binding in LDLR class A and B domains of LRP2. Estrogen binds LRP2 in the inner ear, and estrogen transport reduction may cause a hearing loss.9,10
Compound heterozygous LRP2 variants were detected in both sisters of Family 2 (Figure 1B) c.11798A > G in exon 63, p.(Tyr3933Cys) and a novel predicted splice site variant c.7715 + 3A > T in intron 41. Both variants are absent from gnomAD (Table S3) and are predicted to be damaging. The c.7715 + 3A > T variant is predicted to disrupt the LRP2 exon 41 consensus donor splice site by Splice AI, Human Splice Finder, NetGene2 and Alternative Splice Site Predictor, which was experimentally confirmed by a mini-gene splicing assay (Figure S4). The proband of Family 3 was diagnosed with a hearing loss and retinopathy. She is compound heterozygous for a consensus splice site variant LRP2: c.8452_8452 + 1del and c.9449G > A, p.(Cys3150Tyr, CADD 25.7) (Figures 1C and S1C).
In mouse, Lrp2 mRNA is expressed in the stria vascularis (SV) marginal cells, Reissner’s membrane and spindle cells (Figure S4D), consistent with immunofluorescence localization of LRP2.11 Our scRNA and snRNA-seq data show expression of Lrp2 predominantly in SV marginal cells (Figure S4E).
4 |. DISCUSSION
The longest human LRP2 isoform has 4655 residues encoded by 79 exons (UniProtKB P98164). LRP2 has 4398 extracellular residues which interact with a variety of ligands, a transmembrane domain and a small intracellular cytoplasmic domain at the C-terminus containing an endocytosis signal sequence12 (Figure 1E). LRP2 is internalized upon ligand-binding, controlling functions such as lipoprotein metabolism and endocytic uptake in kidney. LRP2 also modulates sonic hedgehog signaling in mouse and is required for normal development of the inner ear and eye.13 Additionally, in SV marginal cells, LRP2 is the receptor for aminoglycoside, which is cytotoxic to inner hair cells.14
Our RNAseq data suggest a defect in the SV leading to hearing loss in patients with LRP2 mutations. The SV generates the +80 millivolt endocochlear potential driving sound transduction, which may be compromised by biallelic LRP2 variants, suggesting an unreported combination of a strial defect with retinal dystrophy to account for the deaf-blindness phenotype.
Variants of human LRP2 are associated with a panoply of clinically complex disorders (Table S4, Figure S3) including Donnai–Barrow syndrome (DBS, OMIM 222448) characterized by severe myopia, iris coloboma or retinal detachment, sensorineural hearing loss, intellectual disability, craniofacial malformations, agenesis of the corpus callosum (ACC), congenital diaphragmatic hernia (CDH), and omphalocele.7 This phenotypic spectrum indicates functions for LRP2 in many organs (Figures 1D,E and S3). Twenty-three different variants distributed across LRP2 are associated with DBS and most are loss of function alleles (DVD). However, our study probands do not display craniofacial dysmorphology, ACC or CDH suggesting that one of the two variants of LRP2 segregating in each of the three studied families must not fully disable LRP2 function, acting as hypomorphic alleles associated with a more limited deaf-blindness phenotype.
A plausible hypomorphic allele in the Family 1 proband is LRP2 p. (Thr50Ser). There are five p.(Thr50Ser) homozygotes of Finnish ancestry in gnomAD data (v3.1.2). Their hearing and visual status are unknown. ACMG/AMP recommends excluding bottlenecked founder populations including Finnish Europeans as a filtering population where pathogenic allele frequencies may rise above 5%. Moreover, in gnomAD there are 95 individuals homozygous for GJB2 variant p.(Glu114Gly) associated convincingly with recessive deafness DFNB1, 39 homozygotes for CDH23: p.(Val475Met), and 24 homozygotes for SLC26A4: p.(Asn324Tyr).
LRP2 p.(Thr50Ser) has a CADD score of 20.2 suggesting pathogenicity. Nevertheless, we considered two possibilities. p.(Thr50Ser) is a benign variant in disequilibrium with a pathogenic recessive variant elsewhere in LRP2 gene that was not detected by ES analysis, although we had adequate ES coverage for all 79 exons of LRP2. Alternatively, p.(Thr50Ser) is pathogenic when in trans with a more disabling variant of LRP2 such as p.(Asn1669Asp). There are examples of hypomorphic risk alleles that are pathogenic only in compound heterozygosity with a damaging variant.15 For example, the noncoding CEVA SNP haplotype has a 5.6% carrier frequency in controls of European ancestry, is located upstream of the first exon of an otherwise wild-type SLC26A4 gene, and is associated with enlarged vestibular aqueduct when in trans with a pathogenic SLC26A4 variant.16
The conditional silencing of mouse Lrp2 expression in developing ocular tissue caused high myopia and a decrease in bipolar, photoreceptor and retinal ganglion cells, similar to the lrp2 bugeye phenotype in zebrafish.17 Homozygous Lrp2 knockout mice typically die from respiratory failure, but a few survive to adulthood.18 Survivors display brain malformations including forebrain fusion, a common ventricular system, lack of the olfactory bulb and enlarged eyes typical of high myopia and are deaf at 3 months of age further demonstrating the importance of LRP2 in the mammalian eye and ear.9
Data from cohorts of 153 nonsyndromic and syndromic deaf probands from Ghana, 500 families from Pakistan and a multi-ethnic North American cohort of 50 families screened with ES and 654 probands screened with a targeted deafness-gene panel did not yield additional biallelic pathogenic variants of LRP2 associated with deafblindness. Thus, variants of LRP2 appear to be a rare cause of human deaf-blindness in these populations. Nevertheless, the clinical and genetic data of the three unrelated probands reported here and the phenotype of the Lrp2 mutant mouse emphasize the need to evaluate LRP2 sequence in individuals with hearing loss and retinal dystrophy.2
Supplementary Material
ACKNOWLEDGMENTS
We thank the families for participation in this study, and Drs. Roux, Hertzano, and Olszewski for evaluating the manuscript. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the NIH (http://biowulf.nih.gov).
FUNDING INFORMATION
Funded (in part) by the NIDCD, DIR DC000088 to Michael Hoa, DC000039 to Thomas B. Friedman, DC000060 to Andrew J. Griffith, DC000086 to Robert J. Morell, NIDCD/NIDCR Genomics and Computational Biology Core, Wellcome Trust 107755Z/15/Z to Gordon A. Awandare and Ambroise Wonkam, NHGRI/NIH U01-HG-009716, African Academy of Science/Wellcome Trust H3A/18/001 to Ambroise Wonkam, and NIDCD R01s DC01165, DC003594, DC016593 and DC017712 to Suzanne M. Leal.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare there is no potential conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/cge.14312.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Variants identified here are in Clinvar (https://www.ncbi.nlm.nih.gov/clinvar/) SUB12100712, SUB12101044, SUB12114010, SUB12114033, SUB11119334.
REFERENCES
- 1.Medina G, Perry J, Oza A, Kenna M. Hiding in plain sight: genetic deaf-blindness is not always Usher syndrome. Cold Spring Harb Mol Case Stud. 2021;7(4):a006088–a006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith AJ, Friedman TB. Hereditary hearing loss. Ballenger’s Otorhinolaryngology Head and Neck Surgery. 18th ed. People’s Medical Publishing House; 2016:329–345. [Google Scholar]
- 3.Wafa TT, Faridi R, King KA, et al. Vestibular phenotype-genotype correlation in a cohort of 90 patients with Usher syndrome. Clin Genet. 2021;99(2):226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igelman AD, Ku C, da Palma MM, et al. Expanding the clinical phenotype in patients with disease causing variants associated with atypical Usher syndrome. Ophthalmic Genet. 2021;42(6):664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boothe M, Morris R, Robin N. Stickler syndrome: a review of clinical manifestations and the genetics evaluation. J Pers Med. 2020;10(3):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors—an evolutionarily ancient multifunctional receptor family. Biol Chem. 2010;391(11):1341–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarci S, Al-Gazali L, Hill RS, et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculoacoustico-renal syndromes. Nat Genet. 2007;39(8):957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnai D, Barrow M. Diaphragmatic hernia, exomphalos, absent corpus callosum, hypertelorism, myopia, and sensorineural deafness: a newly recognized autosomal recessive disorder? Am J Med Genet. 1993;47(5):679–682. [DOI] [PubMed] [Google Scholar]
- 9.Konig O, Ruttiger L, Muller M, et al. Estrogen and the inner ear: megalin knockout mice suffer progressive hearing loss. FASEB J. 2008;22(2):410–417. [DOI] [PubMed] [Google Scholar]
- 10.Shuster B, Casserly R, Lipford E, et al. Estradiol protects against noise-induced hearing loss and modulates auditory physiology in female mice. Int J Mol Sci. 2021;22(22):12208–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosokawa S, Hosokawa K, Ishiyama G, Ishiyama A, Lopez IA. Immunohistochemical localization of megalin and cubilin in the human inner ear. Brain Res. 2018;1701:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willnow TE, Hilpert J, Armstrong SA, et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA. 1996;93(16):8460–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son EJ, Ma JH, Ankamreddy H, et al. Conserved role of sonic hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc Natl Acad Sci USA. 2015;112(12):3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Ricci AJ. In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective. Proc Natl Acad Sci USA. 2022;119(9):e2117946119–e2117946129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yildiz Bolukbasi E, Karolak JA, Szafranski P, et al. Exacerbation of mild lung disorders to lethal pulmonary hypoplasia by a noncoding hypomorphic SNV in a lung-specific enhancer in trans to the frameshifting TBX4 variant. Am J Med Genet A. 2022;188(5):1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chattaraj P, Munjal T, Honda K, et al. A common SLC26A4-linked haplotype underlying non-syndromic hearing loss with enlargement of the vestibular aqueduct. J Med Genet. 2017;54(10):665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veth KN, Willer JR, Collery RF, et al. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7(2):e1001310–e1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suber T, Mallampalli RK. An emerging role for megalin as a regulator of protein leak in acute lung injury. Am J Respir Cell Mol Biol. 2017;57(5):504–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Variants identified here are in Clinvar (https://www.ncbi.nlm.nih.gov/clinvar/) SUB12100712, SUB12101044, SUB12114010, SUB12114033, SUB11119334.